-
PDF
- Split View
-
Views
-
Cite
Cite
A. Kamal, R. Mansour, I. Fahmy, G. Serour, C. Rhodes, M. Aboulghar, Easily decapitated spermatozoa defect: a possible cause of unexplained infertility, Human Reproduction, Volume 14, Issue 11, November 1999, Pages 2791–2795, https://doi.org/10.1093/humrep/14.11.2791
Close - Share Icon Share
Abstract
We report the first 16 cases of a new sperm abnormality which we call `easily decapitated spermatozoa defect'. This was discovered during intracytoplasmic sperm injection (ICSI) in couples with unexplained infertility. Semen analysis was normal, but minimal micromanipulation for ICSI resulted in decapitation of the spermatozoon during immobilization. For some oocytes the head and tail were injected separately, in others the intact sperm was injected after minimal immobilization. A fertilization rate of 47.5% was obtained using ICSI. Conventional in-vitro fertilization (IVF) on sibling oocytes (three cases) or in a previous cycle (three cases) resulted in total failure of fertilization. All patients reached the embryo transfer stage and three pregnancies resulted. Findings on electron microscopy in four cases included spermatozoa with degeneration or absence of the basal plate, abnormalities of the proximal centriole and degeneration of the midpiece with a large cytoplasmic droplet. We conclude that an occult sperm abnormality presenting as easily decapitated spermatozoa during ICSI could be a cause of unexplained infertility, as it resulted in total failure of fertilization in conventional IVF. Further research is necessary to investigate this sperm abnormality.
Introduction
Following the first reports of intracytoplasmic sperm injection (ICSI) in humans, it has become the standard treatment for male factor infertility (Palermo et al., 1992; Van Steirteghem et al., 1993). The scope of ICSI has widened to include patients with acrosomeless spermatozoa (Lundin et al., 1994), unexplained infertility (Aboulghar et al., 1996), borderline semen (Aboulghar et al., 1995), immunological infertility (Nagy et al., 1995), and previous failure of fertilization in conventional IVF (Cohen et al., 1994). Patients with obstructive and non-obstructive azoospermia have been treated successfully with ICSI using surgically retrieved spermatozoa from the epididymis or testis (Tournaye et al., 1994; Silber et al., 1995; Mansour et al., 1996).
Several types of abnormalities involving separation of the sperm head and tail have been described. These include reports of patients with a spermiogram in which all spermatozoa showed decapitation (Perotti et al., 1981; Baccetti et al., 1984; Toyama et al., 1995). This abnormality, known as decapitated sperm defect, occurs at a testicular level.
We report a new category of sperm head defect in the form of an easily detachable head, which could be related to decapitated sperm defect. This abnormality may be a previously undiscovered cause of unexplained infertility in some patients.
Materials and methods
Patients
The subjects were 16 couples with unexplained infertility. Repeated semen analyses for the husband were normal according to World Health Organization recommendations (World Health Organization, 1993). Tubal patency was confirmed in the women using laparoscopy in eight cases. In eight patients the diagnosis of unexplained infertility was based on the hysterosalpingogram result and clinical findings, and laparoscopy was not performed. All women had regular menstrual cycles and day 21 progesterone was in the ovulatory range. Easily decapitated spermatozoa were found during routine ICSI in all couples. Conventional IVF was attempted in an earlier cycle in three of the couples, with total failure of fertilization. A further three couples had conventional IVF on sibling oocytes at the time of ICSI. This is the policy of our centre for couples with unexplained infertility if an adequate number of oocytes are obtained with no previous failure of IVF.
Ovarian stimulation
All patient cycles were suppressed with a gonadotrophin releasing hormone agonist long protocol, using 200 μg/8 h Suprefact nasal spray (Hoechst AG, Frankfurt am Main, Germany) in the luteal phase of the cycle until the day of human chorionic gonadotrophin (HCG) injection. After ovarian suppression was confirmed by low serum oestradiol concentration and ultrasound, human menopausal gonado-trophin (HMG, Pergonal; E.I.P. Co. Industries, Cairo, Egypt) was given as 150 IU daily i.m. for 6 days. The dose was then modified according to the ovarian response. Monitoring was commenced after six days of HMG injection, using vaginal US and serum oestradiol. Ten thousand units of HCG (Pregnyl; Nile Co., Cairo, Egypt) were given i.m. when two or more follicles reached 18 mm in mean diameter. Transvaginal ultrasound ovum retrieval was scheduled 36 h after HCG injection.
Oocyte handling
For ICSI, the oocytes were denuded of their surrounding cumulus cells 2 h after retrieval, using hyaluronidase 80 IU/ml in HEPES-buffered Earle's balanced salt solution (catalogue no. 1200; Medicult, Copenhagen, Denmark) for 10–15 s. The oocytes were then transferred to Ham's F-10 (Gibco Laboratory, Grand Island, NY, USA) for complete removal of the corona cells by repeated aspiration in a finely pulled pipette. The oocytes were rinsed and incubated in G1 media (Ref 8010; Scandinavian IVF Science AB, Vitrolife AB, Molndalsvagen 30, S-412, 63 Gothenburg, Sweden) under mineral oil (Squibb, Princeton, NJ, USA) until the time of injection, which was done only for oocytes that extruded their first polar bodies. The microinjection procedure has been described previously (Mansour, 1998). In cases of conventional IVF, the oocytes were cultured under mineral oil in G1 media for 4–6 h before insemination. Approximately 5×104 motile spermatozoa were added to each oocyte and incubation was done at 5% CO2 in air at 37°C.
Semen processing
Semen samples were collected on the day of oocyte collection. After complete liquefaction, the semen was washed twice with Ham's F-10 and centrifuged at 300 g for 7 min. The final pellet was resuspended in 0.2 ml medium and layered carefully in the surrounding ring of a Tea tube (RI Limited, Cornwall, UK), filled with Ham's F-10, and incubated until the time of injection. Sperm suspension was aspirated from the central cone of the Tea tube and the count was adjusted from 2 to 6×106/ml. Approximately 2 μl from this sperm suspension was added to the polyvinlpyrrolidone microdroplet (PVP 10%; catalogue no. 1089, Medicult, Copenhagen, Denmark).
Embryo transfer
This was done on day 2 or 3 after oocyte collection using the Wallace catheter (catalogue no. 1816N; H.G.Wallace Ltd, Colchester, Essex, UK) or the Labotect catheter (Labotect, Bonvender-Gottingen, Germany) if the Wallace catheter could not be introduced. Luteal phase support was given routinely in the form of 2500 IU of HCG every fourth day. Cases that were considered at high risk of developing ovarian hyperstimulation syndrome were given a daily progesterone injection (100 mg, progesterone USP; Steris, Phoenix, AZ, USA) in place of the HCG. A serum β-HCG test was done 2 weeks after the transfer, and ultrasound examination was performed after a further 2–3 weeks for patients with a positive test. Clinical pregnancy was diagnosed by the presence of a gestational sac with fetal echoes.
ICSI using easily decapitated spermatozoa
It was very difficult to complete the immobilization and compression steps prior to ICSI in these 16 cases. In some samples, even gentle immobilization of the spermatozoa by touching the midpiece resulted in detachment of the sperm head. The spermatozoa often adhered to the bottom of the dish in the region between the head and tail, and when trying to free a spermatozoon it was invariably decapitated. In order to prevent this, gentle immobilization was done and the spermatozoon was drawn into the injector head-first to free it. Suction was repeated while pushing the injector to the opposite side to free the tail from the bottom of the dish. After freeing the spermatozoon it was released and suction was applied tail-first in order to inject it.
Most oocytes were injected using spermatozoa after gentle immobilization without vigorous compression. A few oocytes were injected using the separated head and tail, if it was technically very difficult to inject an intact spermatozoon.
Electron microscopy
Semen samples were collected for electron microscopic examination in cases 1–4. The samples were examined at Ain Shams University Specialised Hospital Laboratories, Cairo, Egypt.
Results
Sixteen patients were found to have the easily decapitated sperm defect. No abnormality was detected during routine semen analysis and the spermatozoa were intact before micromanipulation.
Demographic, clinical and outcome data are presented in Table I. In three couples previous conventional IVF attempts had resulted in total failure of fertilization. Of the 16 couples in this study, IVF and ICSI on sibling oocytes were performed in three cases with an adequate number of oocytes, but fertilization did not occur with conventional IVF. In the 13 remaining cases, ICSI alone was done. With ICSI, an overall fertilization rate of 47.5% (58/122 injected oocytes) was achieved. Three pregnancies resulted, giving a pregnancy rate of 19%.
In a comparison group of 102 patients at our centre undergoing ICSI for male factor infertility using ejaculated spermatozoa with the standard technique during the same period, the fertilization rate was 56.9% (Aboulghar et al., 1997). This is not statistically different from the rate (47.5%) with easily decapitated spermatozoa using only gentle immobilization (χ2-test: 3.74 after Yates' correction).
Electron microscopy as done on four samples. In case 1, 35% of spermatozoa had normal acrosomes, 40% showed acrosomal degeneration and 25% had irregular and/or abnormally thin acrosomes. The connecting pieces of many spermatozoa showed ultrastructural defects including degeneration of the basal plate, with a gap between the head and tail, although the plasma membrane was still present. Abnormal or absent proximal centrioles were also seen, with separation between the proximal and distal centrioles. The midpieces of many tails were defective with severe degeneration present.
In case 2, 20% of spermatozoa had normal acrosomes with 30% having irregular and/or abnormally thin acrosomes and 50% with acrosomal degeneration. The connecting pieces of some spermatozoa showed basal plate degeneration, shifting of the connecting piece, and degeneration of centrioles. The midpieces of many cells had an enlarged cytoplasmic droplet.
In case 3, only 15% of acrosomes were normal, with 10% having an abnormally thin acrosome, 40% irregular acrosomes and 35% showing acrosomal degeneration. Examination of the connecting pieces revealed absence of the basal plate, abaxial implantation of the midpiece, nuclear degeneration, or separation of the nucleus from the basal plate. Some spermatozoa were decapitated. Examination of the midpieces revealed an incomplete mitochondrial sheath or a large cytoplasmic droplet in some spermatozoa.
In case 4, 30% of sperm heads seen in the sample had a normal acrosome, ~10% had irregular and/or abnormally thin acrosome and ~60% showed acrosomal degeneration. Examination of the connecting pieces revealed that ~25% of spermatozoa showed no basal plate or proximal microtubules; finely granular material in the area of the connecting piece and the beginning of the midpiece was also seen. Examination of the midpieces revealed an abnormal mitochondrial sheath and a large cytoplasmic droplet in some sperm cells.
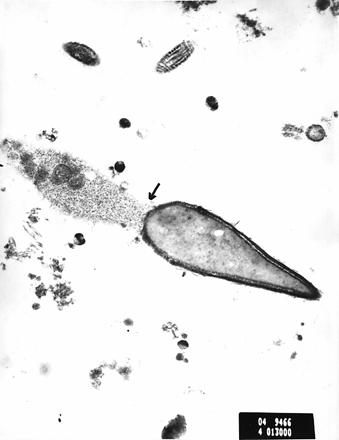
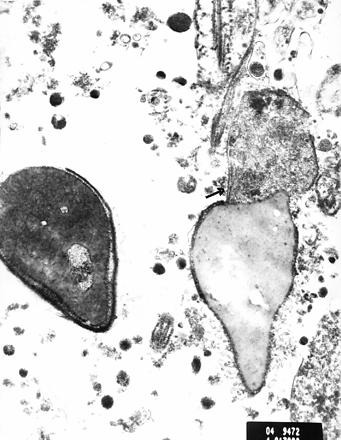
Figures 1 and 2 show electron micrographs of sperm cells from case 4. Figure 1 shows the finely granular material in the region between the connecting piece and the beginning of the midpiece, as shown by the arrow. Figure 2 also shows this finding in another sperm cell. We suggest that this may represent the beginning of the process of degeneration, which could lead to easy decapitation of the spermatozoon on manipulation.
Discussion
In this paper we present a series of patients with unexplained infertility, normal semen analysis, and spermatozoa which were easily decapitated on micromanipulation for ICSI. Unexplained infertility is defined as a couple failing to conceive after 2 years of regular intercourse, with no definite cause for infertility after standard investigations in both partners. Despite improved diagnostic techniques, the average incidence of unexplained infertility is reported as ~15% among infertile couples (Templeton et al., 1982). It is believed that longstanding unexplained infertility may be caused by one or more specific factors, as yet undetected. Easily decapitated spermatozoa could be one of these factors and will only be diagnosed during immobilization of spermatozoa for ICSI.
To the best of our knowledge, this is the first report in the literature that describes the easily decapitated sperm defect. This sperm anomaly may be related to the decapitated sperm defect reported previously in Guernsey bulls (Blom and Brich Anderson, 1970), and subsequently in humans (Perotti et al., 1981). The latter paper was a case report of an infertile man with a spermiogram in which `100% of the spermatozoa displayed separation of head from tail at the level of the proximal centriole. Most tails were normally structured and ended anteriorly with the proximal centriole covered by a continuous plasma membrane'. A variety of defects was found in a small number of tails. No implantation fossa was found in the loose heads. The authors concluded that the separation of heads from tails was due to a specific morphogenetic defect arising at different stages of spermatid differentiation, resulting in the varying types of tail structure.
Toyama et al. reported a case of an infertile man with >90% of headless spermatozoa, with few tailless heads, in an otherwise normal spermiogram (Toyama et al., 1995). Several ultrastructural abnormalities were found in the sperm heads, including abnormalities in the nuclear membrane covering the posterior pole of the nucleus. The implantation fossa and the basal plate were not formed and the decapitation seemed to occur between the basal plate and the proximal centriole. Another case report studied the testicular biopsy specimens of a man with decapitated spermatozoa and found that this malformation was due to `an overproduction of a membraneous vesicles system by the Golgi complex in the region between the centrioles and acrosome during the spermatid stage' (Baccetti et al., 1984). These few case reports in the literature describe semen specimens in which the separation of heads and tails is already present on microscopy, having occurred at a testicular or epididymal level.
Electron microscopy in four of our cases revealed a number of abnormalities in sperm ultrastructure. These included degeneration or absence of the basal plate, abnormalities of the proximal centriole, and degeneration of the midpiece with a large cytoplasmic droplet. These findings are similar to those in the cases above. Further work is needed on more samples to quantify and assess the significance of these results.
In the 16 cases reported here the fertilization rate using ICSI in patients with easily decapitated spermatozoa (47.5%) was not significantly different from a comparison group of 102 patients undergoing ICSI (56.9%) (Aboulghar et al., 1997), despite the omission of vigorous sperm immobilization. The adherence of such spermatozoa to the bottom of the dish and the process of freeing them may be sufficient to accelerate membrane destabilization, and no further manipulation is needed. We wished to inject an intact spermatozoon in order to avoid the possibility of losing genetic material after decapitation, as well as the technical difficulties of injecting a separated head and the problem of the tail adhering to the inside of the injecting pipette.
In this study we have reported a sperm abnormality that could contribute to the cause of complete fertilization failure using conventional IVF in patients with unexplained infertility. In this series, six patients had total failure of fertilization with IVF (three in previous cycles). We suggest that the cause of infertility in these cases may also be failure of fertilization in-vivo due to the easy decapitation of the spermatozoa.
Based on the information gained from this case series, semen testing in couples with a long history of unexplained infertility could be extended to investigate this possibility. After routine examination to assess the motility and morphology, a drop of semen in the micropipette is transferred to the regular injection ICSI dish containing PVP. Using the injecting pipette, as in the normal procedure for ICSI, a spermatozoon is touched for immobilization and then pressed gently against the bottom of the dish. The operator will observe if the sperm head is easily decapitated during the immobilization process and record the rate of decapitation in a number of immobilized spermatozoon. Electron microscopy may also prove useful in the detection of this abnormality.
We have suggested that the easily decapitated sperm defect may be a cause of failure of fertilization in vivo or in vitro, and thus a possible cause of some cases of unexplained infertility. Further research is needed before firmer conclusions are drawn. We have reported this abnormality to highlight a particular sperm problem, which needs further study. Ideally we would have complete data with laparoscopy, IVF and electron microscopy in all cases. We are conducting an ongoing prospective study of cases with decapitated sperm defect, with electron microscopy in all cases.
In conclusion, the easily decapitated sperm defect could be a cause of fertilization failure in some cases of unexplained infertility. The use of ICSI is a treatment option in these cases, and resulted in three successful pregnancies in this case series.
Clinical details and outcome
| Case . | Age (years) . | Infertility duration (years) . | No. of oocytes IVF retrieved . | No. of oocytes . | No. . | Outcome . | ||
|---|---|---|---|---|---|---|---|---|
| no. . | Woman . | Man . | . | . | ICSI (F/UF) . | ET (F/UF) . | . | . |
| aIVF done in previous cycle. | ||||||||
| F = fertilized; UF = unfertilized; ET = embryos transferred; IVF = in-vitro fertilization; ICSI = intracytoplasmic sperm injection. | ||||||||
| 1 | 33 | 38 | 13 | 2 | 1/2 | 1 | no pregnancy | |
| 2 | 35 | 37 | 11 | 5 | 0/10a | 5/5 | 3 | no pregnancy |
| 3 | 32 | 35 | 10 | 5 | 4/5 | 2 | no pregnancy | |
| 4 | 28 | 35 | 3 | 40 | 0/10 | 5/17 | 5 | delivered |
| 5 | 37 | 42 | 13 | 22 | 0/11 | 2/10 | 2 | no pregnancy |
| 6 | 36 | 40 | 14 | 7 | 5/6 | 5 | no pregnancy | |
| 7 | 32 | 42 | 7 | 9 | 4/9 | 4 | pregnant | |
| 8 | 34 | 36 | 8 | 17 | 0/4 | 4/13 | 4 | no pregnancy |
| 9 | 40 | 49 | 10 | 16 | 0/10a | 8/16 | 5 | no pregnancy |
| 10 | 37 | 38 | 8 | 7 | 4/6 | 4 | no pregnancy | |
| 11 | 38 | 32 | 20 | 2 | 1/2 | 1 | no pregnancy | |
| 12 | 32 | 33 | 8 | 7 | 4/7 | 4 | pregnant | |
| 13 | 27 | 30 | 5 | 10 | 0/11a | 3/8 | 3 | no pregnancy |
| 14 | 33 | 40 | 7 | 3 | 1/3 | 1 | no pregnancy | |
| 15 | 36 | 40 | 2 | 9 | 4/9 | 4 | no pregnancy | |
| 16 | 32 | 40 | 5 | 4 | 3/4 | 2 | no pregnancy | |
| Case . | Age (years) . | Infertility duration (years) . | No. of oocytes IVF retrieved . | No. of oocytes . | No. . | Outcome . | ||
|---|---|---|---|---|---|---|---|---|
| no. . | Woman . | Man . | . | . | ICSI (F/UF) . | ET (F/UF) . | . | . |
| aIVF done in previous cycle. | ||||||||
| F = fertilized; UF = unfertilized; ET = embryos transferred; IVF = in-vitro fertilization; ICSI = intracytoplasmic sperm injection. | ||||||||
| 1 | 33 | 38 | 13 | 2 | 1/2 | 1 | no pregnancy | |
| 2 | 35 | 37 | 11 | 5 | 0/10a | 5/5 | 3 | no pregnancy |
| 3 | 32 | 35 | 10 | 5 | 4/5 | 2 | no pregnancy | |
| 4 | 28 | 35 | 3 | 40 | 0/10 | 5/17 | 5 | delivered |
| 5 | 37 | 42 | 13 | 22 | 0/11 | 2/10 | 2 | no pregnancy |
| 6 | 36 | 40 | 14 | 7 | 5/6 | 5 | no pregnancy | |
| 7 | 32 | 42 | 7 | 9 | 4/9 | 4 | pregnant | |
| 8 | 34 | 36 | 8 | 17 | 0/4 | 4/13 | 4 | no pregnancy |
| 9 | 40 | 49 | 10 | 16 | 0/10a | 8/16 | 5 | no pregnancy |
| 10 | 37 | 38 | 8 | 7 | 4/6 | 4 | no pregnancy | |
| 11 | 38 | 32 | 20 | 2 | 1/2 | 1 | no pregnancy | |
| 12 | 32 | 33 | 8 | 7 | 4/7 | 4 | pregnant | |
| 13 | 27 | 30 | 5 | 10 | 0/11a | 3/8 | 3 | no pregnancy |
| 14 | 33 | 40 | 7 | 3 | 1/3 | 1 | no pregnancy | |
| 15 | 36 | 40 | 2 | 9 | 4/9 | 4 | no pregnancy | |
| 16 | 32 | 40 | 5 | 4 | 3/4 | 2 | no pregnancy | |
Clinical details and outcome
| Case . | Age (years) . | Infertility duration (years) . | No. of oocytes IVF retrieved . | No. of oocytes . | No. . | Outcome . | ||
|---|---|---|---|---|---|---|---|---|
| no. . | Woman . | Man . | . | . | ICSI (F/UF) . | ET (F/UF) . | . | . |
| aIVF done in previous cycle. | ||||||||
| F = fertilized; UF = unfertilized; ET = embryos transferred; IVF = in-vitro fertilization; ICSI = intracytoplasmic sperm injection. | ||||||||
| 1 | 33 | 38 | 13 | 2 | 1/2 | 1 | no pregnancy | |
| 2 | 35 | 37 | 11 | 5 | 0/10a | 5/5 | 3 | no pregnancy |
| 3 | 32 | 35 | 10 | 5 | 4/5 | 2 | no pregnancy | |
| 4 | 28 | 35 | 3 | 40 | 0/10 | 5/17 | 5 | delivered |
| 5 | 37 | 42 | 13 | 22 | 0/11 | 2/10 | 2 | no pregnancy |
| 6 | 36 | 40 | 14 | 7 | 5/6 | 5 | no pregnancy | |
| 7 | 32 | 42 | 7 | 9 | 4/9 | 4 | pregnant | |
| 8 | 34 | 36 | 8 | 17 | 0/4 | 4/13 | 4 | no pregnancy |
| 9 | 40 | 49 | 10 | 16 | 0/10a | 8/16 | 5 | no pregnancy |
| 10 | 37 | 38 | 8 | 7 | 4/6 | 4 | no pregnancy | |
| 11 | 38 | 32 | 20 | 2 | 1/2 | 1 | no pregnancy | |
| 12 | 32 | 33 | 8 | 7 | 4/7 | 4 | pregnant | |
| 13 | 27 | 30 | 5 | 10 | 0/11a | 3/8 | 3 | no pregnancy |
| 14 | 33 | 40 | 7 | 3 | 1/3 | 1 | no pregnancy | |
| 15 | 36 | 40 | 2 | 9 | 4/9 | 4 | no pregnancy | |
| 16 | 32 | 40 | 5 | 4 | 3/4 | 2 | no pregnancy | |
| Case . | Age (years) . | Infertility duration (years) . | No. of oocytes IVF retrieved . | No. of oocytes . | No. . | Outcome . | ||
|---|---|---|---|---|---|---|---|---|
| no. . | Woman . | Man . | . | . | ICSI (F/UF) . | ET (F/UF) . | . | . |
| aIVF done in previous cycle. | ||||||||
| F = fertilized; UF = unfertilized; ET = embryos transferred; IVF = in-vitro fertilization; ICSI = intracytoplasmic sperm injection. | ||||||||
| 1 | 33 | 38 | 13 | 2 | 1/2 | 1 | no pregnancy | |
| 2 | 35 | 37 | 11 | 5 | 0/10a | 5/5 | 3 | no pregnancy |
| 3 | 32 | 35 | 10 | 5 | 4/5 | 2 | no pregnancy | |
| 4 | 28 | 35 | 3 | 40 | 0/10 | 5/17 | 5 | delivered |
| 5 | 37 | 42 | 13 | 22 | 0/11 | 2/10 | 2 | no pregnancy |
| 6 | 36 | 40 | 14 | 7 | 5/6 | 5 | no pregnancy | |
| 7 | 32 | 42 | 7 | 9 | 4/9 | 4 | pregnant | |
| 8 | 34 | 36 | 8 | 17 | 0/4 | 4/13 | 4 | no pregnancy |
| 9 | 40 | 49 | 10 | 16 | 0/10a | 8/16 | 5 | no pregnancy |
| 10 | 37 | 38 | 8 | 7 | 4/6 | 4 | no pregnancy | |
| 11 | 38 | 32 | 20 | 2 | 1/2 | 1 | no pregnancy | |
| 12 | 32 | 33 | 8 | 7 | 4/7 | 4 | pregnant | |
| 13 | 27 | 30 | 5 | 10 | 0/11a | 3/8 | 3 | no pregnancy |
| 14 | 33 | 40 | 7 | 3 | 1/3 | 1 | no pregnancy | |
| 15 | 36 | 40 | 2 | 9 | 4/9 | 4 | no pregnancy | |
| 16 | 32 | 40 | 5 | 4 | 3/4 | 2 | no pregnancy | |
Electron micrograph of spermatozoa from case 4. The arrow shows the finely granular material in the area of the connecting piece and the beginning of the midpiece. Scale bar = 1 μm.
The finely granular material in the area of the connecting piece and the beginning of the midpiece is shown in another sperm cell. Scale bar = 1 μm.
To whom correspondence should be addressed
The authors are grateful to Dr Larissa Ananieva of Ain Shams University Specialised Hospital Laboratories, Cairo, for performing electron microscopy.
References
Aboulghar, M.A., Mansour, R.T., Serour, G.I. et al. (
Aboulghar, M.A., Mansour, R.T., Serour, G.I. et al. (
Aboulghar, M.A., Mansour, R.T., Serour, G.I. et al. (
Baccetti, B., Selmi, M.G. and Soldani, P. (
Blom, E. and Brich Anderson, A. (
Cohen, J., Alikani, M., Munné, S. et al. (
Lundin, K., Sjorgren, A., Nilsson, L. et al. (
Mansour, R.T. (
Mansour, R.T., Aboulghar, M.A., Serour, G.I. et al. (
Nagy, Z.P., Verheyen, G., Liu, J. et al. (
Palermo, G., Joris, H., Devroey, P. et al. (
Perotti, M.E., Giarola, A. and Giaricn, M. (
Silber, S.J., Van Steirteghem, A.C., Liu, J. et al. (
Templeton, A.A. and Penney, G.C. (
Tournaye, H., Devroey, P., Liu, J. et al. (
Toyama, Y., Kazama, T., Fuse, H. et al. (
Van Steirteghem, A.C., Nagy, Z., Joris, H. et al. (