-
PDF
- Split View
-
Views
-
Cite
Cite
Johan Defoor, Kevin Martens, Dominika Zielińska, Gert Matthijs, Hilde Van Nerum, Dirk Schepers, Robert Fagard, Luc Vanhees, The CAREGENE study: polymorphisms of the β1-adrenoceptor gene and aerobic power in coronary artery disease, European Heart Journal, Volume 27, Issue 7, April 2006, Pages 808–816, https://doi.org/10.1093/eurheartj/ehi737
Close - Share Icon Share
Abstract
Aims The heritability of aerobic power and of the response to physical training has been shown in healthy subjects. β1-Adrenergic receptor (β1AR) function affects exercise performance. This study aims to investigate whether the Ser49Gly and Gly389Arg polymorphisms of the β1AR gene or their haplotypes are associated with aerobic power or its response to physical training in coronary artery disease (CAD).
Methods and results Nine hundred and thirty-five biologically unrelated Caucasian patients with CAD who had exercised until exhaustion during graded bicycle testing at baseline and after completion of 3 months of exercise training from 1990 to 2001 (n=1095) were eligible for inclusion in the CAREGENE (CArdiac REhabilitation and GENetics of Exercise performance) study. Polymorphisms were detected using the invader assay (Third Wave Technologies™, Madison, Wisconsin, USA). Patients with the Gly49Gly genotype had significantly higher covariate-adjusted aerobic power at baseline than those with Ser49Ser and Ser49Gly (P<0.05). Adjusted aerobic power at baseline was highest in the Ser49–Gly389/Gly49–Gly389 and Gly49–Arg389/Gly49–Arg389 haplotype combinations. Aerobic power increased significantly (P<0.001) with physical training. There was no association with the effect of physical training.
Conclusion Ser49Gly and haplotype combinations of Ser49Gly and Gly389Arg of the β1AR gene are associated with aerobic power, but not with the response to physical training in patients with CAD included in the CAREGENE study.
This paper was guest edited by Prof. Eloisa Arbustini, IRCCS Policlinico S. Matteo, Italy
Introduction
Coronary artery disease (CAD) is currently, more than ever, a global problem with enormous economic consequences. Aerobic power, expressed as peak oxygen uptake (VO2) is an independent prognostic factor of survival in patients with cardiovascular disease.1 It is widely known that physical activity improves survival,2 prevents the progression of CAD,3 improves myocardial oxygen supply,4 and increases aerobic power.2,5 Larger improvements in aerobic power are, furthermore, associated with greater reductions in cardiovascular risk factors6 and mortality risk.7 There is, however, considerable individual variation in aerobic power as well as in the response to physical training6,8 of which, particularly in CAD, the larger part remains unexplained.8
Numerous studies in healthy families9,10 and twins11–14 provide strong indication that the genetic make-up of individuals contributes to the large variation in aerobic power. Estimates of heritability for aerobic power in sedentary subjects in those studies range between 25 and 66%,9–14 with larger resemblance in monozygotic than in dizygotic twins.11–14 The response of aerobic power to physical training has also been shown to be genotype-dependent in monozygotic twins15 and familial aggregation is estimated at 47%.16
Oxygen delivery to working muscles during exercise is increased via augmented cardiac output through a combination of both cardiac and vascular effects of catecholamine-activated adrenergic receptors. The two major subtypes of β-receptors, designated β1- and β2-adrenergic receptors (β1AR and β2AR), are present in varying proportions in different tissues. The β1AR is a 7-transmembrane Gs-protein-coupled receptor expressed in cardiac myocytes.17 β1AR activation elicits excitatory reactions in the heart, resulting in higher cardiac output through increased cardiac inotropy and chronotropy,18 whereas β2AR stimulation has primarily vasodilatory effects.19 In addition, blockers selective for the β1AR or blocking both receptor subtypes impair exercise performance in both normotensive and hypertensive subjects.20
In 1999, two common functional polymorphisms have been identified21 within the coding region of the polymorphic β1AR gene, located at chromosome 10.22 At position 145, an A to G transition substitutes the ‘wild-type’ serine residue at codon 49 (Ser49) by glycine (Gly49) (Ser49Gly) within the extracellular amino-terminal of the receptor,23 which may alter β1AR expression.21 It, furthermore, appears that agonist-promoted downregulation is enhanced with Gly49.24 A G to C substitution at position 1165 replaces glycine by arginine at codon 389 (Gly389Arg) in the intracellular cytoplasmic tail near the seventh transmembrane region of the receptor, which is a critical site for Gs-protein coupling.25 In a biochemical model, the arginine form (Arg389) was associated with a three-fold higher agonist-mediated adenylyl cyclase activity than the glycine form (Gly389).25
Because the β1AR is crucial in regulating cardiac output during exercise, and agents blocking it may impair exercise performance, we hypothesized that genetic variation of the β1AR gene could determine aerobic power in CAD patients. Indeed, in chronic heart failure, the Gly389Arg polymorphism and haplotypes with the Ser49Gly polymorphism were shown to be associated with exercise performance.26 Associations between polymorphisms of the β1AR gene and aerobic power and, particularly, the response to physical training have not been investigated in patients with CAD. The objective of this study was, therefore, to investigate whether the functionally important Ser49Gly and Gly389Arg polymorphisms of the β1AR gene or their haplotypes account for the individual variance in aerobic power or its response to physical training in patients with CAD included in the CAREGENE (CArdiac REhabilitation and GENetics of Exercise performance) study.
Methods
Patients
From 1990 to 2001, a total of 1280 patients have been referred to the ambulatory cardiac rehabilitation programme of the University Hospitals of Leuven. Of these, 1183 (92.5%) patients were referred after myocardial infarction, percutaneous transluminal coronary angioplasty (PTCA), coronary artery bypass grafting, stable angina pectoris which did not limit exercise performance, or a combination of these. A selection among these CAD patients was made, where only biologically unrelated Caucasian patients who had achieved evident exhaustion27 during graded cycle ergometer testing for determination of aerobic power at baseline and after 3 months of exercise training (n=1154) were eligible for inclusion in the CAREGENE study. Finally, patients after heart transplantation, after implantation of a pacemaker, a cardioverter defibrillator or an artificial valve, or after any other cardiac surgery (n=59) were excluded. Among 1095 patients who fulfilled these criteria, 42 were deceased and 11 could not be contacted. The remaining 1042 patients were invited to give a blood sample for genotyping in the CAREGENE study between March 2001 and June 2002. A total of 937 patients agreed to take part, and written informed consent was obtained from each participant before blood sampling. Reasons for non-participation as reported by eligible candidates were mostly related to ambulatory difficulties and transportation, timing, personal reasons, and refusal for genetic testing. Nine hundred and thirty-five blood samples were finally suitable for DNA extraction. Approval for this study was obtained from the Ethics Committee of the Faculty of Medicine.
Exercise testing
The maximal exercise tests on the cycle ergometer (Ergometrics 800S®, Ergometrics, Bitz, Germany) were performed in a laboratory where room temperature was stabilized at 18–22°C. The initial workload of 20 W was increased until exhaustion by 30 W every 3 min or by 20 W every minute, respectively, until or from the year 2000. Blood pressure was regularly measured by use of an STBP-780® device (Colin, Komaki, Japan). Heart rate and a 12-lead electrocardiogram (Max Personal Exercise Testing®, Marquette, WI, USA) were registered continuously. Respiratory data (STPD: ‘standard temperature pressure dry’ conditions of the gas) were measured through breath-by-breath analysis using an Oxycon Alpha® (Jaeger, Mijnhardt, Bunnik, the Netherlands) until 1994 or an 2900Z® (Sensormedics, Bilthoven, the Netherlands) after that date. Individual patients were tested with the same protocol and equipment at baseline and after training. The gas analysers were a paramagnetic O2 analyser and an infrared CO2 analyser. Pulmonary ventilation (VE) was measured by means of a turbine flow meter. The gas analysers and the flow meter were calibrated before each exercise test according to the manufacturer's instructions. VO2 and carbon dioxide output (VCO2) were determined from the continuous measurement of oxygen and carbon dioxide concentration in the inspired and expired air. Peak VO2 was defined as the highest 15-s average of VO2 obtained at the end of the test and was expressed as mL min−1. The percent of predicted peak VO2 was calculated as peak VO2 divided by maximal predicted VO2, using the values reported by Wasserman et al.28 The ventilatory equivalent for oxygen and carbon dioxide (VE divided by VO2 and VCO2, respectively) and respiratory exchange ratio (VCO2/VO2) at peak exercise were also calculated.
Exercise training
Patients took part in an ambulatory, supervised exercise training programme for 3 months. Three exercise sessions per week with a duration of ∼90 min per session were offered. Each session consisted of cycling, running, arm ergometry, rowing, predominantly isotonic callisthenics, and relaxation. Exercise intensity was individually determined by heart rate and progressively increased for each patient separately. Each patient spent on average 45 min above training heart rate during each session. Training frequency averaged 2.27±0.02 times/week and training intensity was 79.7±0.35%. The latter was calculated as: (training heart rate/peak heart rate)×100, where the mean training heart rate of the last three exercise sessions and peak heart rate of the exercise test after training were used.
Genotype determinations
DNA was extracted from white blood cells using the ‘salting-out’29 method. To genotype polymorphisms in the β1AR gene, the Invader™ assay30 (Third Wave Technologies) was used. The Invader assay combines structure-specific cleavage enzymes and a universal fluorescent resonance energy transfer (FRET) system. These enzymes will only cleave when the gene-specific probes bind the target. This mechanism warrants the specificity for distinguishing between alleles, whereas the FRET system generates an amplified readout.31 Synthetic target oligonucleotides were used as controls in every experiment. All reaction components were designed and provided by Third Wave Technologies. Genotyping was performed in a 96-well format. The reaction mixture was prepared by combining Probe Mix (211 µL), FRET mix (317 µL), Cleavase enzyme (79 µL), and MAP buffer (26 µL). Six microlitres of this mixture was added into a 96-well plate. Six microlitres of no target blank, synthetic target oligonucleotides, or genomic DNA samples (50 ng/µL) was added. After short centrifugation and incubation at 63°C for 4 h, fluorescent intensities were measured using a fluorescence microtitre plate reader (Victor2, Perkin Elmer). Genotypes were determined by calculating the ratios of the net wild-type and net mutant signals.32 The analysis was repeated once on those samples where no genotype could be obtained during the initial testing. If genotyping failed twice, samples were excluded from the analysis. In practice, this failure rate varies between 1.4 and 5.1% (data not shown). It seems that either impurities in or the quality of the DNA samples interferes with the Invader reaction to a larger extent than they would do in PCR approaches, at least in our hands.
Statistical methods
Data were analysed using SAS statistical software® version 8.0 for windows (SAS Institute, Inc., Cary, NC, USA). Data are reported as means±SE or as number of patients with percentage for dichotomous variables. A χ2 test with one degree of freedom was used to test whether the observed genotype frequencies were in Hardy–Weinberg equilibrium. Distributions were checked for normality with the Shapiro–Wilk statistic. Comparisons between the exercise test at baseline and after training were made by paired Student's t-test; comparisons across genotypes by analysis of variance (ANOVA), followed by Fisher's protected least significant difference (LSD) if significant. Categorical data were tested by χ2 or by Fisher's exact test where appropriate. To test potential relationships between β1AR variation and aerobic power or the response to training the use of two models was stipulated in advance. One model tested the genotype effect; another tested the effect of haplotype combinations. Each model included analysis of the raw and of the adjusted data by ANOVA and ANCOVA, respectively. Where significant, the latter was followed by Fisher's protected LSD. For the ANCOVA, the general linear model procedure was used where other determinants of the variable of interest were entered as covariates. These covariates had been identified up front by means of a stepwise selection procedure where the following list of independent variables was included: age, gender, height, weight, underlying heart disease, interventions and all types of medication, family history of CAD, history of hypertension or diabetes, previous and current smoking habits, angina or dyspnoea during daily life activities, systolic and diastolic blood pressure at rest, resting heart rate, exercise-induced ST depression or arrhythmia, training intensity, and frequency. The significance level was 12.5% for inclusion and 5% for exclusion from the stepwise building procedure. Haplotypes were constructed using the Phase® (version 2.1) programme.33,34 All statistical tests were two-sided at a significance level of 5%.
Results
CAREGENE study patients
A selection of relevant clinical characteristics of the overall CAREGENE study cohort (n=935) is listed in Table 1. Aerobic power at baseline averaged 1716±16 mL min−1 or 77.9±0.6% of predicted healthy values28 and ranged between 537 and 3326 mL min−1. Aerobic power increased with physical training by 24.2±0.6% (P<0.001), ranging from a decrease of 33.6% to an increase of 111.1%. At peak exercise, respiratory gas exchange ratio was 1.14±0.003 at baseline and 1.13±0.002 after training; peak ventilatory equivalent for oxygen was 38±0.2 and 37±0.2, respectively.
β1AR genotype
The β1AR genotype at positions 49 and 389 was successfully analysed in 892 and 900 patients from the cohort of 935, respectively. Clinical characteristics according to Ser49Gly and Gly389Arg β1AR genotype are shown in Table 1. At position 49, there were 548 (61%) Ser49Ser (homozygous wild-type) patients, 326 (37%) Ser49Gly (heterozygote), and 18 (2%) Gly49Gly patients (homozygous mutant). The allelic prevalence of Ser49 (80%) and Gly49 (20%) was consistent with those previously reported.26 At position 389, the Gly389Gly genotype (homozygous wild-type) was observed in 61 (7%) patients, 390 (43%) were heterozygotes, and 449 (50%) patients were homozygous for Arg389. The allelic prevalence of Gly389 (28%) and Arg389 (72%) was also similar to those observed in previous studies.21,25,26,35,36 Given the similar allelic (P>0.05, χ2 test, d.f.=1) and genotype (P>0.05, χ2 test, d.f.=2) frequency distributions in men and women, the data for both sexes were pooled for statistical analysis. In contrast to Ser49Gly (P<0.05, χ2 test, d.f.=1), the observed genotype distribution for Gly389Arg in all patients and within each sex was in agreement with the prediction by Hardy–Weinberg equilibrium (P>0.05, χ2 test, d.f.=1). Age, sex, body mass index, history of diabetes or hypertension, smoking habits, ejection fraction, cardiac pathology, or intervention and drug therapy were not different across the β1AR genotypes.
Blood pressure and heart rate values according to Ser49Gly and Gly389Arg polymorphisms are shown in Table 2. Resting and submaximal heart rate and resting diastolic blood pressure were lower in Gly389 homozygous patients as compared with carriers of the Arg389 allele. In Gly49 homozygous patients, submaximal heart rate was lower and diastolic blood pressure was higher as compared with Ser49 carriers.
Aerobic power and response to training
Aerobic power at baseline, after physical training, and the response to training stratified by the position 49 genotypes of the β1AR are shown in Table 3. Patients homozygous for the Gly49 allele of the β1AR gene had significantly higher aerobic power at baseline than in heterozygotes (P=0.035) and those homozygous for the Ser49 allele (P=0.026). Table 4, presenting aerobic power at baseline, after training, and the response according to Gly389Arg genotype, shows no differences across the three genotypes.
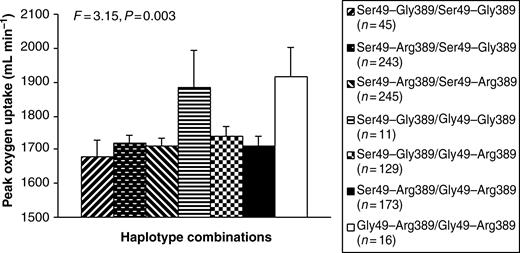
Highest frequency was observed for the Ser49Arg389 (53.1%) haplotype. Frequencies of the Ser49Gly389 and Gly49Arg389 haplotypes were 27.5 and 18.7%, respectively. The Gly49Gly389 haplotype was very rare (0.6%). Of four possible homozygous allele combinations, only three existed in our population. No patient carried the Gly49Gly/Gly389Gly homozygous allele combination. Patients who were homozygous for the Gly49Arg389 allele combination (1916±87 mL min−1) had significantly higher adjusted aerobic power at baseline compared with those with the Ser49Gly389 (1677±49 mL min−1, P=0.026) and Ser49Arg389 (1710±22 mL min−1, P=0.044) homozygous allele combinations (Figure 1). We finally also included heterozygous allele combinations in the analyses. This revealed significantly higher adjusted aerobic power at baseline (P<0.05) in both rarest haplotype combinations, i.e. Ser49–Gly389/Gly49–Gly389 and Gly49–Arg389/Gly49–Arg389, in comparison to other combinations (Figure 1).
Training intensity and training frequency were comparable across β1AR genotypes and haplotypes. Aerobic power increased significantly (P<0.001) with physical training. There was no association of the Ser49Gly (Table 3) and Gly389Arg (Table 4) β1AR genotype or haplotypes (data not shown) with the response to physical training or with post-training results.
Discussion
This study is the first to test the hypothesis of an association between the two common polymorphisms of the β1AR gene and aerobic power and the response to physical training in CAD. In patients with CAD who took part in the CAREGENE study, we show that haplotypes of the β1AR Ser49Gly and Gly389Arg polymorphisms are indeed predictive for aerobic power, however, not for the response to physical training.
Aerobic power and the response to physical training vary largely among individuals with CAD. In a previous study in 1909 cardiac patients, we reported that the response of aerobic power to 3 months of physical training ranged from a decrease of 40% to an increase of 120%.8 A total of 12 determinants accounted for merely 21% of this variation, with exercise performance at baseline and training dosage as the strongest determinants. The remaining variation is most likely the result of many interacting variables such as social, environmental, behavioural, physiological, pathophysiological, metabolic, and genetic factors. The impact of genetic variation on aerobic power and on the response to training amounts to 66%9–14 and 47%,15,16 respectively.
Wagoner et al.26 first reported that two common β1AR polymorphisms are significant determinants of exercise performance in patients with congestive heart failure. Although Gly389 was originally considered the ‘wild-type’ receptor,37 subsequent studies25,26,38 are in agreement with our finding that Arg389 is the more prevalent variant (72%). For reference, however, we considered the Gly389 receptor as ‘wild-type’.
In chronic heart failure, homozygous Arg389 patients showed significantly higher aerobic power and blood pressure in comparison to those with Gly389.26 Also, hypertensive patients homozygous for the Arg389 allele had significantly higher diastolic blood pressure and heart rate as compared with Gly389 carriers.35 In agreement with those findings,26,35 resting and submaximal heart rate and resting diastolic blood pressure in this study were also significantly higher in homozygous Arg389 patients as compared with Gly389 despite similar blood-pressure-lowering treatment. It was hypothesized that increased activity of the Arg389 variant in vivo may result in higher cardiac output and blood pressure.35 This result is in line with original findings at the molecular level, where rodent clonal cell lines expressing only the Arg389 genotype in vitro had both greater basal and isoproterenol-stimulated adenylyl cyclase activity than cells expressing only Gly389.25 This was later replicated in native human receptors where enhanced Gs-protein coupling of the Arg389 variant was also accompanied functionally by a stronger inotropic potency of β1AR activation in vitro.39 The inotropic consequence could, however, not be confirmed in another study in patients with CAD managed with or without β-blockers where the cardiostimulant effects of norepinephrine at β1Ars in the right atrium were conserved across the Gly389Arg genotypes.36 A functional effect of the Gly389Arg polymorphism on aerobic power was not observed in this study either. The present finding is also consistent with previous studies where a functional role for Gly389Arg in vivo was not supported.38,40 Exercise-induced increases in heart rate in healthy young adults38,40 and shortening of the electromechanical systole,38 which are primarily mediated by β1AR stimulation,18 were not different for homozygous Arg389 and Gly389 groups. Furthermore, in two studies in hypertensive patients, the Gly389Arg genotype failed to affect blood pressure or heart rate at baseline41 or the changes in blood pressure and heart rate41,42 during treatment with selective β1-blockers. Despite functional differences between the Gly389Arg receptor variants expressed in vitro, the phenotypic consequences in vivo, including aerobic power in patients with CAD, thus remain inconclusive.
At position 49, aerobic power at baseline was significantly higher in patients homozygous for the Gly49 mutation as compared with homozygous Ser49 and heterozygous patients. There was a tendency for an enhanced aerobic power after 3 months of physical training in Gly49 homozygous patients as well, however, statistical significance was not reached. These results are in line with the findings of Wagoner et al.,26 where aerobic power was higher in a combined group of patients homozygous for Gly49 (1.68%) and the heterozygous groups (‘Gly49 carriers’) vs. Ser49 homozygous patients. Despite similar antihypertensive treatment, resting and submaximal heart rate were higher in homozygous Ser49 and heterozygous patients as compared with Gly49, although statistical significance was only observed for submaximal heart rate. This is comparable with observations in healthy subjects where Ser49 homozygotes also showed the highest mean heart rate.43 Diastolic blood pressure in this study was also significantly higher in homozygous Gly49 as compared with Ser49 and heterozygotes. These observations are unlikely due to differences in Gs-protein coupling across the Ser49Gly genotype. Gly49 and Ser49 β1ARs display identical agonist and antagonist binding affinities. Furthermore, basal and agonist-stimulated adenylyl cyclase activities were the same for these receptors. Long-term agonist-promoted downregulation, however, does appear to be enhanced with Gly49.24
In agreement with observations in chronic heart failure,26 merely three of the four possible homozygous allele combinations existed in our population. Also, in analogy with that study highest aerobic power at baseline was observed in patients homozygous for the Gly49Arg389 allele, whereas in those with the Ser49Gly389 and Ser49Arg389 combinations, aerobic power was lowest. Inclusion of heterozygous combinations showed significantly higher aerobic power at baseline in the two haplotype combinations, i.e. Ser49–Gly389/Gly49–Gly389 and homozygous Gly49–Arg389, in comparison to all other combinations.
Given that peak heart rates in this study were similar across Ser49Gly and Gly389Arg groups, the effect on aerobic power is not due to a primarily chronotropic effect, but rather likely to differences in β1AR-mediated cardiac inotropy and vasodilation conveyed by the polymorphisms.
Aerobic power in this study was increased on average by 24.2±0.6% after physical training, indicating that our training programme provided a sufficient stimulus to induce responses. The range and standard deviation of the improvement in aerobic power in this study were substantial, showing that training responses differ strongly among patients, which compares well to our previous finding in a larger group of cardiac patients.8
This study did not reveal an effect of β1AR variation on the aerobic power response to physical training. This is in agreement with previous observations where it was shown that β-blockade during physical training in patients with CAD does not determine the aerobic power response.8,20,44 Exercise tolerance at baseline, however, has been shown to predict the response to physical training8 and as such was a potentially confounding variable. Aerobic power at baseline was, therefore, included as a covariate in the analyses, which did not alter the outcome of our study. Patient motivation or the ability to perform is another confounding factor in maximal exercise testing. Therefore, only patients who had achieved evident exhaustion at baseline as well as after 3 months of exercise training were included. Maximal exertion in our patients is obvious from the on average high values for ventilatory equivalent for oxygen (>35) and respiratory gas exchange ratio (>1.11) upon termination of exercise.
Numerous factors may account for differences in outcome among various studies. Most plausible explanations relate to methodological issues, such as discrepancies in study design, sample size, subject selection strategies, training programme, phenotype measurement, etc. First, the effect of genetic β1AR variation on aerobic power appears to be very modest and may, therefore, be difficult to detect in studies dealing with a relatively small number of subjects, whereas outliers in smaller studies could falsely affect the outcome. Second, studies in ethnically similar subjects are more likely to detect gene effects that are specific to that particular ethnic subgroup. For these reasons, a large and homogenous cohort consisting only of Caucasian patients with CAD was used in the CAREGENE study. So, our findings should not be generalized to a broader and more diverse population, but require further studies of equal or greater magnitude to investigate the effect of β1AR gene variation in subjects of different ethnicity and cardiac disease.
Limitations
Although there is evidence for more polymorphisms,45 our study focussed only on the two β1AR gene polymorphisms which have been previously well described and investigated, i.e. Ser49Gly and Gly389Arg. It could be argued that a complete phenotypic description of β1AR function requires simultaneous consideration of all polymorphisms. Finally, it is well established that β1AR blockade impairs submaximal endurance performance to a much more important extent as compared with peak performance.20,46 It is, therefore, conceivable that the effect of β1AR variation on endurance performance may be more pronounced than its impact on aerobic power. Because endurance testing was not performed, we can neither confirm nor exclude the possibility of an association with endurance performance.
Conclusions
In this study, we reported evidence that Ser49Gly, but not Gly389Arg, of the β1AR gene and the two haplotype combinations of both polymorphisms are associated with aerobic power, but do not influence the response to physical training in patients with CAD included in the CAREGENE study. Because of the importance of aerobic power as a predictor of survival in patients with cardiovascular disease,1 these present results suggest that genotyping of the β1AR Ser49Gly and Gly389Arg polymorphisms could be used to identify Caucasian CAD patients at risk. The CAREGENE cohort has proven valuable for this type of study.
Acknowledgements
This study was supported by grants from the Fund for Scientific Research—Flanders ‘Fonds voor Wetenschappelijk Onderzoek Vlaanderen’, Belgium (F.W.O. grant G.0124.02) and from the Research Council of the University of Leuven ‘Onderzoeksraad K.U. Leuven’, Belgium (grant OT/01/46). We wish to thank E. Legius, Department of Human Genetics, K.U. Leuven, Belgium, for sharing his experience and providing valuable advice. The assistance of J. Vanslambrouck is gratefully acknowledged. J.D. is research assistant of the Fund for Scientific Research, Flanders (Belgium) (F.W.O. Vlaanderen). L.V. is holder of the Faculty Chair ‘Health and Lifestyle’ of the Faculty of Health Care, University of Professional Education, Utrecht (the Netherlands).
Conflict of interest: none declared.
Figure 1 Covariate-adjusted peak oxygen uptake (mL min−1) at baseline according to homozygous and heterozygous haplotype combinations of the β1AR Ser49Gly and Gly389Arg polymorphisms in biologically unrelated Caucasian CAD patients in the CAREGENE study.
Clinical characteristics according to Ser49Gly and Gly389Arg β1AR genotypes for biologically unrelated Caucasian CAD patients and for the overall cohort (n=935) in the CAREGENE study
| Variable . | Ser49 homozygous (n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | Overall cohort (n=935) . |
|---|---|---|---|---|---|---|---|
| Women | 45 (8) | 28 (9) | 1 (6) | 3 (5) | 29 (7) | 39 (9) | 76 (8) |
| Age (years) | 56±0.4 | 57±0.5 | 57±1.7 | 57±1.3 | 56±0.5 | 56±0.4 | 56±0.3 |
| Body mass index (kg m−2) | 25.9±0.1 | 25.7±0.2 | 26.1±0.6 | 25.9±0.4 | 25.9±0.2 | 25.8±0.2 | 25.8±0.1 |
| History of diabetes | 30 (6) | 13 (4) | 1 (6) | 2 (3) | 20 (5) | 25 (6) | 49 (5) |
| History of hypertension | 152 (28) | 80 (25) | 8 (44) | 13 (21) | 107 (27) | 120 (27) | 251 (27) |
| Current smoking | 26 (5) | 16 (5) | 1 (6) | 4 (7) | 13 (3) | 27 (6) | 45 (5) |
| Past smoking | 396 (72) | 240 (74) | 13 (72) | 46 (75) | 281 (72) | 330 (74) | 681 (73) |
| Complaints of angina | 25 (5) | 15 (5) | 0 (0) | 2 (3) | 16 (4) | 20 (5) | 41 (4) |
| Complaints of dyspnoea | 94 (17) | 46 (14) | 1 (6) | 13 (21) | 70 (18) | 61 (14) | 149 (16) |
| AMI | 382 (70) | 201 (62) | 9 (50) | 40 (66) | 261 (70) | 304 (68) | 630 (67) |
| CK-MB | 1538±113 | 1504±154 | 1868±666 | 1589±317 | 1461±121 | 1524±135 | 1510±86 |
| Anterior | 147 (27) | 83 (25) | 4 (22) | 12 (20) | 106 (27) | 124 (28) | 252 (27) |
| Inferior | 209 (38) | 106 (33) | 5 (28) | 26 (43) | 132 (34) | 162 (36) | 333 (36) |
| CABG | 213 (39) | 145 (45) | 8 (44) | 21 (34) | 169 (43) | 176 (39) | 377 (40) |
| PTCA | 283 (52) | 146 (45) | 12 (67) | 35 (57) | 186 (48) | 227 (51) | 470 (50) |
| Angina | 11 (2) | 10 (3) | 0 (0) | 1 (2) | 9 (2) | 12 (3) | 23 (2) |
| β-Blockers | 464 (85) | 275 (84) | 18 (100) | 49 (80) | 328 (84) | 391 (87) | 794 (85) |
| Antiplatelets | 484 (88) | 292 (90) | 16 (89) | 54 (89) | 343 (88) | 401 (89) | 829 (89) |
| ACE-inhibitors | 136 (25) | 68 (21) | 4 (22) | 14 (23) | 96 (25) | 104 (23) | 222 (24) |
| Hypolipidemic drugs | 102 (19) | 55 (17) | 2 (11) | 7 (12) | 75 (19) | 80 (18) | 167 (18) |
| Calcium antagonists | 46 (8) | 33 (10) | 1 (6) | 5 (8) | 27 (7) | 50 (11) | 84 (9) |
| Molsidomine | 43 (8) | 20 (6) | 0 (0) | 10 (16) | 21 (5) | 35 (8) | 68 (7) |
| Nitrates | 21 (4) | 19 (6) | 0 (0) | 1 (2) | 17 (4) | 23 (5) | 42 (5) |
| Digitalis | 30 (6) | 28 (9) | 2 (11) | 4 (7) | 30 (8) | 30 (7) | 65 (7) |
| Diuretics | 27 (5) | 14 (4) | 1 (6) | 5 (8) | 20 (5) | 14 (3) | 43 (5) |
| Variable . | Ser49 homozygous (n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | Overall cohort (n=935) . |
|---|---|---|---|---|---|---|---|
| Women | 45 (8) | 28 (9) | 1 (6) | 3 (5) | 29 (7) | 39 (9) | 76 (8) |
| Age (years) | 56±0.4 | 57±0.5 | 57±1.7 | 57±1.3 | 56±0.5 | 56±0.4 | 56±0.3 |
| Body mass index (kg m−2) | 25.9±0.1 | 25.7±0.2 | 26.1±0.6 | 25.9±0.4 | 25.9±0.2 | 25.8±0.2 | 25.8±0.1 |
| History of diabetes | 30 (6) | 13 (4) | 1 (6) | 2 (3) | 20 (5) | 25 (6) | 49 (5) |
| History of hypertension | 152 (28) | 80 (25) | 8 (44) | 13 (21) | 107 (27) | 120 (27) | 251 (27) |
| Current smoking | 26 (5) | 16 (5) | 1 (6) | 4 (7) | 13 (3) | 27 (6) | 45 (5) |
| Past smoking | 396 (72) | 240 (74) | 13 (72) | 46 (75) | 281 (72) | 330 (74) | 681 (73) |
| Complaints of angina | 25 (5) | 15 (5) | 0 (0) | 2 (3) | 16 (4) | 20 (5) | 41 (4) |
| Complaints of dyspnoea | 94 (17) | 46 (14) | 1 (6) | 13 (21) | 70 (18) | 61 (14) | 149 (16) |
| AMI | 382 (70) | 201 (62) | 9 (50) | 40 (66) | 261 (70) | 304 (68) | 630 (67) |
| CK-MB | 1538±113 | 1504±154 | 1868±666 | 1589±317 | 1461±121 | 1524±135 | 1510±86 |
| Anterior | 147 (27) | 83 (25) | 4 (22) | 12 (20) | 106 (27) | 124 (28) | 252 (27) |
| Inferior | 209 (38) | 106 (33) | 5 (28) | 26 (43) | 132 (34) | 162 (36) | 333 (36) |
| CABG | 213 (39) | 145 (45) | 8 (44) | 21 (34) | 169 (43) | 176 (39) | 377 (40) |
| PTCA | 283 (52) | 146 (45) | 12 (67) | 35 (57) | 186 (48) | 227 (51) | 470 (50) |
| Angina | 11 (2) | 10 (3) | 0 (0) | 1 (2) | 9 (2) | 12 (3) | 23 (2) |
| β-Blockers | 464 (85) | 275 (84) | 18 (100) | 49 (80) | 328 (84) | 391 (87) | 794 (85) |
| Antiplatelets | 484 (88) | 292 (90) | 16 (89) | 54 (89) | 343 (88) | 401 (89) | 829 (89) |
| ACE-inhibitors | 136 (25) | 68 (21) | 4 (22) | 14 (23) | 96 (25) | 104 (23) | 222 (24) |
| Hypolipidemic drugs | 102 (19) | 55 (17) | 2 (11) | 7 (12) | 75 (19) | 80 (18) | 167 (18) |
| Calcium antagonists | 46 (8) | 33 (10) | 1 (6) | 5 (8) | 27 (7) | 50 (11) | 84 (9) |
| Molsidomine | 43 (8) | 20 (6) | 0 (0) | 10 (16) | 21 (5) | 35 (8) | 68 (7) |
| Nitrates | 21 (4) | 19 (6) | 0 (0) | 1 (2) | 17 (4) | 23 (5) | 42 (5) |
| Digitalis | 30 (6) | 28 (9) | 2 (11) | 4 (7) | 30 (8) | 30 (7) | 65 (7) |
| Diuretics | 27 (5) | 14 (4) | 1 (6) | 5 (8) | 20 (5) | 14 (3) | 43 (5) |
AMI, acute myocardial infarction; CK-MB, creatine kinase; CABG, coronary artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty. Data are presented as means±SE for continuous variables and as numbers (percentage) for dichotomous variables. For comparison between groups, ANOVA (continuous variables) and χ2 or Fisher's exact test(dichotomous variables) was used. Overall F for the comparison of age, body mass index, and CK-MB was 0.89, 0.28, and 0.65, respectively, across Ser49Gly and 0.29, 0.11, 0.10, respectively, across Gly389Arg. None of the patient characteristics at baseline were statistically different between the three genotypes.
Clinical characteristics according to Ser49Gly and Gly389Arg β1AR genotypes for biologically unrelated Caucasian CAD patients and for the overall cohort (n=935) in the CAREGENE study
| Variable . | Ser49 homozygous (n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | Overall cohort (n=935) . |
|---|---|---|---|---|---|---|---|
| Women | 45 (8) | 28 (9) | 1 (6) | 3 (5) | 29 (7) | 39 (9) | 76 (8) |
| Age (years) | 56±0.4 | 57±0.5 | 57±1.7 | 57±1.3 | 56±0.5 | 56±0.4 | 56±0.3 |
| Body mass index (kg m−2) | 25.9±0.1 | 25.7±0.2 | 26.1±0.6 | 25.9±0.4 | 25.9±0.2 | 25.8±0.2 | 25.8±0.1 |
| History of diabetes | 30 (6) | 13 (4) | 1 (6) | 2 (3) | 20 (5) | 25 (6) | 49 (5) |
| History of hypertension | 152 (28) | 80 (25) | 8 (44) | 13 (21) | 107 (27) | 120 (27) | 251 (27) |
| Current smoking | 26 (5) | 16 (5) | 1 (6) | 4 (7) | 13 (3) | 27 (6) | 45 (5) |
| Past smoking | 396 (72) | 240 (74) | 13 (72) | 46 (75) | 281 (72) | 330 (74) | 681 (73) |
| Complaints of angina | 25 (5) | 15 (5) | 0 (0) | 2 (3) | 16 (4) | 20 (5) | 41 (4) |
| Complaints of dyspnoea | 94 (17) | 46 (14) | 1 (6) | 13 (21) | 70 (18) | 61 (14) | 149 (16) |
| AMI | 382 (70) | 201 (62) | 9 (50) | 40 (66) | 261 (70) | 304 (68) | 630 (67) |
| CK-MB | 1538±113 | 1504±154 | 1868±666 | 1589±317 | 1461±121 | 1524±135 | 1510±86 |
| Anterior | 147 (27) | 83 (25) | 4 (22) | 12 (20) | 106 (27) | 124 (28) | 252 (27) |
| Inferior | 209 (38) | 106 (33) | 5 (28) | 26 (43) | 132 (34) | 162 (36) | 333 (36) |
| CABG | 213 (39) | 145 (45) | 8 (44) | 21 (34) | 169 (43) | 176 (39) | 377 (40) |
| PTCA | 283 (52) | 146 (45) | 12 (67) | 35 (57) | 186 (48) | 227 (51) | 470 (50) |
| Angina | 11 (2) | 10 (3) | 0 (0) | 1 (2) | 9 (2) | 12 (3) | 23 (2) |
| β-Blockers | 464 (85) | 275 (84) | 18 (100) | 49 (80) | 328 (84) | 391 (87) | 794 (85) |
| Antiplatelets | 484 (88) | 292 (90) | 16 (89) | 54 (89) | 343 (88) | 401 (89) | 829 (89) |
| ACE-inhibitors | 136 (25) | 68 (21) | 4 (22) | 14 (23) | 96 (25) | 104 (23) | 222 (24) |
| Hypolipidemic drugs | 102 (19) | 55 (17) | 2 (11) | 7 (12) | 75 (19) | 80 (18) | 167 (18) |
| Calcium antagonists | 46 (8) | 33 (10) | 1 (6) | 5 (8) | 27 (7) | 50 (11) | 84 (9) |
| Molsidomine | 43 (8) | 20 (6) | 0 (0) | 10 (16) | 21 (5) | 35 (8) | 68 (7) |
| Nitrates | 21 (4) | 19 (6) | 0 (0) | 1 (2) | 17 (4) | 23 (5) | 42 (5) |
| Digitalis | 30 (6) | 28 (9) | 2 (11) | 4 (7) | 30 (8) | 30 (7) | 65 (7) |
| Diuretics | 27 (5) | 14 (4) | 1 (6) | 5 (8) | 20 (5) | 14 (3) | 43 (5) |
| Variable . | Ser49 homozygous (n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | Overall cohort (n=935) . |
|---|---|---|---|---|---|---|---|
| Women | 45 (8) | 28 (9) | 1 (6) | 3 (5) | 29 (7) | 39 (9) | 76 (8) |
| Age (years) | 56±0.4 | 57±0.5 | 57±1.7 | 57±1.3 | 56±0.5 | 56±0.4 | 56±0.3 |
| Body mass index (kg m−2) | 25.9±0.1 | 25.7±0.2 | 26.1±0.6 | 25.9±0.4 | 25.9±0.2 | 25.8±0.2 | 25.8±0.1 |
| History of diabetes | 30 (6) | 13 (4) | 1 (6) | 2 (3) | 20 (5) | 25 (6) | 49 (5) |
| History of hypertension | 152 (28) | 80 (25) | 8 (44) | 13 (21) | 107 (27) | 120 (27) | 251 (27) |
| Current smoking | 26 (5) | 16 (5) | 1 (6) | 4 (7) | 13 (3) | 27 (6) | 45 (5) |
| Past smoking | 396 (72) | 240 (74) | 13 (72) | 46 (75) | 281 (72) | 330 (74) | 681 (73) |
| Complaints of angina | 25 (5) | 15 (5) | 0 (0) | 2 (3) | 16 (4) | 20 (5) | 41 (4) |
| Complaints of dyspnoea | 94 (17) | 46 (14) | 1 (6) | 13 (21) | 70 (18) | 61 (14) | 149 (16) |
| AMI | 382 (70) | 201 (62) | 9 (50) | 40 (66) | 261 (70) | 304 (68) | 630 (67) |
| CK-MB | 1538±113 | 1504±154 | 1868±666 | 1589±317 | 1461±121 | 1524±135 | 1510±86 |
| Anterior | 147 (27) | 83 (25) | 4 (22) | 12 (20) | 106 (27) | 124 (28) | 252 (27) |
| Inferior | 209 (38) | 106 (33) | 5 (28) | 26 (43) | 132 (34) | 162 (36) | 333 (36) |
| CABG | 213 (39) | 145 (45) | 8 (44) | 21 (34) | 169 (43) | 176 (39) | 377 (40) |
| PTCA | 283 (52) | 146 (45) | 12 (67) | 35 (57) | 186 (48) | 227 (51) | 470 (50) |
| Angina | 11 (2) | 10 (3) | 0 (0) | 1 (2) | 9 (2) | 12 (3) | 23 (2) |
| β-Blockers | 464 (85) | 275 (84) | 18 (100) | 49 (80) | 328 (84) | 391 (87) | 794 (85) |
| Antiplatelets | 484 (88) | 292 (90) | 16 (89) | 54 (89) | 343 (88) | 401 (89) | 829 (89) |
| ACE-inhibitors | 136 (25) | 68 (21) | 4 (22) | 14 (23) | 96 (25) | 104 (23) | 222 (24) |
| Hypolipidemic drugs | 102 (19) | 55 (17) | 2 (11) | 7 (12) | 75 (19) | 80 (18) | 167 (18) |
| Calcium antagonists | 46 (8) | 33 (10) | 1 (6) | 5 (8) | 27 (7) | 50 (11) | 84 (9) |
| Molsidomine | 43 (8) | 20 (6) | 0 (0) | 10 (16) | 21 (5) | 35 (8) | 68 (7) |
| Nitrates | 21 (4) | 19 (6) | 0 (0) | 1 (2) | 17 (4) | 23 (5) | 42 (5) |
| Digitalis | 30 (6) | 28 (9) | 2 (11) | 4 (7) | 30 (8) | 30 (7) | 65 (7) |
| Diuretics | 27 (5) | 14 (4) | 1 (6) | 5 (8) | 20 (5) | 14 (3) | 43 (5) |
AMI, acute myocardial infarction; CK-MB, creatine kinase; CABG, coronary artery bypass grafting; PTCA, percutaneous transluminal coronary angioplasty. Data are presented as means±SE for continuous variables and as numbers (percentage) for dichotomous variables. For comparison between groups, ANOVA (continuous variables) and χ2 or Fisher's exact test(dichotomous variables) was used. Overall F for the comparison of age, body mass index, and CK-MB was 0.89, 0.28, and 0.65, respectively, across Ser49Gly and 0.29, 0.11, 0.10, respectively, across Gly389Arg. None of the patient characteristics at baseline were statistically different between the three genotypes.
Haemodynamic data at baseline according to Ser49Gly and Gly389Arg β1AR genotypes for biologically unrelated Caucasian CAD patients in the CAREGENE study
| Variable . | Ser49 homozygous(n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | F . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | F . |
|---|---|---|---|---|---|---|---|---|
| Heart rate at rest (b.p.m.) | 67±0.5 | 66±0.6 | 64±2.0 | 0.77 | 63±1.4** | 67±0.6 | 67±0.5 | 3.87 |
| Heart rate at 80 W (b.p.m.) | 102±0.7 | 103±1.0 | 96±2.4* | 1.91 | 98±1.8** | 103±0.9 | 102±0.8 | 2.48 |
| Heart rate at peak (b.p.m.) | 128±0.9 | 129±1.1 | 129±4.8 | 0.01 | 126±2.5 | 128±1.0 | 128±1.0 | 0.33 |
| Resting systolic blood pressure (mmHg) | 129±0.8 | 131±1.1 | 135±3.9 | 2.14 | 129±2.3 | 131±1.0 | 129±0.9 | 0.82 |
| Resting diastolic blood pressure (mmHg) | 77±0.5 | 78±0.6 | 83±2.3* | 3.02 | 75±1.4** | 78±0.6 | 77±0.5 | 2.0 |
| Ejection fraction (%) | 61±0.7 | 62±0.9 | 67±4.0 | 1.03 | 62±2.1 | 62±0.8 | 62±0.8 | 0.15 |
| Variable . | Ser49 homozygous(n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | F . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | F . |
|---|---|---|---|---|---|---|---|---|
| Heart rate at rest (b.p.m.) | 67±0.5 | 66±0.6 | 64±2.0 | 0.77 | 63±1.4** | 67±0.6 | 67±0.5 | 3.87 |
| Heart rate at 80 W (b.p.m.) | 102±0.7 | 103±1.0 | 96±2.4* | 1.91 | 98±1.8** | 103±0.9 | 102±0.8 | 2.48 |
| Heart rate at peak (b.p.m.) | 128±0.9 | 129±1.1 | 129±4.8 | 0.01 | 126±2.5 | 128±1.0 | 128±1.0 | 0.33 |
| Resting systolic blood pressure (mmHg) | 129±0.8 | 131±1.1 | 135±3.9 | 2.14 | 129±2.3 | 131±1.0 | 129±0.9 | 0.82 |
| Resting diastolic blood pressure (mmHg) | 77±0.5 | 78±0.6 | 83±2.3* | 3.02 | 75±1.4** | 78±0.6 | 77±0.5 | 2.0 |
| Ejection fraction (%) | 61±0.7 | 62±0.9 | 67±4.0 | 1.03 | 62±2.1 | 62±0.8 | 62±0.8 | 0.15 |
Data are presented as means±SE. Comparisons between the exercise test at baseline and after training were made by means of paired Student's t-test.
*P<0.05 as compared with heterozygous Ser49Gly and Ser49 homozygous patients.
**P<0.05 as compared with heterozygous Gly389Arg and homozygous Arg389 patients.
Haemodynamic data at baseline according to Ser49Gly and Gly389Arg β1AR genotypes for biologically unrelated Caucasian CAD patients in the CAREGENE study
| Variable . | Ser49 homozygous(n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | F . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | F . |
|---|---|---|---|---|---|---|---|---|
| Heart rate at rest (b.p.m.) | 67±0.5 | 66±0.6 | 64±2.0 | 0.77 | 63±1.4** | 67±0.6 | 67±0.5 | 3.87 |
| Heart rate at 80 W (b.p.m.) | 102±0.7 | 103±1.0 | 96±2.4* | 1.91 | 98±1.8** | 103±0.9 | 102±0.8 | 2.48 |
| Heart rate at peak (b.p.m.) | 128±0.9 | 129±1.1 | 129±4.8 | 0.01 | 126±2.5 | 128±1.0 | 128±1.0 | 0.33 |
| Resting systolic blood pressure (mmHg) | 129±0.8 | 131±1.1 | 135±3.9 | 2.14 | 129±2.3 | 131±1.0 | 129±0.9 | 0.82 |
| Resting diastolic blood pressure (mmHg) | 77±0.5 | 78±0.6 | 83±2.3* | 3.02 | 75±1.4** | 78±0.6 | 77±0.5 | 2.0 |
| Ejection fraction (%) | 61±0.7 | 62±0.9 | 67±4.0 | 1.03 | 62±2.1 | 62±0.8 | 62±0.8 | 0.15 |
| Variable . | Ser49 homozygous(n=548) . | Heterozygous (n=326) . | Gly49 homozygous (n=18) . | F . | Gly389 homozygous (n=61) . | Heterozygous (n=390) . | Arg389 homozygous (n=449) . | F . |
|---|---|---|---|---|---|---|---|---|
| Heart rate at rest (b.p.m.) | 67±0.5 | 66±0.6 | 64±2.0 | 0.77 | 63±1.4** | 67±0.6 | 67±0.5 | 3.87 |
| Heart rate at 80 W (b.p.m.) | 102±0.7 | 103±1.0 | 96±2.4* | 1.91 | 98±1.8** | 103±0.9 | 102±0.8 | 2.48 |
| Heart rate at peak (b.p.m.) | 128±0.9 | 129±1.1 | 129±4.8 | 0.01 | 126±2.5 | 128±1.0 | 128±1.0 | 0.33 |
| Resting systolic blood pressure (mmHg) | 129±0.8 | 131±1.1 | 135±3.9 | 2.14 | 129±2.3 | 131±1.0 | 129±0.9 | 0.82 |
| Resting diastolic blood pressure (mmHg) | 77±0.5 | 78±0.6 | 83±2.3* | 3.02 | 75±1.4** | 78±0.6 | 77±0.5 | 2.0 |
| Ejection fraction (%) | 61±0.7 | 62±0.9 | 67±4.0 | 1.03 | 62±2.1 | 62±0.8 | 62±0.8 | 0.15 |
Data are presented as means±SE. Comparisons between the exercise test at baseline and after training were made by means of paired Student's t-test.
*P<0.05 as compared with heterozygous Ser49Gly and Ser49 homozygous patients.
**P<0.05 as compared with heterozygous Gly389Arg and homozygous Arg389 patients.
Peak oxygen uptake (mL min−1) at baseline and after physical training and the percentage response according to β1AR Ser49Gly genotypes for 892 biologically unrelated Caucasian CAD patients in the CAREGENE study
| . | . | Ser49 homozygous . | Heterozygous . | Gly49 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1724±21 | 1697±27 | 1962±113 | 2.68 | 0.07 |
| Adjusteda | 1715±16 | 1724±21 | 1937±89b | 2.99 | 0.05 | |
| After training | Unadjusted | 2108±24 | 2092±31 | 2307±132 | 1.27 | 0.28 |
| Adjusteda | 2097±18 | 2129±24 | 2252±101 | 1.56 | 0.21 | |
| Response | Unadjusted | 23.7±0.8 | 25.2±1.0 | 19.1±4.2 | 1.50 | 0.22 |
| Adjusteda,c | 23.2±0.7 | 25.2±0.9 | 21.9±3.8 | 1.71 | 0.18 |
| . | . | Ser49 homozygous . | Heterozygous . | Gly49 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1724±21 | 1697±27 | 1962±113 | 2.68 | 0.07 |
| Adjusteda | 1715±16 | 1724±21 | 1937±89b | 2.99 | 0.05 | |
| After training | Unadjusted | 2108±24 | 2092±31 | 2307±132 | 1.27 | 0.28 |
| Adjusteda | 2097±18 | 2129±24 | 2252±101 | 1.56 | 0.21 | |
| Response | Unadjusted | 23.7±0.8 | 25.2±1.0 | 19.1±4.2 | 1.50 | 0.22 |
| Adjusteda,c | 23.2±0.7 | 25.2±0.9 | 21.9±3.8 | 1.71 | 0.18 |
aAdjusted for age, sex, height, and weight.
bP<0.05 as compared with homozygous Ser49 and heterozygous patients.
cAdjusted for training intensity and frequency and for aerobic power at baseline. Values are means±SE. Analysis of (co)variance was used to compare means. F-value and level of significance (P value) of the overall AN(C)OVA are presented.
Peak oxygen uptake (mL min−1) at baseline and after physical training and the percentage response according to β1AR Ser49Gly genotypes for 892 biologically unrelated Caucasian CAD patients in the CAREGENE study
| . | . | Ser49 homozygous . | Heterozygous . | Gly49 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1724±21 | 1697±27 | 1962±113 | 2.68 | 0.07 |
| Adjusteda | 1715±16 | 1724±21 | 1937±89b | 2.99 | 0.05 | |
| After training | Unadjusted | 2108±24 | 2092±31 | 2307±132 | 1.27 | 0.28 |
| Adjusteda | 2097±18 | 2129±24 | 2252±101 | 1.56 | 0.21 | |
| Response | Unadjusted | 23.7±0.8 | 25.2±1.0 | 19.1±4.2 | 1.50 | 0.22 |
| Adjusteda,c | 23.2±0.7 | 25.2±0.9 | 21.9±3.8 | 1.71 | 0.18 |
| . | . | Ser49 homozygous . | Heterozygous . | Gly49 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1724±21 | 1697±27 | 1962±113 | 2.68 | 0.07 |
| Adjusteda | 1715±16 | 1724±21 | 1937±89b | 2.99 | 0.05 | |
| After training | Unadjusted | 2108±24 | 2092±31 | 2307±132 | 1.27 | 0.28 |
| Adjusteda | 2097±18 | 2129±24 | 2252±101 | 1.56 | 0.21 | |
| Response | Unadjusted | 23.7±0.8 | 25.2±1.0 | 19.1±4.2 | 1.50 | 0.22 |
| Adjusteda,c | 23.2±0.7 | 25.2±0.9 | 21.9±3.8 | 1.71 | 0.18 |
aAdjusted for age, sex, height, and weight.
bP<0.05 as compared with homozygous Ser49 and heterozygous patients.
cAdjusted for training intensity and frequency and for aerobic power at baseline. Values are means±SE. Analysis of (co)variance was used to compare means. F-value and level of significance (P value) of the overall AN(C)OVA are presented.
Peak oxygen uptake (mL min−1) at baseline and after physical training and the percentage response according to β1AR Gly389Arg genotypes for 900 biologically unrelated Caucasian CAD patients in the CAREGENE study
| . | . | Gly389 homozygous . | Heterozygous . | Arg389 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1723±61 | 1711±24 | 1724±23 | 0.07 | 0.93 |
| Adjusteda | 1724±48 | 1716±19 | 1727±18 | 0.10 | 0.90 | |
| After training | Unadjusted | 2101±71 | 2095±28 | 2116±26 | 0.15 | 0.86 |
| Adjusteda | 2101±54 | 2100±21 | 2121±20 | 0.28 | 0.76 | |
| Response | Unadjusted | 22.1±2.3 | 24.2±0.9 | 24.3±0.8 | 0.44 | 0.64 |
| Adjusteda,b | 22.4±2.1 | 24.0±0.8 | 24.1±0.8 | 0.29 | 0.75 |
| . | . | Gly389 homozygous . | Heterozygous . | Arg389 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1723±61 | 1711±24 | 1724±23 | 0.07 | 0.93 |
| Adjusteda | 1724±48 | 1716±19 | 1727±18 | 0.10 | 0.90 | |
| After training | Unadjusted | 2101±71 | 2095±28 | 2116±26 | 0.15 | 0.86 |
| Adjusteda | 2101±54 | 2100±21 | 2121±20 | 0.28 | 0.76 | |
| Response | Unadjusted | 22.1±2.3 | 24.2±0.9 | 24.3±0.8 | 0.44 | 0.64 |
| Adjusteda,b | 22.4±2.1 | 24.0±0.8 | 24.1±0.8 | 0.29 | 0.75 |
aAdjusted for age, sex, height, and weight.
bAdjusted for training intensity and frequency and for aerobic power at baseline. Values are means±SE. Analysis of (co)variance was used to compare means. F-value and level of significance (P value) of the overall AN(C)OVA are presented.
Peak oxygen uptake (mL min−1) at baseline and after physical training and the percentage response according to β1AR Gly389Arg genotypes for 900 biologically unrelated Caucasian CAD patients in the CAREGENE study
| . | . | Gly389 homozygous . | Heterozygous . | Arg389 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1723±61 | 1711±24 | 1724±23 | 0.07 | 0.93 |
| Adjusteda | 1724±48 | 1716±19 | 1727±18 | 0.10 | 0.90 | |
| After training | Unadjusted | 2101±71 | 2095±28 | 2116±26 | 0.15 | 0.86 |
| Adjusteda | 2101±54 | 2100±21 | 2121±20 | 0.28 | 0.76 | |
| Response | Unadjusted | 22.1±2.3 | 24.2±0.9 | 24.3±0.8 | 0.44 | 0.64 |
| Adjusteda,b | 22.4±2.1 | 24.0±0.8 | 24.1±0.8 | 0.29 | 0.75 |
| . | . | Gly389 homozygous . | Heterozygous . | Arg389 homozygous . | F . | P-value . |
|---|---|---|---|---|---|---|
| Baseline | Unadjusted | 1723±61 | 1711±24 | 1724±23 | 0.07 | 0.93 |
| Adjusteda | 1724±48 | 1716±19 | 1727±18 | 0.10 | 0.90 | |
| After training | Unadjusted | 2101±71 | 2095±28 | 2116±26 | 0.15 | 0.86 |
| Adjusteda | 2101±54 | 2100±21 | 2121±20 | 0.28 | 0.76 | |
| Response | Unadjusted | 22.1±2.3 | 24.2±0.9 | 24.3±0.8 | 0.44 | 0.64 |
| Adjusteda,b | 22.4±2.1 | 24.0±0.8 | 24.1±0.8 | 0.29 | 0.75 |
aAdjusted for age, sex, height, and weight.
bAdjusted for training intensity and frequency and for aerobic power at baseline. Values are means±SE. Analysis of (co)variance was used to compare means. F-value and level of significance (P value) of the overall AN(C)OVA are presented.
References
Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long-term prognosis in 12 169 men referred for cardiac rehabilitation.
Ades PA. Cardiac rehabilitation and secondary prevention of coronary heart disease.
Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease.
Gielen S, Schuler G, Hambrecht R. Exercise training in coronary artery disease and coronary vasomotion.
Giannuzzi P, Saner H, Bjornstad H, Fioretti P, Mendes M, Cohen-Solal A, Dugmore L, Hambrecht R, Hellemans I, McGee H, Perk J, Vanhees L, Veress G. Secondary prevention through cardiac rehabilitation: position paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology.
Wilmore JH, Green JS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao DC, Skinner JS, Bouchard C. Relationship of changes in maximal and submaximal aerobic fitness to changes in cardiovascular disease and non-insulin-dependent diabetes mellitus risk factors with endurance training: the HERITAGE Family Study.
Vanhees L, Fagard R, Thijs L, Amery A. Prognostic value of training-induced change in peak exercise capacity in patients with myocardial infarcts and patients with coronary-bypass surgery.
Vanhees L, Stevens A, Schepers D, Defoor J, Rademakers F, Fagard R. Determinants of the effects of physical training and of the complications requiring resuscitation during exercise in patients with cardiovascular disease.
Bouchard C, Daw EW, Rice T, Perusse L, Gagnon J, Province MA, Leon AS, Rao DC, Skinner JS, Wilmore JH. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study.
Bouchard C, Lesage R, Lortie G, Simoneau JA, Hamel P, Boulay MR, Perusse L, Theriault G, Leblanc C. Aerobic performance in brothers, dizygotic and monozygotic twins.
Fagard R, Van Den BC, Bielen E, Amery A. Maximum oxygen uptake and cardiac size and function in twins.
Fagard R, Bielen E, Amery A. Heritability of aerobic power and anaerobic energy generation during exercise.
Maes HH, Beunen GP, Vlietinck RF, Neale MC, Thomis M, Vanden Eynde B, Lysens R, Simons J, Derom C, Derom R. Inheritance of physical fitness in 10-yr-old twins and their parents.
Prud'homme D, Bouchard C, Leblanc C, Landry F, Fontaine E. Sensitivity of maximal aerobic power to training is genotype-dependent.
Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC. Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study.
Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA. Structure and function of G protein-coupled receptors.
Brodde OE, Bruck H, Leineweber K, Seyfarth T. Presence, distribution and physiological function of adrenergic and muscarinic receptor subtypes in the human heart.
Maqbool A, Hall AS, Ball SG, Balmforth AJ. Common polymorphisms of beta1-adrenoceptor: identification and rapid screening assay.
Hoehe MR, Otterud B, Hsieh WT, Martinez MM, Stauffer D, Holik J, Berrettini WH, Byerley WF, Gershon ES, Lalouel JM. Genetic mapping of adrenergic receptor genes in humans.
Moore JD, Mason DA, Green SA, Hsu J, Liggett SB. Racial differences in the frequencies of cardiac beta(1)-adrenergic receptor polymorphisms: analysis of c145A>G and c1165G>C.
Rathz DA, Brown KM, Kramer LA, Liggett SB. Amino acid 49 polymorphisms of the human beta1-adrenergic receptor affect agonist-promoted trafficking.
Mason DA, Moore JD, Green SA, Liggett SB. A gain-of-function polymorphism in a G-protein coupling domain of the human beta1-adrenergic receptor.
Wagoner LE, Craft LL, Zengel P, McGuire N, Rathz DA, Dorn GW, Liggett SB. Polymorphisms of the beta1-adrenergic receptor predict exercise capacity in heart failure.
Duncan GE, Howley ET, Johnson BN. Applicability of VO2max criteria: discontinuous vs continuous protocols.
Wasserman K, Hansen J, Sue D, Whipp B. Physiology of exercise. In: Wasserman K, Hansen J, Sue D, Whipp B, ed.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells.
de Arruda M, Lyamichev VI, Eis PS, Iszczyszyn W, Kwiatkowski RW, Law SM, Olson MC, Rasmussen EB. Invader technology for DNA and RNA analysis: principles and applications.
Hall JG, Eis PS, Law SM, Reynaldo LP, Prudent JR, Marshall DJ, Allawi HT, Mast AL, Dahlberg JE, Kwiatkowski RW, de Arruda M, Neri BP, Lyamichev VI. Sensitive detection of DNA polymorphisms by the serial invasive signal amplification reaction.
Hessner MJ, Budish MA, Friedman KD. Genotyping of factor V G1691A (Leiden) without the use of PCR by invasive cleavage of oligonucleotide probes.
Stephens M, Donnelly P. A comparison of Bayesian methods for haplotype reconstruction from population genotype data.
Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data.
Bengtsson K, Melander O, Orho-Melander M, Lindblad U, Ranstam J, Rastam L, Groop L. Polymorphism in the beta(1)-adrenergic receptor gene and hypertension.
Molenaar P, Rabnott G, Yang I, Fong KM, Savarimuthu SM, Li L, West MJ, Russell FD. Conservation of the cardiostimulant effects of (−)-norepinephrine across Ser49Gly and Gly389Arg beta(1)-adrenergic receptor polymorphisms in human right atrium in vitro.
Frielle T, Collins S, Daniel KW, Caron MG, Lefkowitz RJ, Kobilka BK. Cloning of the cDNA for the human beta 1-adrenergic receptor.
Buscher R, Belger H, Eilmes KJ, Tellkamp R, Radke J, Dhein S, Hoyer PF, Michel MC, Insel PA, Brodde OE. In vivo studies do not support a major functional role for the Gly389Arg beta 1-adrenoceptor polymorphism in humans.
Sandilands AJ, O'Shaughnessy KM, Brown MJ. Greater inotropic and cyclic AMP responses evoked by noradrenaline through Arg389 beta 1-adrenoceptors vs Gly389 beta 1-adrenoceptors in isolated human atrial myocardium.
Xie HG, Dishy V, Sofowora G, Kim RB, Landau R, Smiley RM, Zhou HH, Wood AJ, Harris P, Stein CM. Arg389Gly beta 1-adrenoceptor polymorphism varies in frequency among different ethnic groups but does not alter response in vivo.
O'Shaughnessy KM, Fu B, Dickerson C, Thurston D, Brown MJ. The gain-of-function G389R variant of the beta1-adrenoceptor does not influence blood pressure or heart rate response to beta-blockade in hypertensive subjects.
Karlsson J, Lind L, Hallberg P, Michaelsson K, Kurland L, Kahan T, Malmqvist K, Ohman KP, Nystrom F, Melhus H. Beta1-adrenergic receptor gene polymorphisms and response to beta1-adrenergic receptor blockade in patients with essential hypertension.
Ranade K, Jorgenson E, Sheu WH, Pei D, Hsiung CA, Chiang FT, Chen YD, Pratt R, Olshen RA, Curb D, Cox DR, Botstein D, Risch N. A polymorphism in the beta1 adrenergic receptor is associated with resting heart rate.
Vanhees L, Fagard R, Amery A. Influence of beta adrenergic blockade on effects of physical training in patients with ischaemic heart disease.
Podlowski S, Wenzel K, Luther HP, Muller J, Bramlage P, Baumann G, Felix SB, Speer A, Hetzer R, Kopke K, Hoehe MR, Wallukat G. Beta1-adrenoceptor gene variations: a role in idiopathic dilated cardiomyopathy?