-
PDF
- Split View
-
Views
-
Cite
Cite
Alberto Priori, Barbara Bossi, Gianluca Ardolino, Laura Bertolasi, Marinella Carpo, Eduardo Nobile-Orazio, Sergio Barbieri, Pathophysiological heterogeneity of conduction blocks in multifocal motor neuropathy, Brain, Volume 128, Issue 7, July 2005, Pages 1642–1648, https://doi.org/10.1093/brain/awh513
Close - Share Icon Share
Abstract
The pathophysiological mechanisms responsible for conduction block in multifocal motor neuropathy (MMN) are still unclear. To clarify the physiological abnormalities at the site of the block, we tested the effects induced by polarizing direct currents on motor conduction along forearm nerves in 25 normal nerves (13 subjects), and at the site of conduction block in six nerves (five patients) with MMN. In healthy controls, whereas nerve depolarization failed to change the conditioned compound muscle action potential (CMAP), hyperpolarization elicited a significant, charge-dependent, decrease in the conditioned CMAP size. Hyperpolarization with 4 mC elicited CMAPs that were 86.76 ± 5.22% (mean ± SEM) of the control unconditioned response (P < 0.05). Analysis of individual MMN nerves showed that polarizing currents elicited markedly heterogeneous effects: depending on the nerve tested, depolarization or hyperpolarization in most cases significantly improved conduction along motor fibres across the conduction block. In three MMN nerves, pathophysiological abnormalities were consistent with a hyperpolarizing block, in two with a depolarizing block, and in one with a mixed block. Our observations indicate that the pathophysiological abnormalities at the site of conduction block in MMN may arise from depolarization or hyperpolarization, probably depending on the course of disease.
Introduction
Multifocal motor neuropathy (MMN) is a rare disorder of the PNS characterized by the presence of focal conduction blocks along motor fibres leading to weakness and eventually muscle atrophy in the territory of individual nerves (Nobile-Orazio et al., 2005). Although its aetiopathogenesis is still obscure, MMN is thought to arise from dysimmune mechanisms and responds dramatically to treatment with intravenous immunoglobulin (IVIg).
Knowledge is also lacking on the pathophysiology of axonal conduction in MMN. Hyperpolarization or depolarization can both lead to failure of action potential conduction along motor axons and consequently to conduction block. Recent studies assessing axonal excitability, despite some controversial findings, consistently showed hyperpolarization of the motor axonal membrane outside the region of conduction block (Kiernan et al., 2002; Priori et al., 2002). No information is available on the pathophysiology of the axonal membrane within the conduction block mainly because at this level the marked threshold/rheobase increase makes motor fibres almost unexcitable (Yokota et al., 1996; Kaji et al., 2003). Several indirect observations nonetheless suggest that the conduction block in MMN arises from axonal depolarization (Kimura et al., 2001; Priori et al., 2002; Kaji et al., 2003). According to this hypothesis, abnormal depolarization at the site of conduction block in turn leads to a compensatory hyperpolarization outside the block. However, direct electrophysiological evidence documenting depolarization at the site of block is still lacking. In addition, motor axonal hyperpolarization induced by the firing of motor fibres worsens the conduction block in MMN (Kaji et al., 2000). The pathophysiological mechanisms leading to conduction block in MMN therefore remain unclear. Their understanding can be relevant for developing new therapeutic strategies.
Polarizing direct currents (DCs) induce remarkable excitability changes in the PNS (Kiernan et al., 2000) and CNS (Priori, 2003). At the PNS level, anodal DCs block motor conduction (anodal block or anodal depression) (Lloyd, 1950) by eliciting membrane hyperpolarization (Manfredi, 1970, Lorente de Nó, 1947; Petruska et al., 1998; Kimura, 2001). By reversing membrane depolarization at the site of a depolarizing block, anodal DCs should in theory improve motor conduction in MMN. Vice versa, a hyperpolarizing block should worsen during hyperpolarizing anodal DCs.
In this study, to clarify the pathophysiological mechanisms responsible for conduction block in MMN, we assessed the effects elicited by focal nerve polarization on motor fibre conduction at the site of block in patients with MMN. Motor conduction was assessed by recording the compound motor action potential (CMAP) elicited in small hand muscles by nerve stimulation proximal to the site of block and results were compared with those from healthy control subjects.
Subjects and methods
Subjects
We studied 25 nerves in 13 normal subjects (seven men, six women) aged 26–44 years and six nerves in five patients (four men, one woman) with MMN aged 29–63 years. The diagnosis of MMN fulfilled the criteria proposed by the American Association of Electrodiagnostic Medicine on MMN (Olney et al., 2003). The site of conduction block along the forearm nerves was identified with the ‘inching’ technique (Kimura, 2001). Patients selected had to have a CMAP evoked by distal stimulation with peak-to-peak amplitude >3 mV. Four patients were under treatment with IVIg and they had the last therapeutic cycle at variable intervals (range 2–8 weeks, mean ± SEM, 3.7 ± 1.4) before the study. One patient (nerve number 5) was treated with immunosuppressive therapy. The mean duration of disease since the diagnosis was 8 ± 2.5 years (range 5–15). Four patients had at least one motor conduction block >50% peak-to-peak amplitude with a temporal dispersion <30% (Olney et al., 2003) at the time of the diagnosis. One patient (nerve number 6) had a probable partial conduction block, characterized by moderate temporal dispersion of 45% and a peak-to-peak amplitude reduction of 51% (Olney et al., 2003).
Because conduction blocks varied after the diagnosis during the course of the disease, for the purpose of the analysis we divided the nerves into two groups according to the degree of conduction block at the time of the study: nerves with marked conduction block (peak-to-peak decrement in size >50%; temporal dispersion <30%) and nerves with mild conduction block (peak-to-peak decrement in size <50% or temporal dispersion >30%, or both abnormalities). To assess the reproducibility of the neurophysiological finding, two nerves were tested on different occasions. The control (nerve number 25) was studied twice on the same day, whereas the MMN (nerve number 1) was tested twice at an interval of 44 weeks.
All the participants gave their informed consent and the procedure was approved by the Ethics Committee of the IRCCS Ospedale Maggiore di Milano.
Conditioning DC nerve polarization
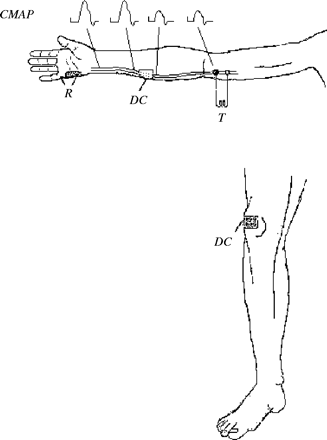
Graded DC currents (maximum intensity 10 mA, 400 ms, maximum charge density ≤0.16 mC/cm2) generated by a Grass Stimulator connected to an isolation unit (Grass SIU5) and to a constant current device (Grass CCU) were delivered through one saline-soaked, square sponge (25 cm2) electrode over the median or ulnar nerve. The other electrode was placed over the skin of the ipsilateral knee joint (Fig. 1). Four MMN nerves were tested using polarizing strengths of 0.4, 0.8, 1.6 and 4 mC; two nerves (1 and 6) were studied only up to 1.6 mC. Throughout the text, hyperpolarizing DC pulses refer to stimuli delivered over the median or ulnar nerve through an electrode connected to the anodal (+) output of the stimulator; depolarizing DC pulses refer to stimuli delivered over the nerve through an electrode connected to the cathodal (−) output of the stimulator. In the patients with MMN, nerves were focally polarized at the point where a preliminary ‘inching’ study showed a focal conduction block. The variables used for delivering conditioning DC stimulation were within the safety range of transthoracic currents (Knickerbocker, 1973).
A schematic diagram illustrating the experimental design. The upper portion of the panel shows the upper limb with recording electrodes (R) over the hypothenar muscles. The polarizing electrode (DC) over the ulnar nerve is placed over the point where the inching study demonstrated the presence of a focal conduction block of the CMAP. Test nerve stimulation (T) is delivered at the elbow. Except for the position of the recording electrodes over the thenar muscles and of the electrodes for test stimulation (T) over the anterior part of the elbow, the same experimental design was also used for studying the median nerve.
Test nerve stimulation
Given the relative rarity of MMN, we had to enrol patients having a conduction block either in the median or in the ulnar nerve. Consequently, we also studied both nerves in the control group. The nerves were stimulated at the wrist and at the elbow to elicit a test CMAP either in the abductor pollicis brevis muscle for median nerve stimulation or in the abductor digiti minimi for ulnar nerve stimulation. The nerve was stimulated bipolarly through a pair of gold-plated non-polarizable surface electrodes 10 mm in diameter (Technomed Europe TE/C32-434). Electrodes were placed above the nerve with an interelectrode distance of 20 mm: the cathode was distal and the anode proximal. Electrodes were connected through a Grass SIU5 isolation unit to a Grass Stimulator generating square pulses (0.1–0.7 ms). Control unconditioned CMAP, elicited by proximal stimulation, was ∼50% of the maximum CMAP evoked by proximal stimulation. To detect suppression or facilitation of the test CMAP without a ‘ceiling’ effect, we used a submaximal test response. Submaximal test responses in both groups of nerves were stable. Test stimuli at the elbow were delivered 350 ms after the 400 ms polarizing DC pulses started: hence the test CMAP was evoked during the last 50 ms of conditioning DC polarization over the block. The volley of action potentials elicited by stimulation at the elbow and travelling along motor axons therefore crossed the conduction block after >350 ms of fibre polarization. At each polarization intensity, and for each DC polarity, five unconditioned control CMAPs and three conditioned CMAPs were acquired in a pseudorandom sequence.
The electromyographic (EMG) signal
The surface EMG signal from the abductor pollicis brevis and from the abductor digiti minimi muscle was captured through a pair of non-polarizable surface electrodes (Nicolet-Biomedical, diameter 10 mm, 1.0 m cable). The signal was then pre-amplified, bandpassed (10 Hz–5 kHz), A/D converted, analysed on-line and stored on a Nicolet Viking IV System. Data were also analysed off-line. CMAP amplitude was measured peak-to-peak and the effects elicited by nerve polarization were estimated by measuring the changes in CMAP size. The size of the conditioned CMAP elicited by elbow stimulation during the delivery of DC polarization in the mid forearm is expressed as a percentage of the control unconditioned CMAP elicited in the absence of DC polarization by elbow stimulation. The latency of the conditioned CMAP elicited by elbow stimulation during the delivery of DC polarization in the mid forearm is expressed as a percentage of the latency of the control unconditioned CMAP elicited in the absence of DC polarization by elbow stimulation.
In all the experiments, skin temperature of the arm was maintained at >32°C. Data were analysed using the Mann–Whitney U test because the variances differed among groups. The Kruskal–Wallis test was used to determine whether the effect of polarization strength at all intensities tested differed in different groups of nerves. The one-sample Wilcoxon signed rank test was used to test whether single values for each patient differed significantly from those of the healthy group. A linear correlation analysis was used to test the correlation between two variables (i.e. total anodal polarization charge and decrease in CMAP). Spearman rank correlation was used to test the correlation between polarization-dependent changes and the time elapsed after the last IVIg treatment). Data throughout the text are the mean ± SEM. P values <0.05 were considered to indicate statistical significance.
Results
Polarization of normal nerves
All the nerves tested had distal latency, CMAP amplitude, motor conduction velocity, and temporal and amplitude dispersion within the normal reference ranges for our laboratory.
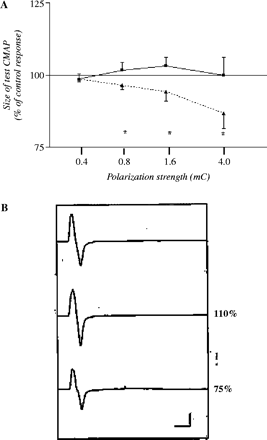
Anodal but not cathodal polarization markedly changed motor conduction along nerves in the forearm (Fig. 2A), without significant differences between median and ulnar nerves (P > 0.05, Kruskal–Wallis test). Whereas nerve depolarization failed to change the conditioned CMAP, hyperpolarization elicited a significant, charge-dependent, decrease in the conditioned CMAP size. The amount of charge delivered by polarizing pulse in healthy subjects linearly correlated with the degree of anodal block (r2 = 0.9941, P = 0.003) and it appeared at charge ≥0.8 mC. The reduction in the test CMAP was significant at charge ≥0.8 mC (at 0.8 mC, P = 0.0045; at 1.6 mC, P = 0.0197; at 4 mC, P = 0.014 Wilcoxon signed rank test) (Fig. 2A).The reproducibility of the effects elicited by polarizing DC pulses was assessed in one normal nerve that was tested twice in the same day and yielded substantially the same effect (T1, 4 mC: anodal polarization = 62%, cathodal polarization = 128%; T2, 4 mC: anodal polarization = 74%, cathodal polarization = 105%).
Changes in motor conduction during focal polarization of healthy nerves. (A) The plot shows the average effects of polarization on nerve conduction expressed as a function of the polarization strength (depolarization, solid line/squares; hyperpolarization, dashed line/triangles). x-axis = polarization strength expressed as total charge delivered (mC); y-axis = size of the conditioned test compound muscle action potential (CMAP) evoked by proximal nerve stimulation expressed as a percentage of the control unconditioned CMAP. Error bars are SEM, n = 25 nerves, *P < 0.05, Wilcoxon signed rank test. (B) Raw data for a representative healthy nerve: the top trace shows the control unconditioned CMAP evoked by proximal stimulation of the median nerve at the elbow, the middle trace is the CMAP conditioned by depolarizing pulse at the mid forearm, and the bottom trace is the CMAP conditioned by hyperpolarizing current. Values on the right of the middle and bottom trace are the peak-to-peak size of the control response. Note that whereas depolarization leaves the CMAP substantially unchanged, hyperpolarization, by inducing anodal block, decreases the test CMAP size.
Conduction time across the polarized segment remained unchanged during the delivery of the conditioning pulse (4 mC hyperpolarization, 100 ± 0.12%, P = 1.00; 4 mC depolarization, 99.92 ± 0.17%, P = 0.6 Mann–Whitney test).
Polarization of MMN nerves
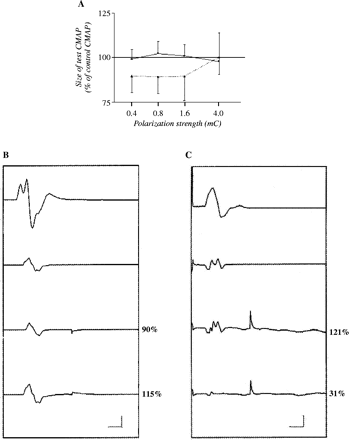
In contrast to normal subjects, in patients with MMN, group statistics failed to show significant changes in CMAP amplitude either during depolarization or during hyperpolarization (Fig. 3A). During delivery of the conditioning pulse, conduction time across the polarized segment remained unchanged (4 mC hyperpolarization, 100 ± 0%; 4 mC depolarization, 100 ± 0%). Nonetheless, responses of individual affected nerves to polarizing DCs showed either hyperpolarization or depolarization at the site of the block (Table 1). Hyperpolarization significantly and consistently worsened the conduction block in nerves 1 and 5 whereas depolarization improved motor conduction in nerve 4. Hence, the responses of nerves 1, 5 and 4 to polarizing DCs indicated hyperpolarization at the site of the block. Conversely, hyperpolarization significantly and consistently improved the conduction block in nerve 2 and depolarization worsened the conduction block in nerve 3. Hence, the responses of nerves 2 and 3 to polarizing currents were consistent with depolarization at the site of the block. Although each nerve was maximally sensitive to one polarity of the conditioning DC, the direction of changes induced by either depolarization or hyperpolarization was opposite in most nerves (four out of six).
The effect of focal polarization on motor conduction in multifocal motor neuropathy (MMN) nerves. (A) The plot shows the average effects of polarization on MMN nerve conduction expressed as a function of the polarization strength. The rest of the legend is as for Fig. 2A. (B) A representative MMN nerve (2) with polarization-induced effects indicating a depolarizing block. The top trace is the compound muscle action potential (CMAP) elicited by distal nerve stimulation at the wrist, the second trace from the top shows the control unconditioned test CMAP elicited by proximal nerve stimulation at the elbow (note the conduction block), the third trace from the top shows the slightly decreased response size during depolarizing conditioning pulses, and the fourth trace from the top its increase during hyperpolarizing conditioning pulses. Vertical calibration is 2 mV, horizontal calibration is 10 ms. Note the small artefact at 50 ms latency elicited by the end of the conditioning polarizing DC pulse delivered to the nerve. The improved conduction block along motor fibres during hyperpolarization argues for a depolarizing block. (C) A representative MMN nerve (1) with polarization-induced effects indicating a hyperpolarizing block. The top trace is the CMAP elicited by nerve stimulation at the wrist, the second trace from the top shows the control test CMAP elicited by proximal nerve stimulation at the elbow (note the conduction block), the third trace from the top shows the response during depolarizing conditioning pulses, and the fourth trace from the top its abolition during hyperpolarizing conditioning pulses. Vertical calibration is 2 mV for the top trace and 0.5 mV for other traces, horizontal calibration is 10 ms. Note the artefact at 50 ms latency elicited by the end of the conditioning polarizing DC pulse delivered to the nerve. The worsening conduction block along motor fibres during further membrane hyperpolarization is consistent with a hyperpolarizing block.
Qualitative changes elicited by polarizing currents in individual nerves with multifocal motor neuropathy
| No. 1, 90% | H | ↓↓ |
| D | ↑ | |
| No. 2, 79% | H | ↑↑ |
| D | ↓ | |
| No. 3, 56% | H | – |
| D | ↓↓ | |
| No. 4, 60% | H | ↓ |
| D | ↑↑ | |
| No. 5, 48% | H | ↓↓ |
| D | ↑ | |
| No. 6, 51% | H | ↓ |
| D | ↓ |
| No. 1, 90% | H | ↓↓ |
| D | ↑ | |
| No. 2, 79% | H | ↑↑ |
| D | ↓ | |
| No. 3, 56% | H | – |
| D | ↓↓ | |
| No. 4, 60% | H | ↓ |
| D | ↑↑ | |
| No. 5, 48% | H | ↓↓ |
| D | ↑ | |
| No. 6, 51% | H | ↓ |
| D | ↓ |
Amount of conduction block = compound muscle action potential (CMAP) size for stimulation proximal to the site of the block expressed as a percentage of the CMAP elicited by distal stimulation; H = hyperpolarizing conditioning DC; D = depolarizing conditioning DC; ↓↓ or ↑↑ = significant (P < 0.05) and consistent decrease or increase in the test CMAP size elicited by proximal stimulation during nerve polarization at all the polarization strengths tested; ↓ or ↑ = decrease or increase in the test CMAP size elicited by proximal stimulation during nerve polarization estimated by the algebraic sum of statistically significant change at each current strength in one direction with non-significant change; – = no change.
Qualitative changes elicited by polarizing currents in individual nerves with multifocal motor neuropathy
| No. 1, 90% | H | ↓↓ |
| D | ↑ | |
| No. 2, 79% | H | ↑↑ |
| D | ↓ | |
| No. 3, 56% | H | – |
| D | ↓↓ | |
| No. 4, 60% | H | ↓ |
| D | ↑↑ | |
| No. 5, 48% | H | ↓↓ |
| D | ↑ | |
| No. 6, 51% | H | ↓ |
| D | ↓ |
| No. 1, 90% | H | ↓↓ |
| D | ↑ | |
| No. 2, 79% | H | ↑↑ |
| D | ↓ | |
| No. 3, 56% | H | – |
| D | ↓↓ | |
| No. 4, 60% | H | ↓ |
| D | ↑↑ | |
| No. 5, 48% | H | ↓↓ |
| D | ↑ | |
| No. 6, 51% | H | ↓ |
| D | ↓ |
Amount of conduction block = compound muscle action potential (CMAP) size for stimulation proximal to the site of the block expressed as a percentage of the CMAP elicited by distal stimulation; H = hyperpolarizing conditioning DC; D = depolarizing conditioning DC; ↓↓ or ↑↑ = significant (P < 0.05) and consistent decrease or increase in the test CMAP size elicited by proximal stimulation during nerve polarization at all the polarization strengths tested; ↓ or ↑ = decrease or increase in the test CMAP size elicited by proximal stimulation during nerve polarization estimated by the algebraic sum of statistically significant change at each current strength in one direction with non-significant change; – = no change.
In the remaining nerve with mild conduction block (number 6), depolarization and hyperpolarization both worsened motor conduction across the block.
The effects induced by nerve polarization did not significantly correlate with the interval after the last IVIg treatment (4 mC hyperpolarizing current, rs = −0.74, P = 0.33; 4 mC depolarizing current, rs = 0.95, P = 0.08).
The reproducibility of the effects elicited by polarizing DC pulses was assessed in one MMN nerve (number 1) that was tested twice at an interval of 44 weeks and yielded substantially the same effect (T1, 1.6 mC anodal polarization = 33%, cathodal polarization = 102%; T2, 1.6 mC, anodal polarization = 39%, cathodal polarization = 128%).
No difference was found in the frequency of fasciculations in hyperpolarized and depolarized nerves of the tested limb.
Discussion
Whereas in the healthy human motor axons we studied focal nerve depolarization left motor fibre conduction substantially unchanged, hyperpolarization significantly and consistently impaired conduction across the polarized nerve segment. Conversely, in MMN nerves, hyperpolarizing DC pulses elicited differential changes in motor conduction. In more severely affected nerves, they either improved or worsened motor fibre conduction across the block itself. These findings suggest that conduction blocks in MMN arise from more than one pathogenetic mechanism (hyperpolarization or depolarization) that can change during the course of the disease.
In the normal human nerves we studied, depolarizing DCs left motor fibre conduction unchanged. Early studies have already described the effects of hyperpolarizing DCs on frog nerves (Lorente de Nó, 1947). Hyperpolarization impairs motor conduction through anodal block (Manfredi et al., 1970; Petruska et al., 1998; Kimura, 2001; Bhadra and Kilgore, 2004). We presume that in our study, depolarization, conversely, failed to improve motor conduction in normal subjects because in normal motor fibres, whereas hyperpolarization blocks the conduction of a nerve impulse, depolarization (at least in the range of polarization strength used here) cannot facilitate action potentials because they are already all-or-none, maximum responses. Hence, under normal conditions, segmental depolarization cannot further enhance a submaximal impulse volley travelling along motor axons owing to a ‘ceiling’ effect.
Interestingly, the decrease in the conditioned CMAP size during hyperpolarizing DCs was not related to changes in the conduction time across the polarized nerve segment. A possible explanation for this finding is that—in line with previous experimental findings (Manfredi 1970)—the hyperpolarizing DCs used in our experiments block the conduction of the impulse volley travelling along motor axons only in a fraction of nerve fibres, leaving conduction along the remaining fibres unaltered.
In the patients with MMN, polarizing DC pulses delivered over conduction blocks induced heterogeneous changes in motor conduction. Several lines of thought argue against the possibility that the effects of conditioning polarizing pulse spread proximally. First, the electrode delivering the conditioning polarizing current in our experiments was placed at least 10–12 cm distally to the proximal electrode delivering the test shock at the elbow. Hence, the conditioning charge density at the elbow below the electrode delivering the test shock would be extremely low, or almost negligible, especially at the low intensity we used for the conditioning current. Secondly, the same polarity of conditioning current induced opposite effects in different nerves (Fig. 3B and 3C). In two nerves (numbers 2 and 3) with marked conduction block (79% and 56%, respectively), from the same subject, hyperpolarization improved, whereas depolarization worsened motor conduction across the block. These findings support Kaji et al.'s hypothesis that depolarization leads to a depolarizing block in MMN (Kaji, 2003).
Yet, surprisingly, two other MMN nerves (numbers 1 and 4) with marked conduction block and one nerve with mild conduction block (number 5) responded to polarizing currents in an opposite manner: hyperpolarization worsened, whereas depolarization improved motor conduction across the block. Hence, this second type of response to polarizing currents suggests that MMN also causes hyperpolarizing blocks. The hyperpolarizing origin of the conduction block agrees with the observation of activity-dependent worsening of conduction blocks (Kaji et al., 2000). The pathophysiological mechanisms leading to hyperpolarization at the site of block probably resemble those thought responsible for hyperpolarization outside the conduction block in MMN, namely, the immune-mediated impairment of Na+ conductances (Priori et al., 2002) or the hyperactivity of the Na+/K+ pump (Kiernan et al., 2002). Scarring at the site of conduction block could impair ionic conductances, thus segregating an axolemmal segment from the extracellular space. The segregated segment would communicate only with the rest of the hyperpolarized intracellular space, thereby accentuating hyperpolarization. One nerve (number 6) with mild conduction block but with the longest disease duration worsened with polarizing DCs of either polarity, arguing for a conduction block involving both depolarization and hyperpolarization, suggesting that the two mechanisms can even co-exist in the same conduction block. The heterogeneity of the pathological abnormalities we observed fits with the variability of the pathological findings in MMN at the site of conduction block (Taylor et al., 2004).
Because both mild and marked conduction blocks can have the features of a hyperpolarizing block, the degree of conduction block does not correlate with the underlying pathophysiological abnormality. Yet, interestingly, MMN nerves with shorter disease duration had depolarizing blocks, whereas nerves with longer disease duration had hyperpolarizing blocks (Table 2), suggesting that the pathophysiological mechanisms underlying the conduction blocks change during the course of the disease, or also in relation to the treatment. Understanding the pathophysiological mechanisms of conduction blocks could also explain the variability of the response to IVIg treatment (Van den Berg-Vos et al., 1998).
Qualitative correlation between the duration of the disease and axonal abnormalities in nerves of patients with multifocal motor neuropathy
. | Disease duration (years) . | Pathophysiological abnormality . |
|---|---|---|
| No. 1, 90% | 13 | Hyperpolarization |
| No. 2, 79% | 2 | Depolarization |
| No. 3, 56% | 2 | Depolarization |
| No. 4, 60% | 5 | Hyperpolarization |
| No. 5, 48% | 5 | Hyperpolarization |
| No. 6, 51% | 15 | Hyperpolarization and depolarization |
. | Disease duration (years) . | Pathophysiological abnormality . |
|---|---|---|
| No. 1, 90% | 13 | Hyperpolarization |
| No. 2, 79% | 2 | Depolarization |
| No. 3, 56% | 2 | Depolarization |
| No. 4, 60% | 5 | Hyperpolarization |
| No. 5, 48% | 5 | Hyperpolarization |
| No. 6, 51% | 15 | Hyperpolarization and depolarization |
Qualitative correlation between the duration of the disease and axonal abnormalities in nerves of patients with multifocal motor neuropathy
. | Disease duration (years) . | Pathophysiological abnormality . |
|---|---|---|
| No. 1, 90% | 13 | Hyperpolarization |
| No. 2, 79% | 2 | Depolarization |
| No. 3, 56% | 2 | Depolarization |
| No. 4, 60% | 5 | Hyperpolarization |
| No. 5, 48% | 5 | Hyperpolarization |
| No. 6, 51% | 15 | Hyperpolarization and depolarization |
. | Disease duration (years) . | Pathophysiological abnormality . |
|---|---|---|
| No. 1, 90% | 13 | Hyperpolarization |
| No. 2, 79% | 2 | Depolarization |
| No. 3, 56% | 2 | Depolarization |
| No. 4, 60% | 5 | Hyperpolarization |
| No. 5, 48% | 5 | Hyperpolarization |
| No. 6, 51% | 15 | Hyperpolarization and depolarization |
In conclusion, our experiments using segmental nerve polarization show that opposite types of pathophysiological abnormalities exist at the site of conduction block in MMN: their underlying mechanisms could reflect disease evolution. A depolarizing block possibly precedes a hyperpolarizing block in the course of MMN.
References
Bhadra N, Kilgore KL. Direct current electrical conduction block of peripheral nerve.
Kaji R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies.
Kaji R, Bostock H, Kohara N, et al. Activity-dependent conduction block in multifocal motor neuropathy.
Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons.
Kiernan MC, Guglielmi JM, Kaji R, Murray NMF, Bostock H. Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block.
Kimura J. Electrodiagnosis in disease of nerve and muscle: principles and practice. 3rd edn. New York: Oxford University Press;
Knickerbocker GG. Fibrillating parameters of direct and alternating (20 Hz) currents separately and in combination—an experimental study.
Lloyd DPC. Electrical properties of nerve and muscle. In: Fulton JF, editor. Textbook of physiology. 16th edn. Philadelphia: W. B. Saunders Company;
Lorente De Nó R. A study of nerve physiology. New York: Rockefeller Institute for Medical Research;
Manfredi M. Differential block of conduction of larger fibers in peripheral nerve by direct current.
Nobile-Orazio E, Cappellari A, Priori A. Multifocal motor neuropathy: current concepts and controversies. Muscle Nerve. Mar 15 [E Pub]
Olney RK, Lewis RA, Putnam TD, Campellone R. Consensus criteria for the diagnosis of multifocal motor neuropathy.
Petruska JC, Hubscher CH, Johnson RD. Anodally focused polarization of peripheral nerve allows discrimination of myelinated and unmyelinated fiber input to brainstem nuclei.
Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability.
Prori A, Cinnante C, Pesenti A, et al. Distinctive abnormalities of motor axonal strength-duration properties in multifocal motor neuropathy and in motor neurone disease.
Taylor BV, Dyck JB, Engelstad J, Grunener G, Grant I, Dyck PJ. Multifocal motor neuropathy: pathological alterations at the side of conduction block.
Van den Berg-Vos RM, Franssen H, Wokke JHJ. The long-term effect of intravenous immunoglobulin treatment in multifocal motor neuropathy.
Author notes
1Dipartimento di Scienze Neurologiche, Università degli Studi di Milano, IRCCS Ospedale Maggiore di Milano, Milan and 2Dipartimento di Scienze Neurologiche e della Visione, Università degli Studi di Verona, Verona, Italy
3Present address: Unità di Neurologia 2, Istituto Clinico Humanitas, via Manzoni, 20 Rozzano (MI), 20080 Italy