-
PDF
- Split View
-
Views
-
Cite
Cite
Phan-Kiet Tran, Victor T. Tsang, Patricia R. Cornejo, Ryo Torii, Troy Dominguez, Karin Tran-Lundmark, Tain-Yen Hsia, Marina Hughes, Nagarajan Muthialu, Martin Kostolny, Midterm results of the Ross procedure in children: an appraisal of the subannular implantation with interrupted sutures technique, European Journal of Cardio-Thoracic Surgery, Volume 52, Issue 4, October 2017, Pages 798–804, https://doi.org/10.1093/ejcts/ezx113
Close - Share Icon Share
Abstract
The support of the pulmonary autograft root by the fibromuscular left ventricular outflow tract is emphasized to address the concern related to the dilatation of the pulmonary autograft structures in the paediatric population.
This retrospective study analyses the outcomes of 75 children who were operated between 1998 and 2012 with the subannular interrupted sutures technique at a median age of 10.2 years (range, 5.3 months–18.0 years). Median follow-up time was 5.2 years (range, 3 days–13.2 years).
There were no deaths, but there were 3 reinterventions on the autograft for regurgitation and 2 resections of left ventricular outflow tract obstruction. There was no significant autograft stenosis, and freedom from moderate-to-severe regurgitation was 95% (95% confidence interval: 89–100) and 88% (95% confidence interval: 77–99) at 5 and 10 years, respectively. Median z-scores at the latest follow-up examination were, at the annulus, 0.31 [interquartile range (IQR) = −0.81 to 1.2]; at the sinus of Valsalva, 2.7 (IQR = 1.5–3.5); and at the sinotubular junction, 3.1 (IQR = 1.7–4.2). The correlation between z-scores and time after the operation was negative at the level of the annulus (r = −0.29, P = 0.034) but positive at the level of the sinus (r = +0.37, P = 0.005) and the sinotubular junction (r = +0.26, P = 0.068). The median rate of change in the z-score at the annulus was low, 0.065 z-score/year (IQR = −0.13 to 0.43).
The subannular interrupted sutures implantation technique is associated with acceptable risks and, in the midterm, delivers limited annular dilatation, autograft regurgitation and delayed need for autograft reintervention.
INTRODUCTION
The advantages of the pulmonary autograft procedure in the treatment of aortic valve disease in children are well proven [1–4]. One of the long-standing concerns regarding this procedure is the dilatation of the pulmonary autograft [5]. It is important to recognize that neoaortic root dilatation was not an issue in the original series of adult patients who were operated on by Donald Ross [6]. With the recent exception of Sievers and co-workers [7], the inherent challenge with Ross’s original subcoronary implantation technique has proven too difficult to reproduce. Moreover, in a small child, the level of difficulty is even higher.
The root transfer technique is less complex and more resistant to distortions of the valve geometry and is also applicable for a small child [6, 8, 9]. Unfortunately, this simplified method has its own inherent disadvantage, namely dilatation. The autograft root consists of a thinner pulmonary arterial wall [10] and a more distensible muscular infundibulum [11]. Intuitively, the supra-infundibular position of the original pulmonary valve, now the pulmonary autograft valve, must be placed within the fibromuscular left ventricular outflow tract (LVOT) for support. We prefer to use interrupted sutures to facilitate a clear visualization of the subannular LVOT position, especially in small infants and in cases with discrepancies in the sizes of the pulmonary autograft and the native aortic valves, to have good alignment of the autograft root.
The purpose of this study was to evaluate this method of interrupted sutures in the subannular implantation with specific focus on the changes in root dimensions and development of neoaortic valve regurgitation.
MATERIALS AND METHODS
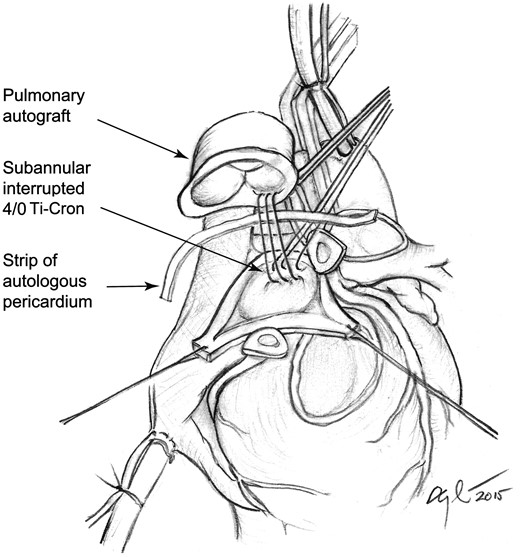
We conducted a single-centre, cross-sectional, retrospective study. All patients were operated at the Great Ormond Street Hospital between 1998 and 2012. The study was approved by an institutional review board, and consent was waived due to the retrospective nature of the study. The surgical technique of subannular implantation of the pulmonary autograft with interrupted 4/0 Ti-Cron sutures is shown in Fig. 1. In the presence of a dilated ascending aorta at the time of the Ross procedure, we would routinely plicate the distal autograft suture line either by a triangular aortic resection or by a horizontal pledgeted Prolene sutures to minimize the potential problem with dilatation over time. This procedure was described in detail in the textbook [12]. Our experience with external support is limited.
Illustration of the subannular implantation technique as it is performed at the Great Ormond Street Hospital, London, UK. Interrupted 4/0 Ti-Cron sutures are used to position the pulmonary autograft within the left ventricular outflow tract. A single pericardial strip is used as a tissue buffer to improve haemostasis of the proximal anastomosis.
Aortic regurgitation was graded as trivial, mild, moderate or severe according to American Heart Association guidelines [14]. Clinical notes from the last follow-up examination and the National Health Service's databases were used to confirm survival.
Statistical methods
Data are presented as mean with 95% confidence interval (CI) for normally distributed data, otherwise as median with range or interquartile range (IQR). Categorical or ordinal data are presented as n (%). Graphical analyses and the Shapiro–Wilk test were used to test for a non-normal distribution. The Kaplan–Meier method was used to analyse time-to-event data (i.e. reintervention or moderate-to-severe aortic regurgitation). Correlation between continuous variables was performed using Spearman’s correlation coefficients. Kruskal–Wallis and posthoc Mann–Whitney tests were used to compare the z-scores from the latest follow-up examination and the rate of change in z-scores across aortic root structures. A P-value < 0.05 was considered significant. All analyses were made in SPSS version 23 (IBM 2015).
RESULTS
Between 1998 and 2012, 75 children (median age, 10.2 years; range, 5.3 months–18.0 years) were operated with the subannular interrupted sutures technique. Of these, 10 patients were younger than 2 years at the time of the operation. The median follow-up time was 5.2 years (range, 3 days–13.2 years; Tables 1 and 2).
Preoperative patient characteristics
| . | n = 75 . | % . |
|---|---|---|
| Gender | ||
| Male | 59 | 79 |
| Primary diagnosis | ||
| Congenital valvular stenosis | 57 | 76 |
| Regurgitation | 7 | 9.3 |
| Endocarditis, rheumatic | 11 | 15 |
| Primary intervention | ||
| Balloon dilatation | 44 | 59 |
| Surgical valvuloplasty | 13 | 17 |
| Previous surgical procedures | ||
| Ventricular septal defect repair | 5 | 6.7 |
| Coarctation | 9 | 12 |
| Left ventricular outflow tract obstruction resection | 5 | 6.7 |
| Aortic cusp suspension | 3 | 3.0 |
| Aortic valve repair | 1 | 1.3 |
| Supravalvular stenosis repair | 1 | 1.3 |
| Mitral valve repair | 1 | 1.3 |
| . | n = 75 . | % . |
|---|---|---|
| Gender | ||
| Male | 59 | 79 |
| Primary diagnosis | ||
| Congenital valvular stenosis | 57 | 76 |
| Regurgitation | 7 | 9.3 |
| Endocarditis, rheumatic | 11 | 15 |
| Primary intervention | ||
| Balloon dilatation | 44 | 59 |
| Surgical valvuloplasty | 13 | 17 |
| Previous surgical procedures | ||
| Ventricular septal defect repair | 5 | 6.7 |
| Coarctation | 9 | 12 |
| Left ventricular outflow tract obstruction resection | 5 | 6.7 |
| Aortic cusp suspension | 3 | 3.0 |
| Aortic valve repair | 1 | 1.3 |
| Supravalvular stenosis repair | 1 | 1.3 |
| Mitral valve repair | 1 | 1.3 |
Preoperative patient characteristics
| . | n = 75 . | % . |
|---|---|---|
| Gender | ||
| Male | 59 | 79 |
| Primary diagnosis | ||
| Congenital valvular stenosis | 57 | 76 |
| Regurgitation | 7 | 9.3 |
| Endocarditis, rheumatic | 11 | 15 |
| Primary intervention | ||
| Balloon dilatation | 44 | 59 |
| Surgical valvuloplasty | 13 | 17 |
| Previous surgical procedures | ||
| Ventricular septal defect repair | 5 | 6.7 |
| Coarctation | 9 | 12 |
| Left ventricular outflow tract obstruction resection | 5 | 6.7 |
| Aortic cusp suspension | 3 | 3.0 |
| Aortic valve repair | 1 | 1.3 |
| Supravalvular stenosis repair | 1 | 1.3 |
| Mitral valve repair | 1 | 1.3 |
| . | n = 75 . | % . |
|---|---|---|
| Gender | ||
| Male | 59 | 79 |
| Primary diagnosis | ||
| Congenital valvular stenosis | 57 | 76 |
| Regurgitation | 7 | 9.3 |
| Endocarditis, rheumatic | 11 | 15 |
| Primary intervention | ||
| Balloon dilatation | 44 | 59 |
| Surgical valvuloplasty | 13 | 17 |
| Previous surgical procedures | ||
| Ventricular septal defect repair | 5 | 6.7 |
| Coarctation | 9 | 12 |
| Left ventricular outflow tract obstruction resection | 5 | 6.7 |
| Aortic cusp suspension | 3 | 3.0 |
| Aortic valve repair | 1 | 1.3 |
| Supravalvular stenosis repair | 1 | 1.3 |
| Mitral valve repair | 1 | 1.3 |
Characteristics at the time of the procedure
| . | n (%) . | Median (range) . |
|---|---|---|
| Age | ||
| All | 75 | 10.2 (0.44–18) years |
| Number <2 years | 10 (13) | 0.84 (0.44–1.8) year |
| Number >2 years | 65 (87) | 11.2 (3.5–18) years |
| Weight | 24.9 (5.7–94) kg | |
| Intraoperative parameters | ||
| Extracorporeal circulation time | 144 (97–280) min | |
| Cross-clamp time | 107 (49–208) min | |
| Cooling temp | 30.9 (25.0–34.3)°C | |
| RV-PA conduits used | ||
| Pulmonary homograft | 70 (93) | 20 (12–27) mm |
| Aortic homograft | 1 (1.3) | 21 mm |
| Bovine jugular vein | 4 (5.3) | 17 (14–22) mm |
| Complications | ||
| Atrioventricular block III | 1 (1.3) | |
| Pneumothorax | 1 (1.3) | |
| Postoperative bleeding | 2 (2.6) | |
| Postoperative ECMO | 0 (0) | |
| Mortality | 0 (0) | |
| Length of stay | ||
| Intensive care unit | 1.2 (0.22–6.6) days | |
| Total in hospital | 6.0 (4–17) days | |
| . | n (%) . | Median (range) . |
|---|---|---|
| Age | ||
| All | 75 | 10.2 (0.44–18) years |
| Number <2 years | 10 (13) | 0.84 (0.44–1.8) year |
| Number >2 years | 65 (87) | 11.2 (3.5–18) years |
| Weight | 24.9 (5.7–94) kg | |
| Intraoperative parameters | ||
| Extracorporeal circulation time | 144 (97–280) min | |
| Cross-clamp time | 107 (49–208) min | |
| Cooling temp | 30.9 (25.0–34.3)°C | |
| RV-PA conduits used | ||
| Pulmonary homograft | 70 (93) | 20 (12–27) mm |
| Aortic homograft | 1 (1.3) | 21 mm |
| Bovine jugular vein | 4 (5.3) | 17 (14–22) mm |
| Complications | ||
| Atrioventricular block III | 1 (1.3) | |
| Pneumothorax | 1 (1.3) | |
| Postoperative bleeding | 2 (2.6) | |
| Postoperative ECMO | 0 (0) | |
| Mortality | 0 (0) | |
| Length of stay | ||
| Intensive care unit | 1.2 (0.22–6.6) days | |
| Total in hospital | 6.0 (4–17) days | |
RV-PA: right ventricular to pulmonary artery; ECMO: extracorporeal membrane oxygenation.
Characteristics at the time of the procedure
| . | n (%) . | Median (range) . |
|---|---|---|
| Age | ||
| All | 75 | 10.2 (0.44–18) years |
| Number <2 years | 10 (13) | 0.84 (0.44–1.8) year |
| Number >2 years | 65 (87) | 11.2 (3.5–18) years |
| Weight | 24.9 (5.7–94) kg | |
| Intraoperative parameters | ||
| Extracorporeal circulation time | 144 (97–280) min | |
| Cross-clamp time | 107 (49–208) min | |
| Cooling temp | 30.9 (25.0–34.3)°C | |
| RV-PA conduits used | ||
| Pulmonary homograft | 70 (93) | 20 (12–27) mm |
| Aortic homograft | 1 (1.3) | 21 mm |
| Bovine jugular vein | 4 (5.3) | 17 (14–22) mm |
| Complications | ||
| Atrioventricular block III | 1 (1.3) | |
| Pneumothorax | 1 (1.3) | |
| Postoperative bleeding | 2 (2.6) | |
| Postoperative ECMO | 0 (0) | |
| Mortality | 0 (0) | |
| Length of stay | ||
| Intensive care unit | 1.2 (0.22–6.6) days | |
| Total in hospital | 6.0 (4–17) days | |
| . | n (%) . | Median (range) . |
|---|---|---|
| Age | ||
| All | 75 | 10.2 (0.44–18) years |
| Number <2 years | 10 (13) | 0.84 (0.44–1.8) year |
| Number >2 years | 65 (87) | 11.2 (3.5–18) years |
| Weight | 24.9 (5.7–94) kg | |
| Intraoperative parameters | ||
| Extracorporeal circulation time | 144 (97–280) min | |
| Cross-clamp time | 107 (49–208) min | |
| Cooling temp | 30.9 (25.0–34.3)°C | |
| RV-PA conduits used | ||
| Pulmonary homograft | 70 (93) | 20 (12–27) mm |
| Aortic homograft | 1 (1.3) | 21 mm |
| Bovine jugular vein | 4 (5.3) | 17 (14–22) mm |
| Complications | ||
| Atrioventricular block III | 1 (1.3) | |
| Pneumothorax | 1 (1.3) | |
| Postoperative bleeding | 2 (2.6) | |
| Postoperative ECMO | 0 (0) | |
| Mortality | 0 (0) | |
| Length of stay | ||
| Intensive care unit | 1.2 (0.22–6.6) days | |
| Total in hospital | 6.0 (4–17) days | |
RV-PA: right ventricular to pulmonary artery; ECMO: extracorporeal membrane oxygenation.
There were no deaths, but a total of 5 reinterventions: 3 on the autograft for regurgitation and 2 on the LVOT, which required resection to relieve obstruction. In the 3 cases of reoperation on the autograft, the children received their primary Ross procedure at the age of 5, 10 and 12 years, and the times to their reoperations were 50 days, 7 months and 5.4 years, respectively. The indication in the case of earliest reoperation was aortic regurgitation caused by a dehiscence of the non-coronary cusp, probably due to endocarditis. The indication for the second case was the formation of a pseudoaneurysm caused by dehiscence of the posterior aspect of the autograft, probably due to a surgical technical error. The indication in the third case was related to a technical error and injury to the left coronary cusp at the time of the Ross procedure that later required repair in the midterm (Table 3). In all the 3 cases, the autograft could be repaired, and good valvular function was restored. In both cases of LVOT obstruction, the children were operated with the Ross procedure at a young age, 0.9 and 1.8 years, and the times to their reoperations were 1.8 and 10.1 years, respectively. In the case of the youngest child who required early reoperation, the cause was related to unfavourable angulation of the LVOT; in the other case, the anomaly was related to the late development of a subvalvular membrane (Table 3). After the reoperations, and over the course of several years, both patients developed a recurrence of mild-to-moderate LVOT obstruction (Table 3).
Autograft and LVOT reinterventions
| Case no. . | Age at Ross procedure . | Time to reoperation . | Indication/anomaly . | Operation . | Result . |
|---|---|---|---|---|---|
| Autograft reintervention | |||||
| 1 | 15.2 years | 50 days | Regurgitation/dehiscence of the non-coronary cusp and suspected endocarditis | Repair | Good valvular function |
| 2 | 10.2 years | 6.7 years | Regurgitation/pseudo-aneurysmal dehiscence of posterior aspect of autograft | Repair | Good valvular function |
| 3 | 12.0 years | 5.4 years | Regurgitation/perforation of left coronary cusp at time of Ross procedure | Repair | Good valvular function |
| LVOT reintervention | |||||
| 1 | 11.2 months | 1.8 years | Stenosis/borderline LVOT with unfavourable angulation at time of Ross procedure | Resection | 6 years after reoperation: v-max 3.6 m/s |
| 2 | 1.8 years | 10.1 years | Late development of subvalvular membrane | Resection | 2.5 years after reoperation: v-max 3 m/s |
| Case no. . | Age at Ross procedure . | Time to reoperation . | Indication/anomaly . | Operation . | Result . |
|---|---|---|---|---|---|
| Autograft reintervention | |||||
| 1 | 15.2 years | 50 days | Regurgitation/dehiscence of the non-coronary cusp and suspected endocarditis | Repair | Good valvular function |
| 2 | 10.2 years | 6.7 years | Regurgitation/pseudo-aneurysmal dehiscence of posterior aspect of autograft | Repair | Good valvular function |
| 3 | 12.0 years | 5.4 years | Regurgitation/perforation of left coronary cusp at time of Ross procedure | Repair | Good valvular function |
| LVOT reintervention | |||||
| 1 | 11.2 months | 1.8 years | Stenosis/borderline LVOT with unfavourable angulation at time of Ross procedure | Resection | 6 years after reoperation: v-max 3.6 m/s |
| 2 | 1.8 years | 10.1 years | Late development of subvalvular membrane | Resection | 2.5 years after reoperation: v-max 3 m/s |
LVOT: left ventricular outflow tract.
Autograft and LVOT reinterventions
| Case no. . | Age at Ross procedure . | Time to reoperation . | Indication/anomaly . | Operation . | Result . |
|---|---|---|---|---|---|
| Autograft reintervention | |||||
| 1 | 15.2 years | 50 days | Regurgitation/dehiscence of the non-coronary cusp and suspected endocarditis | Repair | Good valvular function |
| 2 | 10.2 years | 6.7 years | Regurgitation/pseudo-aneurysmal dehiscence of posterior aspect of autograft | Repair | Good valvular function |
| 3 | 12.0 years | 5.4 years | Regurgitation/perforation of left coronary cusp at time of Ross procedure | Repair | Good valvular function |
| LVOT reintervention | |||||
| 1 | 11.2 months | 1.8 years | Stenosis/borderline LVOT with unfavourable angulation at time of Ross procedure | Resection | 6 years after reoperation: v-max 3.6 m/s |
| 2 | 1.8 years | 10.1 years | Late development of subvalvular membrane | Resection | 2.5 years after reoperation: v-max 3 m/s |
| Case no. . | Age at Ross procedure . | Time to reoperation . | Indication/anomaly . | Operation . | Result . |
|---|---|---|---|---|---|
| Autograft reintervention | |||||
| 1 | 15.2 years | 50 days | Regurgitation/dehiscence of the non-coronary cusp and suspected endocarditis | Repair | Good valvular function |
| 2 | 10.2 years | 6.7 years | Regurgitation/pseudo-aneurysmal dehiscence of posterior aspect of autograft | Repair | Good valvular function |
| 3 | 12.0 years | 5.4 years | Regurgitation/perforation of left coronary cusp at time of Ross procedure | Repair | Good valvular function |
| LVOT reintervention | |||||
| 1 | 11.2 months | 1.8 years | Stenosis/borderline LVOT with unfavourable angulation at time of Ross procedure | Resection | 6 years after reoperation: v-max 3.6 m/s |
| 2 | 1.8 years | 10.1 years | Late development of subvalvular membrane | Resection | 2.5 years after reoperation: v-max 3 m/s |
LVOT: left ventricular outflow tract.
There were in total 4 other complications in the entire patient cohort: 2 had postoperative bleeding, 1 had pneumothorax and 1 had atrioventricular block III. All patients recovered uneventfully following reoperations for bleeding, drain insertion and pacemaker implantation (Table 2).
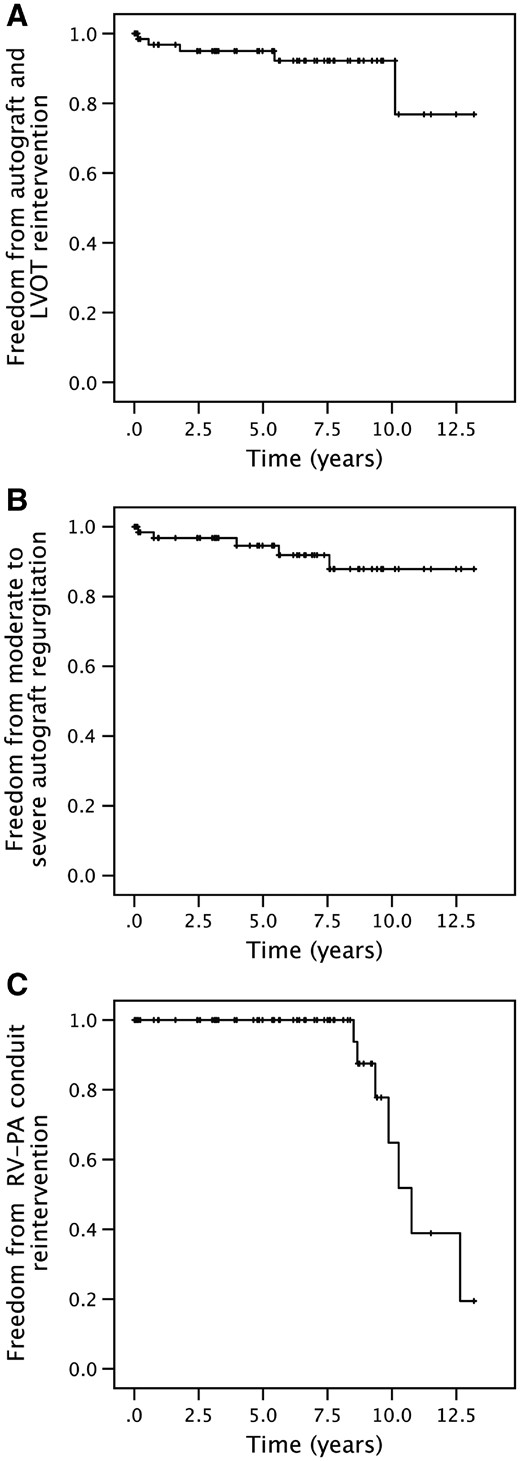
Freedom from autograft and LVOT reintervention was 95% (95% CI: 90–100) and 92% (95% CI: 85–100) at 5 and 10 years, respectively (Fig. 2A). There was no significant autograft stenosis at the valvular level. Freedom from moderate-to-severe autograft regurgitation was 95% (95% CI: 89–100) and 88% (95% CI: 77–99) at 5 and 10 years, respectively (Fig. 2B). Median freedom from right ventricle to pulmonary artery (RV-PA) reintervention time was 10.7 years (95% CI: 9.6–11.9), where freedom from RV-PA conduit reintervention was 65% (95% CI: 35–95) at 10 years (Fig. 2C).
Kaplan–Meier survival analyses. (A) Freedom from autograft and LVOT reintervention. (B) Freedom from moderate-to-severe autograft regurgitation. (C) Freedom from RV-PA conduit reintervention. RV-PA: right ventricular to pulmonary artery.
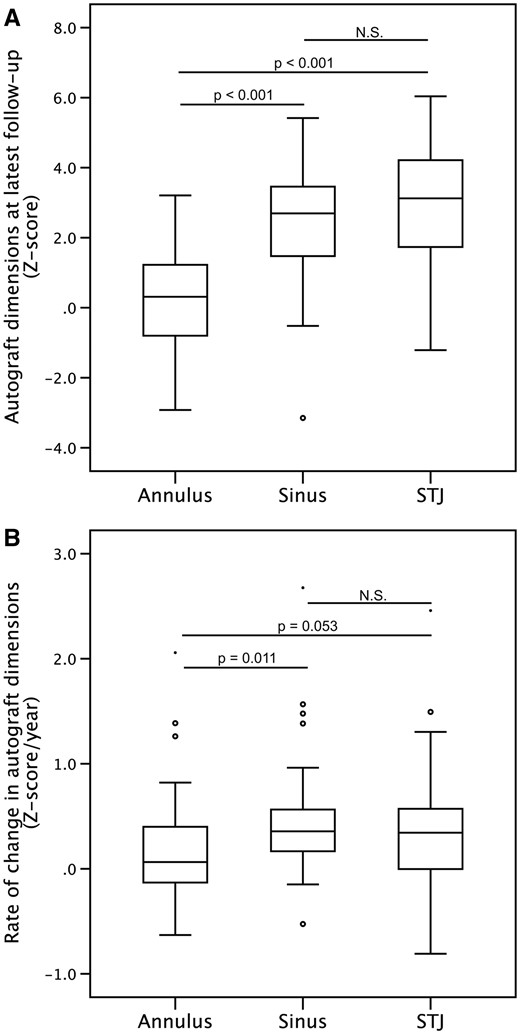
Z-scores of aortic root structures at the latest postoperative follow-up at median 4.6 years (range, 1 day–13.2 years) were, at the annulus, 0.31 (IQR = −0.81 to 1.2), sinus of Valsalva 2.7 (IQR = 1.5–3.5) and sinotubular junction 3.1 (IQR = 1.7–4.2). The z-score at the annulus was significantly lower and closer to normal compared with those at the sinus and the sinotubular junction (Fig. 3A).
Boxplot analysis. (A) Z-scores of the different aortic root structures at the latest available echocardiographic examination. (B) The rate of change in the z-scores of the different aortic root structures between the earliest and latest available echocardiographic examinations. Outliers are displayed as circles and asterisk.
The median rate of change in the z-score was significantly lower at the annulus, 0.065 z-score/year (IQR = −0.13 to 0.43) compared with the rate observed at the level of the sinus of Valsalva, 0.36 z-score/year (IQR = 0.12–0.56), but not the sinotubular junction, 0.34 z-score/year (IQR = 0.0033–0.60) (Fig. 3B).
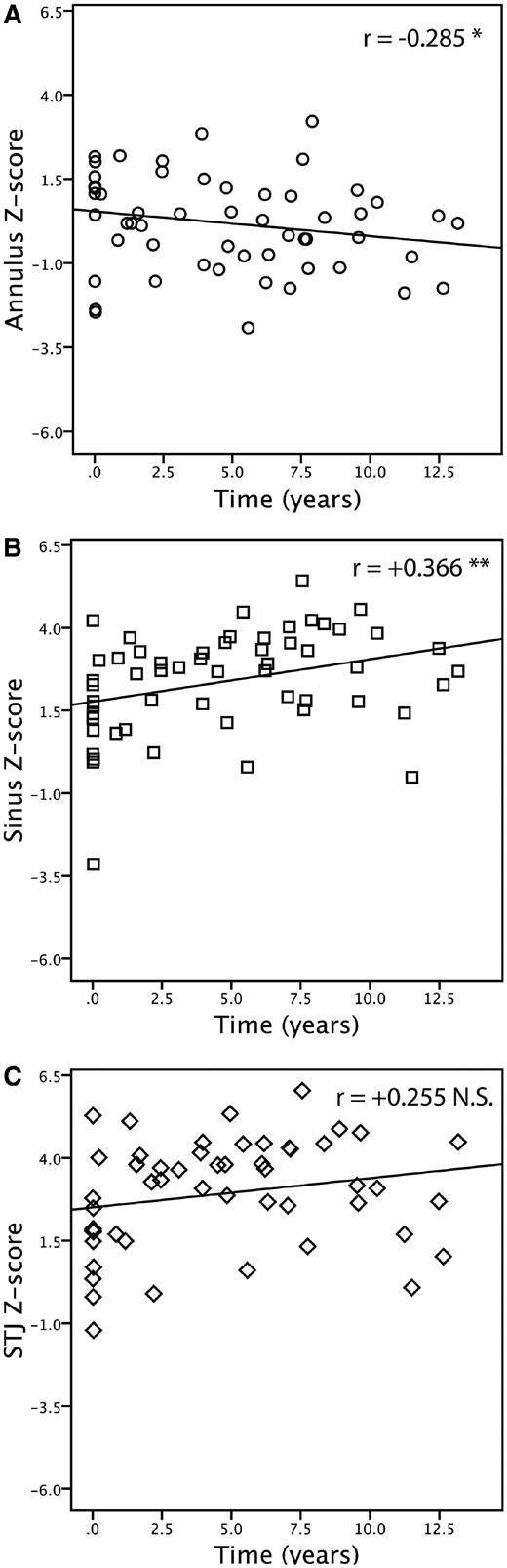
A correlation between the z-score of the root structures and the time after surgery was found to be positive at the level of the sinus (r = +0.366, P = 0.005) and at the sinotubular junction (r = +0.255, P = 0.068) but negative at the annulus (r = −0.285, P = 0.034) (Fig. 4A–C).
Spearman’s rho correlation analysis. (A) Annulus z-score and time after surgery. (B) Sinus of Valsalva z-score and time after surgery. (C) Sinotubular junction z-score and time after surgery.
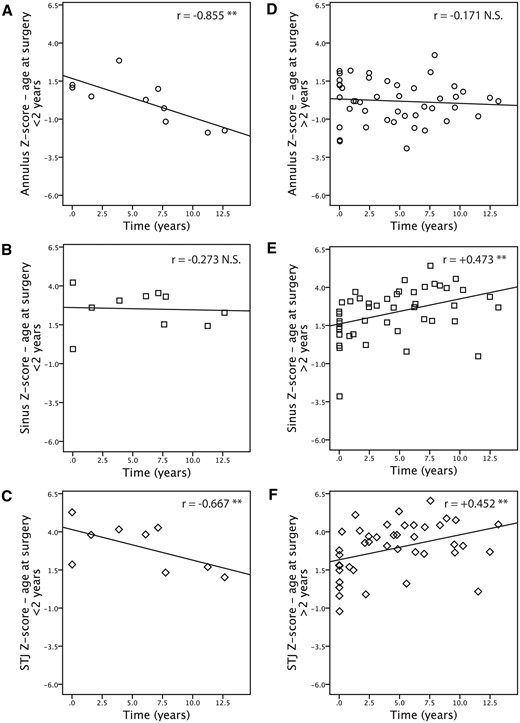
Subgroup analyses of children operated before the age of 2 years revealed consistently negative correlations between the z-scores at all root structure levels and time after surgery, where the correlations at the level of the annulus (r = −0.855, P = 0.002) and the sinotubular junction (r = −0.667, P = 0.05) were strong and significant (Fig. 5A–C). In contrast, children operated after the age of 2 years displayed no correlation at the level of the annulus (r = −0.171, P = 0.26), but a moderate positive and significant correlation at the level of the sinus (r = +0.473, P = 0.001) and the sinotubular junction (r = +0.452, P = 0.002) (Fig. 5D–F). The z-scores at the latest follow-up and the rate of change in z-scores across all root structures did not differ between those younger versus older than 2 years at the time of Ross procedure (data not shown).
Spearman’s rho correlation—subgroup analysis. Panels on the left represent children under 2 years of age (A–C); those on the right represent children over 2 years of age (D–F). Annulus z-score and time after surgery (A, D). Sinus of Valsalva z-score and time after surgery (B, E). Sinotubular junction z-score and time after surgery (C, F).
DISCUSSION
In this study, we show that subannular autograft implantation with interrupted sutures provides the structural support for the annulus. The dimension of the autograft stayed within the normal limits, and an average annulus z-score of +0.31 was observed, at a median time of 4.6 years after the operation. Strictly speaking, this is not a comparison but may be an improvement based on the previously reported annulus z-scores, which were between +2.0 and more than +3.0, where the autograft had been implanted with running sutures [15, 16]. Favourable changes were also found at the level of the sinus of Valsalva and the sinotubular junction, where the z-scores in our cohort were +2.7 and +3.1, respectively. Reported z-scores for these structures with the other implantation methods were +4.5 and +7.0, respectively [16]. In support of our findings, a study using an intra-annular implantation strategy showed that the growth profile of autograft structures ran parallel to that of normal somatic growth [17].
Consistent with the preceding findings, we also observed a lower rate of change in z-scores at the level of the annulus, +0.065 z-score/year with the background of +0.31 z-score/year in the case of implantation with running sutures [16]. However, the use of a different formula to calculate the z-score may partially explain the observed differences between the operative methods.
The subannular positioning of the autograft does not increase the risk of LVOT obstruction, valvular stenosis or dysfunction. In our series, 2 cases of LVOT obstruction required resection. One was related to the unusual morphological angulation of the LVOT, irrespective of the Ross approach, and the other was a subvalvular membrane that developed after more than 10 years. Both children were <2 years at the time of the Ross procedure. In hindsight, a concomitant Konno procedure would have been preferable. However, the overall freedom from autograft and LVOT reintervention was 92% at 10 years, which compares well with results from other studies [1, 4]. The incidence of moderate-to-severe aortic regurgitation was low in our cohort, being approximately 12% at 12.5 years. Only longer term follow-up data would offer an insight into the adaptation of the valve unit, because recent reported results ranged from 18% to more than 24% [15, 16].
The subannular interrupted sutures implantation technique was associated with acceptable risks because there were no deaths in our cohort. Early reoperations aside, the complication rate was low and ranged from trivial pneumothorax to the most serious, a single case of atrioventricular block. Having said that, autograft and especially the arterial wall dilatation are ongoing processes. As is evident from the longer follow-up into the second decade, we noted an expected small increment in the rate of autograft dilatation and development of regurgitation and the need for operation beginning at around 5 years after the Ross procedure [18]. Although our midterm data seem promising, the true test of improved stability lies ahead.
Some data suggest that having the procedure at a young age may take advantage of a time window when the pulmonary root can adapt structurally to the systemic blood pressure and thereby resist dilatation [15, 19]. Our observations provide some preliminary data to support this hypothesis. Nevertheless, understanding when one should operate is still a difficult task, especially in the asymptomatic child [20]. The incentive for an early procedure on the assumption of an improved chance of tissue adaptation and prevention of dilatation in the long-term is also countered by the higher technical demands on the surgeon [21, 22].
In the midterm, analysis of explanted autografts (3–6 years after implantation) from adult patients showed normal cusps with trilaminar structure and near-normal cellularity [23]. Only future, long-term analysis will prove whether the autograft and the arterial wall can adapt. So far, with our observational data and the clinical performance of the valve unit, we can confirm that the subannular interrupted sutures implantation technique can deliver good midterm results.
Many innovative strategies to wrap and protect the autograft root and sinuses from dilatation have been tested in the adult population [24, 25]. However, none or only a limited number of these augmentations can be used in the growing child. The autograft root must be free from a fixed external restraint and be allowed to grow. In fact, the fibromuscular annulus of the native LVOT can provide that desired external support without restricting normal growth. Later in life, the continuing dilation of the autograft sinuses, if indicated, can possibly be managed with a valve-sparing root procedure, and the patient can continue to live without anticoagulation.
The need for reoperations in this patient group is also dependent on the lifespan of the RV-PA conduit. The majority of children in our cohort received a pulmonary homograft. The overall need for reintervention was related to the discrepancy between the original small conduit dimensions and somatic growth, which was not different from that previously reported by other groups [26, 27]. Catheter intervention with repeated implantations of stent-mounted valves can be used to extend the life of the RV-PA conduit and defer surgical reinterventions [28].
In contrast to our era of technological innovations, some reports have shown that the autograft valve replacement strategy may be underused [29, 30]. This may change. With expanding indications, transcatheter aortic valve implantation may in the future be applicable for the young. Hence, at some point, these stent-mounted valves may need to be surgically removed, a procedure with a potentially high risk. Surgeons may need to become comfortable with the autograft/homograft implantation technique to offer an alternative treatment but also to deal with complex, redo aortic root lesions.
CONCLUSION
In conclusion, our data indicate that the subannular interrupted sutures technique can deliver good functional valve performance and prevent excessive dilatation at the level of the aortic annulus. Even though the mild-to-moderate dilatation at the sinus and the sinotubular junction of the autograft valve are out of proportion to the somatic growth, the valve unit remains functionally stable, at least in the midterm.
Funding
This work was supported by the Swedish Heart-Lung Foundation [P.-K.T.]; the Swedish Heart-Lung Foundation [K.T.-L.]; Skane’s County Council [K.T.-L.]; Thure Carlsson Memorial Foundation [K.T.-L.]; and Fanny Ekdahl’s Foundation [K.T.-L.].
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the Meeting of the American Heart Association Scientific Session, Orlando, FL, USA, 7–11 November 2015.