-
PDF
- Split View
-
Views
-
Cite
Cite
Claudio Muneretto, Gianluigi Bisleri, Fabrizio Rosati, Ralf Krakor, Laura Giroletti, Lorenzo Di Bacco, Alberto Repossini, Massimo Moltrasio, Antonio Curnis, Claudio Tondo, Gianluca Polvani, European prospective multicentre study of hybrid thoracoscopic and transcatheter ablation of persistent atrial fibrillation: the HISTORIC-AF trial, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 4, October 2017, Pages 740–745, https://doi.org/10.1093/ejcts/ezx162
Close - Share Icon Share
Abstract
The HISTORIC-AF trial is a prospective, multicentre, single-arm study designed to evaluate the outcomes of a staged endoscopic and transcatheter ablation in patients with stand-alone, persistent or long-standing persistent atrial fibrillation (AF).
From 2012 to 2015, 100 consecutive patients were enrolled and underwent thoracoscopic left atrial epicardial isolation (‘box lesion’) followed by transcatheter ablation in case of AF recurrency. The safety end point was the composite outcome of freedom from major adverse events at 30-days, while efficacy end points were: (i) primary: freedom from AF and stable sinus rhythm following isolated thoracospic ablation >60% and (ii) secondary: freedom from AF and stable sinus rhythm >80% following hybrid ablation (as per HRS criteria).
No death occurred and surgical thoracoscopic procedure was successfully completed in all patients. Survival free from major adverse events at 30 days was 94%: there were 3 permanent pacemaker implants, 2 episodes of stroke and 1 revision for bleeding. At discharge, 87% of patients were in sinus rhythm. A staged transcatheter ablation was carried out in all patients with AF recurrences at the end of 3 months blanking period (17% of patients). At 12-months follow-up, a stable restoration of sinus rhythm was achieved in 75% and 88% of patients following isolated thoracoscopic ablation and hybrid ablation, respectively.
The HISTORIC-AF trial showed that thoracoscopic isolated surgical ablation reached both the safety and the efficacy end points. Hybrid ablation steadily improved rhythm outcomes and may be considered in the future as the treatment of choice for patients with persistent and long-standing persistent AF.
NCT01622907.
INTRODUCTION
The treatment of persistent and long-standing persistent atrial fibrillation (AF) still represents a major challenge in current clinical practice. Pharmacological therapies showed limited efficacy in this subset of patients, with a limited success in terms of rate control and relevant side effects [1–3].
Despite the consistent improvements in technology over the past decade, catheter-based procedures in persistent and long standing persistent AF have shown unsatisfactory outcomes, with a success rate ranging from 30% after a single procedure and up to 50–60% following multiple transcatheter reinterventions [4–6].
The innovations in the technologies and techniques for surgical treatment of AF allowed for less invasive approaches being developed, with the possibility to perform an effective lesion set via a minimally invasive thoracoscopic approach [7]. In an attempt to steadily improve the results of minimally invasive surgical ablation, a hybrid approach combining surgical epicardial and transcatheter ablation has recently demonstrated excellent outcomes in terms of sinus rhythm restoration (about 90% of cases) at mid-term follow-up [8, 9].
Therefore, we designed a multicentre, prospective, single arm study to evaluate the outcome of a staged hybrid approach combining minimally invasive epicardial surgical ablation (as the first step) with transcatheter ablation (as a second step) in patients with persistent and long-standing persistent AF.
METHODS
Study protocol
The HybrId STaged Operating Room and Interventional Catheter Ablation for Atrial Fibrillation (HISTORIC-AF) trial is an investigator-driven, prospective, multicentre, single arm study designed to evaluate the clinical outcomes of combined surgical-transcatheter (hybrid) approach in patients with stand-alone persistent or long-standing persistent AF. This trial recruited 100 patients at 3 different European sites (University of Brescia Medical School, Brescia, Italy; THG Staedtisches Klinikum Dortmund, Germany and Centro Cardiologico Monzino-University of Milan, Milan, Italy).
All subjects were required to meet the following inclusion criteria in order to be considered eligible for participation in the trial:
Documented failure of at least 1 Vaughan-Williams Class I or III anti-arrhythmic drug;
Symptomatic recurrent long standing persistent AF as defined by the HRS/EHRA/ECAS Expert Consensus Statement on catheter and surgical ablation of atrial fibrillation [10]; duration longer than 1 year but less than 5 years;
Absence of left atrial thrombus as documented by an imaging study (e.g. transthoracic echocardiogram (TTE), transoesophageal echocardiogram (TEE), thoracic computed tomography scan, magnetic resonance imaging, or left atrial angiography) within 30 days prior to enrolment;
Age 18–75 years;
Willing and capable of providing informed consent to undergo surgery and participate in all examinations and follow-up visits associated with this clinical trial.
The exclusion criteria were:
History of long-standing persistent AF for more than 5 years;
Documented left atrial (LA) size (antero-posterior diameter) greater than 55 mm;
Documented left ventricular ejection fraction of 40% or less;
History of cerebrovascular disease, including stroke or transient ischaemic attack (TIA) within 6 months prior to enrolment;
Significant underlying structural heart disease requiring surgical or procedural intervention;
Previous heart surgery;
Chronic obstructive pulmonary disease (<70% predicted lung function)
Known contraindication to anticoagulant therapy, or inability to comply with anticoagulant therapy;
Other clinical conditions precluding inclusion (e.g. organ disease, disturbances of haemostasis, etc.);
Pregnancy, planned pregnancy or breastfeeding;
Concomitant cardiac surgery procedure planned.
A physical examination, echocardiography (transthoracic or transoesophageal), cardiac assessment by means of computed tomography scan or magnetic resonance imaging were performed before surgery in order to rule out any potential cardiac structural disease as well as the anatomy of all 4 pulmonary veins (PV), left atrial appendage and posterior aspect of the left atrium.
Subjects enrolled in the trial were planned to participate up to 1-year follow-up, including: baseline evaluation, surgical ablation procedure and follow-up evaluations at 30 days, at the end of the 3-months blanking period, and at 6–9–12 months. Rhythm analysis during the follow-up period was based on 24/72-h ECG Holter monitoring at 6 and 12 months, according to HRS criteria. Implantation of continuous loop recorders (Reveal XT, Medtronic, Minneapolis, MN, USA) was not mandatory but recommended and was used for rhythm analysis only in addition to Holter monitoring.
Intraoperative ablation endpoints included, upon completion of the box lesion, the evaluation of exit-block (mandatory) and entrance-block (strongly recommended). Transcatheter ablation end points included PVs isolation (mandatory).
The primary efficacy end point was a rate of therapeutic success of thoracoscopic ablation >60% (defined as freedom from AF according to HRS criteria, without any subsequent electrophysiological (EP) ablation, during the first 12 months following surgical ablation). Secondary efficacy end point was a target rate of therapeutic success >80% following hybrid ablation (surgical thoracoscopic followed by EP ablation), defined as freedom from AF according to HRS criteria during the first 12 months.
The primary composite safety endpoint was the composite of the following major adverse events occurring within 30 days from the radiofrequency (RF) ablation procedure for subjects in the sequential hybrid treatment protocol: death (any cause), pericardial tamponade/effusion requiring intervention, stroke/TIA, myocardial infarction, excessive bleeding requiring reoperation, access site infection, pacemaker (PM) implantation, PV stenosis, oesophageal fistula.
Surgical procedure
The minimally invasive surgical approach consists of an epicardial, beating-heart surgical ablation which is performed exclusively via a right monolateral thoracoscopic approach, as previously reported [9]. Briefly, following the insertion of three 5- or 10-mm trocars in the right chest, the pericardial sac is widely opened parallel to the phrenic nerve to expose the superior vena cava and inferior vena cava. The pericardial reflections around the superior vena cava and inferior vena cava are then bluntly dissected to gain access to the transverse and oblique pericardial sinuses, respectively; epicardial fat is then removed at the level of the Waterstone’s groove. A dedicated introducer is then placed inside the chest in order to properly position the surgical ablation probe (Cobra Fusion, Estech/Atricure, San Ramon, CA, USA) around all 4 PVs and the posterior aspect of the left atrium. A vacuum of minimum −500 mmHg is initiated in order to achieve an effective adherence of the device to the epicardium and sequential RF ablation is started either with bipolar and unipolar mode: all lesions are repeated at least twice, and extensive overlapping is performed.
Upon completion of the box lesion, entrance and exit blocks are assessed and confirmed using a tetra-polar EP catheter or the AFfirm Testing Probe (Estech, San Ramon, CA, USA) placed on the epicardial surface within the box lesion and with a stimulation up to 10 V or 10 mA (at least 20 min after the end of the ablation procedure): as per protocol, it was mandatory to achieve at least unidirectional block.
An implantable loop recorder (Reveal XT, Medtronic, Minneapolis, MN, USA) was implanted at the completion of the surgical procedure, according to the site preference and availability. Even in patients with implantable loop recorders, 24 and 72 h ECG Holter monitoring served as the primary mean for detection of AF recurrences as per HRS recommendations [10].
Transcatheter procedure
Staged transcatheter ablation was performed only in patients showing AF recurrences at the end of the 3 months blanking period following surgical thoracoscopic ablation.
The EP procedure was carried out with the purpose to:
Assess integrity of the box lesion and PV isolation;
Target gaps in the surgical lesion line and provide effective isolation of PVs;
Terminate fragmented potentials, if deemed appropriate and at discretion of the electrophysiologist;
Perform a cavo-tricuspid isthmus lesion line, especially in patients with history of typical atrial flutter.
Upon completion of this procedure, integrity of the lesions was reassessed just prior to withdrawing the EP catheters from the LA. An electro-anatomical evaluation (Carto-Merge or NaveX) was utilized rather than a standard analysis with a Lasso catheter.
Statistical analysis
Continuous variables are expressed as mean and standard deviation; categorical data are summarized by reporting absolute frequency distribution and percentage.
Survival analysis was performed with Kaplan–Meier curves and populations were compared with the log-rank test.
Statistical findings were considered significant if the P-value was <0.05. Statistical analysis was performed with SPSS software (Version 20, IBM, New York, NY, USA).
RESULTS
Perioperative outcomes
Hundred consecutive patients were included in the current analysis. A detailed breakdown of patients’ characteristics is shown in Table 1, in particular: mean age was 60.9 ± 9 years and mean of AF duration was 48 months. Of note 45% of patients underwent transcatheter ablation before surgery and 3 patients had previous stroke.
Baseline patients’ characteristics
| Total patients . | 100 . |
|---|---|
| Age | 60.9 ± 9 |
| Male | 70 |
| Obesity (BMI >30) | 26 |
| Hypertension | 60 |
| Dyslipidaemia | 11 |
| Diabetes | 11 |
| Chronic renal failure | 4 |
| COPD | 9 |
| Previous TE events | 3 |
| Previous electrical cardiovertion | 66 |
| Previous EP ablation | 45 |
| AF type | |
| Persistent | 32 |
| Long-standing persistent | 68 |
| Total patients . | 100 . |
|---|---|
| Age | 60.9 ± 9 |
| Male | 70 |
| Obesity (BMI >30) | 26 |
| Hypertension | 60 |
| Dyslipidaemia | 11 |
| Diabetes | 11 |
| Chronic renal failure | 4 |
| COPD | 9 |
| Previous TE events | 3 |
| Previous electrical cardiovertion | 66 |
| Previous EP ablation | 45 |
| AF type | |
| Persistent | 32 |
| Long-standing persistent | 68 |
COPD: chronic obstructive pulmonary disease; TE: thromboembolic event; EP: electrophysiological; AF: atrial fibrillation.
Baseline patients’ characteristics
| Total patients . | 100 . |
|---|---|
| Age | 60.9 ± 9 |
| Male | 70 |
| Obesity (BMI >30) | 26 |
| Hypertension | 60 |
| Dyslipidaemia | 11 |
| Diabetes | 11 |
| Chronic renal failure | 4 |
| COPD | 9 |
| Previous TE events | 3 |
| Previous electrical cardiovertion | 66 |
| Previous EP ablation | 45 |
| AF type | |
| Persistent | 32 |
| Long-standing persistent | 68 |
| Total patients . | 100 . |
|---|---|
| Age | 60.9 ± 9 |
| Male | 70 |
| Obesity (BMI >30) | 26 |
| Hypertension | 60 |
| Dyslipidaemia | 11 |
| Diabetes | 11 |
| Chronic renal failure | 4 |
| COPD | 9 |
| Previous TE events | 3 |
| Previous electrical cardiovertion | 66 |
| Previous EP ablation | 45 |
| AF type | |
| Persistent | 32 |
| Long-standing persistent | 68 |
COPD: chronic obstructive pulmonary disease; TE: thromboembolic event; EP: electrophysiological; AF: atrial fibrillation.
Echocardiographic assessment was performed in all patients before surgery in order to evaluate left atrial parameters, as listed in Table 2: of note, mean LA antero-posterior diameter was 46.3 ± 4.9 mm, mean LA area was 25.7 ± 5.1 cm2.
Echocardiographic parameters
| . | Patients (normal) . |
|---|---|
| Number of patients = 100 . | |
| LA AP diameter | 46.3 ± 4.9 mm (30–40 mm) |
| LA area | 25.7 ± 5.1 cm2 (20 cm2) |
| LA maximum volume | 92.4 ± 31.9 ml (44 ± 10 ml) |
| LA minimum volume | 58.6 ± 26.4 ml (22 ± 8 ml) |
| . | Patients (normal) . |
|---|---|
| Number of patients = 100 . | |
| LA AP diameter | 46.3 ± 4.9 mm (30–40 mm) |
| LA area | 25.7 ± 5.1 cm2 (20 cm2) |
| LA maximum volume | 92.4 ± 31.9 ml (44 ± 10 ml) |
| LA minimum volume | 58.6 ± 26.4 ml (22 ± 8 ml) |
LA: left atrial; AP: antero-posterior.
Echocardiographic parameters
| . | Patients (normal) . |
|---|---|
| Number of patients = 100 . | |
| LA AP diameter | 46.3 ± 4.9 mm (30–40 mm) |
| LA area | 25.7 ± 5.1 cm2 (20 cm2) |
| LA maximum volume | 92.4 ± 31.9 ml (44 ± 10 ml) |
| LA minimum volume | 58.6 ± 26.4 ml (22 ± 8 ml) |
| . | Patients (normal) . |
|---|---|
| Number of patients = 100 . | |
| LA AP diameter | 46.3 ± 4.9 mm (30–40 mm) |
| LA area | 25.7 ± 5.1 cm2 (20 cm2) |
| LA maximum volume | 92.4 ± 31.9 ml (44 ± 10 ml) |
| LA minimum volume | 58.6 ± 26.4 ml (22 ± 8 ml) |
LA: left atrial; AP: antero-posterior.
A thoracoscopic procedure was performed in all patients and no one required conversion to median sternotomy. The uni-bipolar RF device was used to achieve an epicardial ‘box lesion’ set around the PVs, as previously described. Half of the patients received an implantable continuous loop recorder (REVEAL XT, Medtronic, Minneapolis, MN, USA). Mean ablation time was 17 ± 5.5 min with an overall procedural time of 122.3 ± 56.6 min.
At the end of the surgical procedure, effective isolation of the left atrium was assessed according to the intraprocedural EP techniques and end points as previously described [8, 9]: entrance block was assessed in 78 patients and achieved in 91% of patients (71/78), while the exit block was assessed in all patients, as mandatory, and confirmed in 94% of patients.
Mean ICU stay was 11.2 ± 6 h with a mean hospitalization of 5.9 ± 3.8 days. Hospital mortality was 0%. At discharge from hospital, 87% of patients were in sinus rhythm.
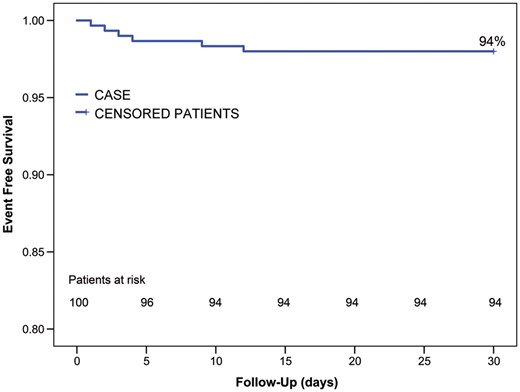
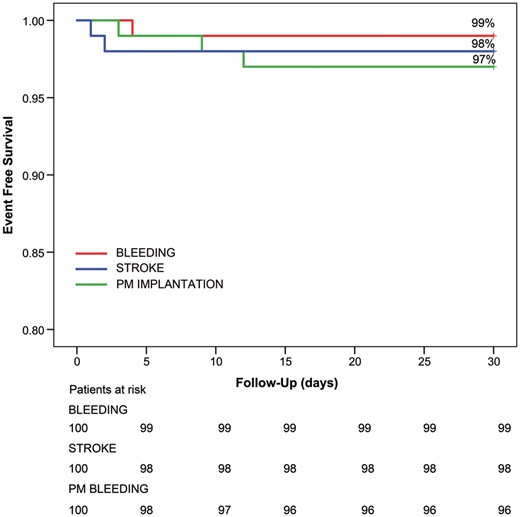
Freedom from major adverse events at 30 days was 94%, as depicted in Fig. 1. In particular, 30-days mortality was 0%, 1 patient required surgical revision for bleeding on postoperative day 4, while 3 patients required postoperative PM implantation (due to atrioventricular block in 2 patients and a sinus node dysfunction in the other patient). Two perioperative strokes/TIAs occurred during the second and third postoperative day respectively (Fig. 2): 1 patient experienced a perioperative stroke 2 days after the procedure with permanent neurological sequelae; the second patient had only a TIA with complete resolution within 12 h.
Survival freedom from major adverse events (bleeding/stroke/PM implantation) at 30 days. PM: pacemaker.
Staged electrophysiological procedure
At the end of the 3 months blanking period, 17 patients needed an EP due to AF recurrences: cavo-tricuspid isthmus ablation was performed in 9 patients while gaps in the box lesion set were identified only in 3 patients. Finally, sites of focal triggers outside the posterior left atrium and initiating AF were identified in 3 cases, and complex fractionated atrial electrograms were targeted only in 2 patients.
Follow-up
As previously outlined, patients were monitored over the follow-up period, as per protocol: in particular, 24 and 72-h ECG Holter was used as the primary rhythm monitoring strategy at 6 and 12 months, even in the presence of continuous loop recorders, as per HRS recommendations [10]. Additional data could be retrieved from loop recorder evaluations, in particular, a monthly AF burden of less than 0.5% was used as a threshold for significant AF burden, as previously reported in literature [10].
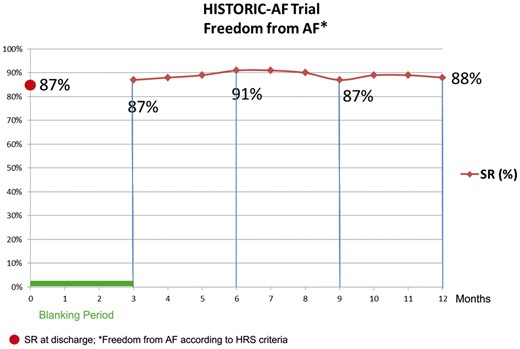
Following surgical ablation, a stable restoration of sinus rhythm was achieved in 77% and 75% of patients at 6 and 12 months follow-up respectively. Instead, following hybrid ablation sinus rhythm was restored in 91% and 88% of patients at 6 and 12 months follow-up respectively, as shown in Fig. 3.
Freedom from AF at 12 months in patients who underwent hybrid ablation. AF: atrial fibrillation.
DISCUSSION
The most appropriate treatment for non-paroxysmal AF still represents a major issue of debate: despite novel drugs being introduced in clinical practice, the rate of success of medical therapy is still limited, ranging around 20% [1, 2]. Similarly, transcatheter approaches seemed to yield promising results, albeit the few studies in literature reporting the long-term results of percutaneous treatment of AF clearly depicted the limited efficacy of such approach at mid-long term, with an estimated success rate around 30–40% after a single procedure and of 50–60% after multiple ones [4–6].
Despite the Cox-Maze III and IV being the most successful therapeutical options from the surgical standpoint [11], the technical complexity and degree of invasiveness of such approaches hampered a wide acceptance among patients and cardiologists; therefore, there has been an increased interest towards less invasive, off-pump surgical techniques for AF treatment over the past decade [12].
Moreover, in an effort to improve the efficacy the minimally invasive surgical procedure, which allows only for a limited lesion set to be performed, the possibility to merge surgical and EP procedures in hybrid fashion has emerged over the past few years [13]. Several approaches have been proposed, either as simultaneous or sequential staged procedures [14]: a sequential-staged approach (combining an endoscopic surgical ablation followed by an EP evaluation and ablation if required) previously demonstrated success rates ranging around 90%, thereby confirming excellent results at short- and mid-term [9, 15].
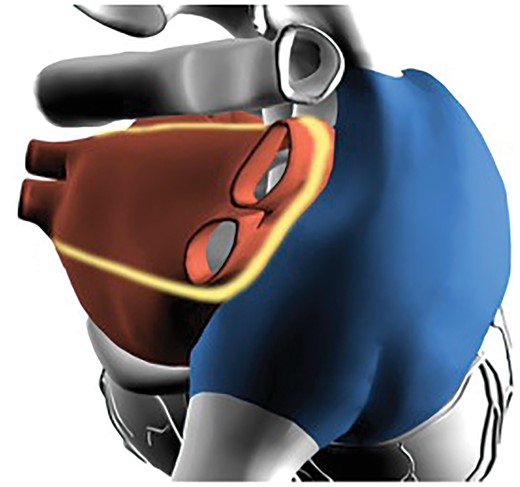
To validate the efficacy of the hybrid ablation and minimize the biases of patients’ selection and single centre experience, we designed a prospective, multicentre clinical trial (HISTORIC-AF: ClinicalTrials.gov Identifier: NCT01622907). The study was designed to combine the current less invasive surgical approach with a staged transcatheter approach, if required. The surgical approach consisted of a mono-lateral right side thoracoscopic epicardial approach which allowed for an effective epicardial LA isolation (box lesion—Fig. 4). The device and energy source used for the surgical ablation was a combination of uni-bipolar RF, designed to provide a continuous transmural ablation line.
From the surgical standpoint, the box lesion consisted of a continuous epicardial ablation line around all 4 PVs, thus allowing a complete isolation of the 4 PVs, the entire posterior left atrial wall and half of the lateral wall of the left atrium, thus decreasing the critical mass of the LA by nearly 40%. The complete isolation of the posterior LA wall may be relevant from the EP stand-point: in fact, in presence of an abnormal LA substrate, LA fibrosis, electroanatomical heterogeneity and fragmented potentials are mainly located in the LA posterior wall.
Despite the box lesion set reproduces only part of the original lesion set of the Maze III and IV, the rate of sinus rhythm restoration following such procedure is encouraging, especially in combination with a sequential staged transcatheter approach, leading to a success rate of 80% without anti-arrhytmhic drugs.
With respect to the EP touch-up procedure, the possibility to be performed in a staged fashion allows for several advantages: first, early inducible arrhythmias can lead to both false-positives (due to the need for a ‘maturation’ of the lesion) and false-negative (due to a transient block); moreover, the concomitant presence of the surgical and EP team performing ablations can lead to prolonged operative times and potentially unnecessary procedures.
Recently, some authors reported novel relevant insights in the pathophysiology of AF: in fact, they demonstrated in the experimental setting that a more ‘chronic’ substrate of persistent AF is associated with a higher likelihood of transmural (epicardial–endocardial and vice versa) conduction in re-entry circuits and even a different pattern of muscle fibres orientation could be detected in long-standing fibrillating atria; moreover, such re-entrant circuits were more likely to occur in the epicardial layers, especially in the setting of ‘chronic’ AF. Such observations are therefore leading to a more complex scenario of re-entry circuits in long-standing persistent AF, which is ‘multiplanar’ rather than bidimensional as considered up to date. Additionally, such studies provided a potential explanation of the high success rates of the hybrid epicardial–endocardial approach as described above, with better outcomes than multiple endocardial-only EP ablations [16, 17].
Unlike previously published series which required all patients to undergo a second EP ‘step’ [9], the current protocol mandated patients to receive a staged EP procedure only in the presence of AF recurrences, which occurred only in 17% of the study population. Our approach seems to potentially avoid patients’ ‘overtreatment’ and the related associated risk: of note, even in our previously published series with a systematic surgical ablation followed by transcatheter evaluation in all instances, 30–40% of those patients did not receive any additional ablation [15]. Furthermore, the HISTORIC-AF trial showed excellent success rate (88%) in terms of sinus rhythm restoration of the ‘tailored’ hybrid approach, suggesting that this approach may provide similar results to the ‘intention to treat’ hybrid strategy (with mandatory transcatheter ablation) [15], thus minimizing the procedural risks and overutilization of financial resources related to unnecessary treatment.
In the current study, the rate of perioperative complications was low and in particular there were no conversions to sternotomy indicating that the thoracoscopic mono-lateral approach was safe and reproducible. There were no in-hospital deaths and the incidence of perioperative strokes/TIAs (2%) is comparable to the results of transcatheter ablation [18, 19], despite no exclusion of the left atrial appendage being performed at the time of surgery. The perioperative stroke/TIA occurring in our series may be justified by an inadequate management of low molecular weight heparin administration during the early postoperative period following successful conversion to sinus rhythm, when transient atrial standstill may have occurred.
The HISTORIC-AF trial reached both the safety and efficacy end points, demonstrating that at 12 months follow-up isolated thoracoscopic surgical ablation has a success rate of 75% and the tailored hybrid approach steadily improves rhythm outcome with a success rate up to 88%.
Limitations
The lack of a matched catheter-only control group represents an obvious limitation of this trial, which was primarily designed to assess the safety and efficacy of a sequential-staged hybrid procedure in complex subset of patients, such as those with persistent and long-standing persistent AF. Furthermore, the presence of previous transcatheter ablations may represent a potential confounding factor in the interpretation of the results, albeit the current population did not receive any transcatheter treatment in the 6 months prior to the enrolment in the present trial.
CONCLUSION
In conclusion, the results of the HISTORIC-AF trial demonstrate the safety and the efficacy of this sequential tailored hybrid approach, with a low rate of morbidity, limited need for transcatheter reintervention, and excellent results up to 1-year follow-up.
Conflict of interest: Claudio Muneretto, Gianluigi Bisleri and Ralf Krakor disclose financial interest with Atricure/Estech, San Ramon, CA, USA.
REFERENCES
Author notes
Presented at the 30th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 1–5 October 2016.