- Split View
-
Views
-
Cite
Cite
Matthew H. Persons, Sean E. Walker, Ann L. Rypstra, Fitness costs and benefits of antipredator behavior mediated by chemotactile cues in the wolf spider Pardosa milvina (Araneae: Lycosidae), Behavioral Ecology, Volume 13, Issue 3, May 2002, Pages 386–392, https://doi.org/10.1093/beheco/13.3.386
Close - Share Icon Share
Abstract
Animals may exhibit a variety of defensive behaviors in the presence of indirect predator cues. Such behavior offers immediate fitness benefits but may also incur substantial foraging and reproductive costs. We measured shifts in space use (vertical climbing) by the wolf spider Pardosa milvina induced by chemotactile cues (silk and excreta) from a co-occurring predatory wolf spider Hogna helluo. We then measured foraging and reproductive costs, as well as survival benefits, of this behavior. For 2 weeks, we maintained mated adult female Pardosa in plastic containers with one of three treated peat moss substrates: a container previously occupied by a conspecific for 3 days, a container previously occupied by an adult Hogna for 3 days, and a container devoid of either cue (control). We measured prey capture efficiency, body condition, egg sac production, egg sac weight, and egg number for individuals in each treatment. We also counted the number of Pardosa that survived and exhibited climbing behavior in the presence of a live Hogna with and without silk and excreta cues. Pardosa climbed container walls significantly more often in the presence of Hogna silk and excreta relative to other treatments. Pardosa exposed to Hogna cues coupled with live Hogna survived significantly longer than spiders that had no predator cues available. Pardosa placed in containers with Hogna cues, but no Hogna, lost weight more quickly, ate fewer prey, were in poorer body condition, produced lighter egg sacs, and produced fewer eggs than spiders in control or conspecific treatments.
Prey often exhibit behavioral adaptations to avoid predation such as avoidance (Brown et al., 1995; Gore, 1966), reduced activity (Holomuzki and Short, 1990; Malmqvist, 1992), cover seeking (Kats et al., 1988; Wahle, 1992), or increased vigilance (Sweitzer and Berger, 1992). Early predator detection is often critical for effective use of these tactics. Therefore, many prey have evolved the ability to detect a variety of metabolic by-products associated with the presence of predators and exhibit appropriate behavior even in the absence of the predator itself (reviewed in Chivers and Smith, 1998; Kats and Dill, 1998). Preemptive antipredator tactics may incur substantial fitness costs such as reduced foraging success (reviewed in Lima, 1998; Lima and Dill, 1990) or impaired reproduction (reviewed in Magnhagen, 1991; Kats and Dill, 1998; Repka et al., 1994). These costs may, in turn, translate into a number of life-history changes (Crowl and Covich, 1990) such as delayed development, slower growth (Barry, 1994; Spitze, 1992) or postponed reproduction (Koskela and Ylönen, 1995; Ylönen and Ronkainen, 1994).
Despite an extensive body of literature on prey responses to indirect predator cues, few such studies exist among terrestrial arthropods (Kats and Dill, 1998; Persons et al., 2001; Venzon et al., 2000), and even fewer studies document the costs incurred by arthropods exhibiting chemical or tactile-mediated defensive behavior (Grostal and Dicke, 1999; Hoffmeister and Roitberg, 1997).
Wolf spiders provide an interesting taxon to examine indirectly mediated predator-prey interactions because, in addition to excreta, they also leave a silk dragline behind them while moving through the environment. Because wolf spiders are common prey as well as predators of other species of wolf spider (Persons and Rypstra, 2000), they may be especially likely and able to detect and respond to spider silk and excreta cues. The wolf spider species Hogna helluo (Walckenaer) (Araneae: Lycosidae) and Pardosa milvina (Hentz) (Araneae: Lycosidae) are syntopic intraguild predators. Both species live in early successional habitats and are often the two most abundant cursorial spiders in agricultural systems of the eastern and central United States (Dondale and Redner, 1990; Marshall and Rypstra, 1999; Young and Edwards, 1990). Neither species constructs a web, and both are typically found on the soil surface or residing on low-lying vegetation, where they feed on a wide variety of ground-dwelling arthropods including cursorial spiders, collembola, and crickets (Persons et al., personal observations). Hogna is a large wolf spider (adult female ca. 300-800 mg) and has frequently been observed to prey on the much smaller Pardosa (ca. 20 mg) (Persons et al., 2001). Pardosa exhibits a variety of defensive tactics when exposed to silk and excreta cues produced by Hogna, including prolonged periods of immobility, reduced walking speeds, and substrate avoidance (Persons et al., 2001). These behaviors minimize predation in the presence of Hogna (Persons et al., 2001). Pardosa has also been anecdotally observed to shift space use by climbing vertical surfaces when exposed to Hogna silk and excreta. Behavioral responses may be mediated by chemicals found within silk and excreta, localized structural changes to the substratum, or tactile information embedded within the predator cues. For this reason, we refer to behaviorally relevant information found in silk and excreta as chemotactile cues. To date, the potential direct and indirect fitness costs of chemotactile-mediated antipredator behavior has not been experimentally demonstrated in any species of spider.
Female Pardosa, like all lycosids, attach their egg sacs to their spinnerets. Egg sacs are energetically expensive to produce, constituting more than 30% of the mass of the spider (Edgar, 1971; Marshall and Gittleman, 1994). Therefore, any behavior that reduces foraging success or results in less energy being available for reproduction should result in a reduction in egg number or egg mass, or a delay in the timing of egg sac production.
Here we addressed possible foraging and reproductive costs as well as survival benefits associated with a shift in space use (vertical climbing) by Pardosa when exposed to silk and excreta produced by Hogna. We tested the five hypotheses: (1) Pardosa should shift their locations to vertical surfaces (climbing movement) in the presence of silk and excreta cues from Hogna compared to conspecific silk and excreta or substrates lacking either cue; (2) shifts in space use should result in either reduced foraging success and/or increased energy expenditure as measured by spider body condition; (3) Pardosa should reduce feeding in the presence of cues from Hogna compared to conspecific cues; (4) long-term exposure to predator chemotactile cues should delayed reproduction and reduce reproductive output; and (5) vertical climbing should increase survival in the presence of live Hogna compared to spiders that do not exhibit this behavior.
General spider collection and maintenance
For all experiments we collected adult female Pardosa with egg sacs from soybean fields at Miami University's Ecology Research Center (Oxford, Butler County, Ohio, USA) in August 1998. Egg sacs were removed from females within 48 h of any experiment unless otherwise indicated. Adult female Hogna with spiderlings or egg sacs were collected from the same soybean fields in August 1997, and their offspring were raised in the lab and used as experimental predators or predator stimuli for all experiments. Pardosa were maintained in 5.5 cm high × 5.5 cm diam containers with a 2-cm layer of moistened peat moss covering the bottom. Each container was covered with a clear plastic lid. Hogna were maintained in 8 cm high × 12 cm diam white plastic cups with clear lids and also had a 2 cm deep layer of moistened peat moss provided as a source of water and burrowing substrate. Both species were maintained on a diet of domestic house crickets (Acheta domesticus).
Experiment 1: measuring behavioral changes and fitness consequences of Pardosa exposure to Hogna cues
Methods
We measured shifts in space use of mated adult female Pardosa when exposed to containers with peat moss substrates previously occupied by one of the following: an adult female Hogna helluo, a conspecific female Pardosa, or a substrate lacking either cue. Over a 2-week period, we quantified the following for each Pardosa within each peat moss treatment: changes in body condition (morphometric measure of weight loss scaled to body size), latency to produce an egg sac, number of egg sacs produced, egg sac weight, and egg number per egg sac. Additionally, Pardosa were observed either on the side or bottom of the container, and we used this as our behavioral response variable across each peat moss treatment.
Peat moss stimulus preparation. All test Pardosa were randomly assigned among containers with three treatment groups of moistened peat moss (n = 25/treatment): (1) control, containers devoid of any spider cues; (2) conspecific cues, containers previously occupied for 3 days by a mated adult female; and (3) predator cues, containers previously occupied by an adult female Hogna for 3 days. Each treatment container consisted of a 12 cm diam × 8 cm high white plastic cup with a clear lid. Before adding peat moss, all plastic cups were rinsed with 95% ethanol to remove any residual odor cues from prior use. We then placed 2 cm of commercially obtained moist peat moss in the bottom of each container. For containers requiring a stimulus, a single Pardosa or Hogna was satiated with crickets during a 24-h period, then introduced into each cup. All stimulus spiders were maintained in the cup without food for 3 days and subsequently removed. A test Pardosa was introduced into each of the three peat moss treatment containers (control, Pardosa cues, or Hogna cues). A different individual Hogna and Pardosa were used for each container. Every 4 days, a new set of peat moss containers was set up to maintain fresh cues for each treatment group. Immediately after transfers to new containers, test spiders were fed to satiation through ad libitum feeding within the treatment containers. Thus spiders were transferred to a new container with 3-day-old cues every 4 days and fed to excess with additional prey added every 4 days (3 times during the 14-day test period). All spiders were maintained in environmental chambers with a 13:11h light:dark photoregimen at stable humidity (50-60%) and temperature (22-25°C).
Measurement of behavior, body condition, and egg sac production. After the initial 4 days within each treatment, but before the first feeding within the stimulus containers, we measured body condition (a size-independent measure of nutritional state) for each test Pardosa (described in Jakob et al., 1996). Cephalothorax width and abdomen width were measured with dial calipers. Both measures were taken at the greatest width for both cephalothorax and abdomen. We used cephalothorax width as a measure of spider body size because it remains constant with food intake within an instar (Hagstrum, 1971; Jakob et al., 1996). Abdomen width increases as a function of feeding and was used as an index of body condition.
Within each treatment group, the location of the spider—on the substrate or on the side of the container—was noted, and the presence or absence of an egg sac was recorded. A spider was recorded as being on the side of the container only if all eight legs were in contact with the wall and none with the substrate. We recorded spider location three times daily at 0800, 1200, and 2100 h. Environmental chamber lighting was set on a timer. Morning recordings were completed 30 min before artificial daylight and evening recordings were recorded 30 min after artificial nightfall. Morning and evening measurements were taken using a 15-watt flashlight as the sole light source. Once egg sacs had been deposited, the spider and egg sac were removed and weighed separately on an analytical balance and then placed in 70% alcohol so that the number of eggs could be determined at a later date. We collected spiders producing egg sacs during a 14-day period, after which point the experiment was terminated. Measurement of climbing behavior was discontinued after 8 days due to a reduction in sample size from spiders producing egg sacs.
Statistical analysis. Significant differences in climbing behavior for each chemotactile cue treatment were determined with a chi-square contingency table for each time period immediately following replacement of the substrate (the mornings of day 1, day 4, and day 8). We determined differences in spider abdomen width (body condition), egg sac mass, and egg number within each treatment using one-way ANOVAs and Tukey post-hoc comparison of means tests. Data were natural-log transformed to conform to ANOVA assumptions of normality when necessary. We compared differences in the number of individuals producing an egg sac within each treatment using a chi-square two-by-three contingency table. We used failure-time analysis using the Kaplan-Meier product limit estimator to compare differences in the median time to produce an egg sac, and we tested for a significant treatment effect and then compared the distribution of time to egg sac production using the log-rank (Mantel-Cox) test.
Results
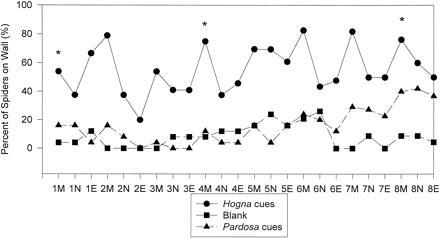
Pardosa milvina exhibited significant differences in climbing behavior across the three treatment groups for each time period tested (Figure 1). Spiders spent significantly more time on the sides of the containers in the presence of Hogna cues than either the blank control treatment or the conspecific treatment (Figure 1), and this behavior appeared to continue over much of the 8-day recording period.
Percentage of spiders exhibiting climbing behavior in containers with three peat moss treatments (n = 25/treatment): Hogna predator cues present, conspecific cues present, and blank control. Spider positions were recorded over an 8-day period three times a day—morning (M), noon (N), and evening (E). Statistical differences in the number of spiders on the sides of the container were determined with a chi-square test after each time the substrate was replaced (indicated by an asterisk). Significantly more spiders were on the wall after 1 day (χ2 = 19.60, p <.0001), day 4 (χ2 = 31.60, p <.00001), and day 8 (χ2 = 23.86, p <.00001) in the predator cue treatments compared to other treatments.
We compared cephalothorax width of spiders across treatment groups to test for the possibility that spiders differed in body size across groups by chance. Spiders were not significantly different in cephalothorax width across treatments (ANOVA, F2,71 = 0.39, p =.68), demonstrating that body size was not significantly different across treatments. Therefore, we used abdomen width alone rather than cephalothorax width to abdomen width ratio as an index for body condition (Jakob et al., 1996). After being maintained for 4 days, Pardosa exhibited significant differences in abdomen width between treatments (Table 1). Because spiders were not significantly different in cephalothorax width across treatments, this indicates a significant difference in body condition between treatments (Table 1). Tukey post-hoc comparison of means test indicated that Pardosa maintained in the Hogna cue treatment had significantly smaller abdomens than either conspecific or control treatments (Table 1).
Foraging, body condition, and reproductive costs of adult female Pardosa milvina placed in containers previously occupied by another adult female Pardosa, a single predatory adult Hogna, or no spider (control)
| . | Treatment . | . | . | ||
|---|---|---|---|---|---|
| . | Control . | Pardosa cues . | Hogna cues . | Test statistic . | p . |
| Means ± SEs are presented. See text for further details. | |||||
| aAbdomen change as a result of 4 days in the container without food. | |||||
| bFruit flies eaten within 1.5 h while in each treatment. | |||||
| cChange in abdomen width before and after exposure to fruit flies. | |||||
| d,eDifferent superscript letters next to each treatment mean indicate significant differences between groups based on an ANOVA and Tukey post-hoc comparison of means test. | |||||
| Mean abdomen widtha (mm) | 2.50d ± 0.05 | 2.43d ± 0.042 | 2.14e ± 0.049 | F2, 71 = 15.96 | .0001 |
| Mean egg sac weight (mg) | 9.21d ± 0.61 | 8.39d,e ± 0.54 | 6.62e ± 0.68 | F2, 53 = 7.75 | .0011 |
| Mean no. of eggs | 21.16d ± 1.53 | 20.91d ± 1.331 | 14.13e ± 2.522 | F2, 53 = 5.76 | .0055 |
| Mean no. of flies eatenb | 3.62 ± 0.368 | 1.3850 ± 0.417 | χ 2 = 26.81 | .0001 | |
| Mean change in abdomen widthc (mm) | 0.64 ± 0.088 | 0.32 ± 0.110 | T1,24 = 2.289 | .0312 | |
| . | Treatment . | . | . | ||
|---|---|---|---|---|---|
| . | Control . | Pardosa cues . | Hogna cues . | Test statistic . | p . |
| Means ± SEs are presented. See text for further details. | |||||
| aAbdomen change as a result of 4 days in the container without food. | |||||
| bFruit flies eaten within 1.5 h while in each treatment. | |||||
| cChange in abdomen width before and after exposure to fruit flies. | |||||
| d,eDifferent superscript letters next to each treatment mean indicate significant differences between groups based on an ANOVA and Tukey post-hoc comparison of means test. | |||||
| Mean abdomen widtha (mm) | 2.50d ± 0.05 | 2.43d ± 0.042 | 2.14e ± 0.049 | F2, 71 = 15.96 | .0001 |
| Mean egg sac weight (mg) | 9.21d ± 0.61 | 8.39d,e ± 0.54 | 6.62e ± 0.68 | F2, 53 = 7.75 | .0011 |
| Mean no. of eggs | 21.16d ± 1.53 | 20.91d ± 1.331 | 14.13e ± 2.522 | F2, 53 = 5.76 | .0055 |
| Mean no. of flies eatenb | 3.62 ± 0.368 | 1.3850 ± 0.417 | χ 2 = 26.81 | .0001 | |
| Mean change in abdomen widthc (mm) | 0.64 ± 0.088 | 0.32 ± 0.110 | T1,24 = 2.289 | .0312 | |
Foraging, body condition, and reproductive costs of adult female Pardosa milvina placed in containers previously occupied by another adult female Pardosa, a single predatory adult Hogna, or no spider (control)
| . | Treatment . | . | . | ||
|---|---|---|---|---|---|
| . | Control . | Pardosa cues . | Hogna cues . | Test statistic . | p . |
| Means ± SEs are presented. See text for further details. | |||||
| aAbdomen change as a result of 4 days in the container without food. | |||||
| bFruit flies eaten within 1.5 h while in each treatment. | |||||
| cChange in abdomen width before and after exposure to fruit flies. | |||||
| d,eDifferent superscript letters next to each treatment mean indicate significant differences between groups based on an ANOVA and Tukey post-hoc comparison of means test. | |||||
| Mean abdomen widtha (mm) | 2.50d ± 0.05 | 2.43d ± 0.042 | 2.14e ± 0.049 | F2, 71 = 15.96 | .0001 |
| Mean egg sac weight (mg) | 9.21d ± 0.61 | 8.39d,e ± 0.54 | 6.62e ± 0.68 | F2, 53 = 7.75 | .0011 |
| Mean no. of eggs | 21.16d ± 1.53 | 20.91d ± 1.331 | 14.13e ± 2.522 | F2, 53 = 5.76 | .0055 |
| Mean no. of flies eatenb | 3.62 ± 0.368 | 1.3850 ± 0.417 | χ 2 = 26.81 | .0001 | |
| Mean change in abdomen widthc (mm) | 0.64 ± 0.088 | 0.32 ± 0.110 | T1,24 = 2.289 | .0312 | |
| . | Treatment . | . | . | ||
|---|---|---|---|---|---|
| . | Control . | Pardosa cues . | Hogna cues . | Test statistic . | p . |
| Means ± SEs are presented. See text for further details. | |||||
| aAbdomen change as a result of 4 days in the container without food. | |||||
| bFruit flies eaten within 1.5 h while in each treatment. | |||||
| cChange in abdomen width before and after exposure to fruit flies. | |||||
| d,eDifferent superscript letters next to each treatment mean indicate significant differences between groups based on an ANOVA and Tukey post-hoc comparison of means test. | |||||
| Mean abdomen widtha (mm) | 2.50d ± 0.05 | 2.43d ± 0.042 | 2.14e ± 0.049 | F2, 71 = 15.96 | .0001 |
| Mean egg sac weight (mg) | 9.21d ± 0.61 | 8.39d,e ± 0.54 | 6.62e ± 0.68 | F2, 53 = 7.75 | .0011 |
| Mean no. of eggs | 21.16d ± 1.53 | 20.91d ± 1.331 | 14.13e ± 2.522 | F2, 53 = 5.76 | .0055 |
| Mean no. of flies eatenb | 3.62 ± 0.368 | 1.3850 ± 0.417 | χ 2 = 26.81 | .0001 | |
| Mean change in abdomen widthc (mm) | 0.64 ± 0.088 | 0.32 ± 0.110 | T1,24 = 2.289 | .0312 | |
There was no significant difference in the number of egg sacs produced across treatment groups (χ2 = 3.571, p =.167). Spiders maintained in the Hogna cue treatment produced 15 egg sacs compared to 18 and 21 for control and Pardosa treatments, respectively. There was also no significant difference in the median time to produce an egg sac across treatment groups (Mantel-Cox test for time to egg sac production, χ 2 = 1.264, df = 2, p =.5316; median = 12.00 ± 0.518 days for control treatment, 11.00 ± 0.414 days for Pardosa treatment, and 11.00 ± q1.861 days for the Hogna treatment). However, we did find significant differences in mean egg sac weight between treatments (Table 1), with spiders from the Hogna treatment producing significantly lighter egg sacs than spiders in the other two treatment groups (Table 1). Pardosa maintained in containers with Hogna cues also produced significantly fewer eggs per egg sac than individuals from either of the other treatments (Table 1).
Experiment 2: effects of perceived predation risk on feeding behavior
Methods
Experiment 1 demonstrated that Pardosa lost weight significantly faster in the presence of Hogna cues than conspecific cues or blank controls. However, we were uncertain if differences in egg sac mass and egg number were due to differences in the rate of weight loss alone or if Pardosa also captured less prey in containers with Hogna cues compared to the other treatments. Therefore, we examined the effect of Hogna cues versus Pardosa cues on the feeding behavior of Pardosa.
Stimulus preparation. We compared the number of prey consumed in 1.5 h by Pardosa in containers that had been previously occupied by either a Hogna or a conspecific (n = 13/treatment). Spiders were placed in opaque, colorless plastic food-service containers (5 cm high × 9 cm diam) with a 1 cm peat moss substratum. Hogna and Pardosa were fed crickets of an appropriate size once weekly before use in the experiment. The Pardosa were satiated, then fasted for 5 days before testing. To reduce variation in the production of Hogna and Pardosa excreta and silk, spiders to be used as stimuli for both treatments were sated with crickets 24 h before being placed in the containers. We introduced a single Hogna or Pardosa into the plastic container with a moistened peat moss substratum for 12 h. After 12 h we removed these spiders and placed a single test Pardosa into each container.
Measurement of feeding behavior and statistics. Five minutes after Pardosa were introduced, five vestigial-winged fruit flies (Drosophila melanogaster) were given to each spider. We then counted the number of flies consumed by each spider after 1.5 h. We compared the proportion of flies eaten between the two stimulus treatments (i.e., Hogna versus conspecific) using logistic regression with an indicator variable. This type of procedure is analogous to doing an ANOVA using the proper error distribution for data which consist of the number of successes/number of trials (Collet, 1991; Stokes et al., 1995). To verify that the number of prey killed was correlated with prey biomass consumed, we also tested for a change in the nutritional status of the test Pardosa by comparing body condition before and after the experiment. Because abdomen width is an indicator of recent foraging success (Jakob et al., 1996), we measured abdomen width of each spider before and after the experiment and calculated the change in width (width after — width before = change in abdomen width). We then compared the changes in abdomen width between treatment groups using a t test.
Results
Pardosa in containers that previously contained Hogna consumed fewer fruit flies than did spiders in containers that previously contained Pardosa (Table 1). Pardosa placed in containers with conspecific cues not only killed more fruit flies, but also consumed more prey biomass, as evidenced by an increase in abdomen width. The change in mean abdomen width was greater for spiders in containers previously occupied by Pardosa compared to abdomen width changes of spiders in containers previously occupied by Hogna (Table 1).
Experiment 3: survival value of predator chemical cue detection and climbing behavior
Methods
Because Pardosa behavior in the presence of Hogna cues is sufficient to induce weight loss, reduce feeding efficiency, reduce prey capture, and affect reproductive output, we were interested in the possible immediate survival benefits of such behavior. We tested the survival of Pardosa exposed to the risk of predation by Hogna with and without substratum-borne cues from Hogna and also measured survival of Pardosa that exhibited climbing behavior versus those spiders that did not. We collected and maintained 60 adult female Pardosa with egg sacs as described in experiment 1 and removed the egg sacs 24 h before testing. Pardosa were fed crickets until sated 12 h before to testing.
Stimulus preparation. Sixty Hogna were maintained in 12 cm diam × 8 cm high white plastic cups with clear lids (same containers used to house Pardosa in experiment 1). To standardize hunger levels, Hogna were sated with crickets, then fasted for 2 weeks before being used as experimental predators. We used two treatments for this experiment (n = 30/treatment): containers previously occupied by Hogna for 3 days and containers devoid of Hogna cues (control). Hogna helluo used as stimulus cues were fully satiated 24 h before introduction into the container, but were removed and replaced with a Hogna that had been sated then fasted for 2 weeks.
Measurement of Pardosa survival and statistics. After removal of the stimulus spider, but before adding the test Pardosa and predator, we increased heterogeneity by adding a small plastic vial wrapped in tape (4 cm long, 2.5 cm diam). Because the vial was added after the removal of the stimulus Hogna, it was devoid of predator silk or excreta. The vial was placed horizontally with its long axis close to the wall. The tape served to provide traction for the spider to climb on if it chose to do so. The space formed between the vial and wall was large enough to be occupied by Pardosa, but not Hogna. The opening of the vial was of a size that the predator could enter only with some difficulty. We placed a single adult, mated female Pardosa in each container that had an egg sac removed 24 h before the beginning of the experiment. The spider was allowed to acclimate for 1 h, after which we noted the position of the spider as being in the vial, on top of the vial, between the vial and wall on the ground, elsewhere on the ground, or on the wall exposed. Because spiders were only observed on the wall, on top of the vial, or elsewhere on the ground, we collapsed categories to wall, vial (on top), and ground. A single Hogna was introduced into the container at noon. Each container was monitored continuously for a 1 h period, every 5 min for the second and third hour, and once every 30 min thereafter for a 24-h period. Observations were suspended at 2200 h, and the room lighting was turned off and resumed at 0800 h when lighting was again turned on the next day. To compare the effectiveness of various Pardosa positions in the container as possible predator deterrents, we recorded the positions of spiders killed within the first 5 min of the experiment.
To analyze the effectiveness of being in a vertical position in deterring predation, we compared differences in Pardosa predation latency across peat moss treatments based on both the position of Pardosa and the presence of Hogna cues. We compared differences in the median time of predation by Hogna for each treatment using a Kaplan-Meier failure time analysis (log-rank test). Differences in space use in the container were tested using a chi-square test.
Results
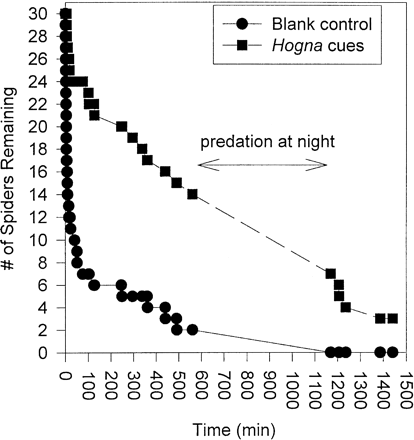
Pardosa milvina survived much longer in the presence of Hogna chemotactile cues than in containers lacking such cues. Based on a Kaplan-Meier failure-time analysis, there was a highly significant difference in the median time to predation between Hogna and control treatments (log-rank test, χ2 = 26.96, df = 1; p <.0001; Figure 2). Pardosa survived a median of only 8.83 ± 1.96 min in the container without substratum-borne cues from Hogna, but survived a median of 9.34 ± 0.709 h when Hogna cues were present.
Number of Pardosa remaining alive over time in the presence of Hogna helluo during a 3.5-h period. Spiders either had access (Hogna treatment) or did not have access (blank control) to the presence of Hogna chemical cues during the experiment.
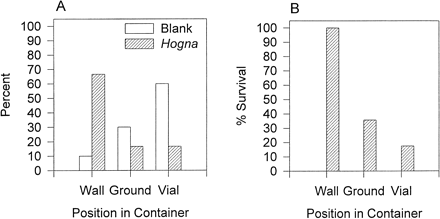
Pardosa showed a significant difference in their initial positions in the containers with or without predator chemotactile cues (χ2 = 21.86, p <.0009; Figure 3A). In containers without Hogna cues, most spiders were found resting on the top of the vial, with fewer on the ground, and the fewest number positioned vertically on the wall (Figure 3A). In the Hogna treatment, the majority of spiders were occupying the wall with equal numbers on the ground or vial top (Figure 3A). After the first 5 min of introducing the predator, there were significant differences in survival of spiders based on their position within the container (χ2 = 32.80, p <.0001, n = 23). All Pardosa that were vertically oriented on the walls survived, whereas only 4 out of 19 spiders on the vial and 6 out of 14 spiders on the ground survived (Figure 3B). Chemotactile cues from predators elicit climbing behavior, but vertical positions in the container are not favored by Pardosa when predators are not present (Figure 3A). The vertical orientation of Pardosa did significantly reduce predation by Hogna (Figure 3B).
(A) Differences in space use among Pardosa in the presence of Hogna cues or blank control containers. Percentage of Pardosa on the sides of the container (wall), ground, or on top of a vial. (B) Percentage of Pardosa that survived Hogna predation while occupying the wall, ground, or top of vial in the container.
Hogna were found to engage in nocturnal foraging because they preyed on Pardosa in nearly complete darkness between 2200 and 0800 h (Figure 2). During this time, nine Pardosa were captured and eaten in the absence of a light source. This indicates that Hogna probably located Pardosa through vibratory, tactile, and/or chemical cues.
DISCUSSION
Fitness-related costs due to chemically induced antipredator behavior have been commonly found within aquatic systems (Chivers and Smith, 1998), but far fewer analogous studies have been conducted in terrestrial systems. Further, although many shifts in behavior have been documented in the presence of either an actual predator or indirect cues associated with a predator, few studies document if such changes in behavior actually result in reduced predation when the predator is present (Persons et al., 2001; Venzon et al., 2000). Here we document the occurrence of behavioral changes by prey due to chemotactile cues from predators in a terrestrial arthropod. We also document that although there are significant fitness-related costs to this shift in space use, it is also an extremely effective antipredator behavior against the Hogna producing the cues.
Our results suggest that climbing behavior is both energetically costly and reduces prey capture efficiency. Spiders in experiment 1 were fed before initial introduction into each treatment and were measured before a second feeding. Measurements indicate that Pardosa were losing weight more quickly in containers that previously contained Hogna. The difference in weight loss could be attributed to physiological costs borne by the stress of being under constant perceived predation risk and therefore may be independent of whether spiders were exhibiting climbing behavior. However, the majority of spiders climbed the sides of the container in the Hogna treatment, unlike their behavior in the other treatment, which suggests a larger energetic cost associated with maintaining a position on the side of the container rather than the ground. Minimally, results indicate that the spider's preferred location is not on the side of the container. Wolf spiders are generally ground dwellers (Lowrie, 1968), and, unlike other ground-dwelling spiders such as gnaphosids, clubionids, and corinnids, wolf spiders lack scopular hairs on the most distal portion of the leg. Scopular hairs aid in adhering to smooth surfaces (Foelix, 1996). Even lycosid species such as Rabidosa rabida that typically are found in herbaceous vegetation seem to prefer horizontal or gently sloping surfaces on plants (Rovner and Knost, 1974). Pardosa lack scopular hairs at the tips of their tarsi and were observed to have some difficulty climbing the sides of the plastic containers.
The energetic costs of climbing behavior are compounded by the fact that the presence of predator cues either inhibits feeding behavior or reduces capture success. The feeding experiment indicates that spiders not only kill fewer prey, but also eat fewer prey when in the presence of chemotactile cues from a predator than in the presence of conspecific cues. There are a number of nonmutually exclusive reasons that Pardosa may exhibit reduced fruit fly predation in the containers with Hogna cues. Spiders may have had difficulty capturing prey because they were residing on the side of the container and therefore used their legs for maintaining their position rather than lunging at prey. Pardosa that do lunge at prey while on the side of the container are probably more likely to drop to the ground, which, as our data show, is a more dangerous place to be if a Hogna is present. Pardosa may have shown a decrease in activity in the container with Hogna cues. Previous studies indicate that Pardosa reduce activity levels in the presence of Hogna chemotactile cues and that such behavior has a survival benefit (Persons et al., 2001). Decreased movement would have a negative impact on prey capture.
Increased energetic costs and the decreased foraging efficiency that results from residing in the presence of Hogna cues may have both contributed to reduced reproductive output. Spiders in the presence of Hogna cues produced fewer (though not significantly fewer) egg sacs than spiders in either conspecific or control treatments. Pardosa produced significantly lighter egg sacs and fewer eggs when exposed to predator cues for 2 weeks. We found no evidence that predator cues affect the latency to produce an egg sac, suggesting that the presence of Hogna chemical cues is not likely to have a large impact on the phenology of Pardosa. However, the presence of large numbers of predators could have a significant impact on Pardosa populations even if actual predation seldom occurs.
Although the reproductive and foraging costs for climbing behavior are high, the survival benefit of climbing may also be high. Spiders that climbed the sides of the container had 100% survival compared to individuals on the ground (42%) or vial top (21%) (Figure 3B). Climbing behavior appeared to be mediated primarily, if not exclusively, by the presence of Hogna cues on the substrate. Although Pardosa could presumably detect Hogna in the container through tactile cues, visual cues, vibratory cues through the substratum, and/or possibly even airborne volatiles, these sensory channels combined were insufficient to elicit high levels of climbing behavior. This suggests that detection of substratum-borne predator cues by the prey is the primary means of predator detection and is extremely important for survival in a Hogna—Pardosa encounter.
It is likely that the field equivalent to climbing behavior may be either avoidance of the substrate or moving into the nearest available patch of vertical vegetation. Adult Pardosa will avoid substrates containing Hogna silk and excreta (Persons et al., 2001). However, we suggest that simple avoidance of the substrates containing predator cues does not fully explain observed climbing behavior. Pardosa did not climb on the vial top, which was both devoid of cues and horizontally oriented when Hogna cues were present on the peat moss. This indicates that Pardosa did not climb the walls and assume a vertical orientation only to avoid direct contact with Hogna cues, but rather that vertical orientation may deter prey capture by Hogna. Under natural conditions, it is unknown if Pardosa will exhibit climbing behavior on the nearest vegetation when encountering Hogna silk or excreta. Previous studies show that Pardosa may show other effective defensive behaviors in the presence of Hogna as well such as avoidance and decreased activity (Persons et al., 2001). During our collection of Hogna and Pardosa, we did observe that Pardosa could be found in large numbers in soybean plants and high in vegetation on some occasions, and strictly found on the ground at other times. The cause for this shift in space use is currently unknown, but this study suggests that predator avoidance cannot be ruled out as an explanation.
Our results are important in understanding predator—prey dynamics among these two species. This study confirms the results of other experiments that suggest that chemotactile cues alone induce antipredator behavior, not the predator itself (Persons et al., 2001). Also, Pardosa are capable of fine discrimination between predators that pose different levels of predation risk. Spiders show graded levels of activity depending on the diet of the Hogna (Persons et al., 2001) producing the cues. In light of our finding that defensive behaviors incur significant fitness costs, exhibition of graded responses to different levels of perceived risk may be a means of mitigating these costs. Other studies suggest that indirectly mediated predator—prey interactions are much more common in ground-dwelling spiders than previously realized. Punzo (1997) found that wolf spiders Schizocosa avida showed increased avoidance of substrates previously occupied by the scorpion Centruroides vittatus if they had been previously attacked by the scorpion than if they had never encountered it. Wolf spiders may use chemotactile cues to locate prey more efficiently as well. Persons and Uetz (1996) found that the wolf spider Schizocosa ocreata spends longer periods of time on substrates previously occupied by house crickets compared to controls. Punzo and Kukoyi (1996) found that the wolf spider Trochosa parthensus and the oxyopid Oxyopes salticus spend longer periods of time on substrates previously occupied by prey common to their natural habitat compared to cues from novel prey. Persons and Rypstra (2000) found that H. helluo prefer substrates previously occupied by their most recent prey and also show attraction to chemotactile cues from Pardosa when fed Pardosa.
These results clearly show that chemotactile detection of predators is critical for spiders to perform effective defensive behavior and that both the costs and benefits of climbing behavior are high. Spiders tended to remain on the sides of the containers for the entire time that Hogna cues were present, even when the actual predator was absent. This suggests that Pardosa may tend to overestimate predation risk and weigh the benefits of climbing behavior more heavily than the reproductive and feeding costs incurred by it. Selection pressure for Pardosa to respond to indirect predator cues is likely to be high. Future field studies will be necessary to verify the adaptive significance of chemotactilely mediated shifts in space use.
Thanks are extended to Sam Marshall for technical assistance on this article. We also thank Doug Meikle for the use of lab space for this study and M. Brueseke, E. Henley, and Dean Ferrera for their help in collecting and maintaining spiders in the lab. Special thanks to Jon Hlivko for data collection. This research was funded by National Science Foundation grant DEB 9527710 (to A.R. and S. Marshall), the Department of Zoology, Miami University Research Challenge Grant, and the Hamilton Campus of Miami University.
References
Brown GE, Chivers DP, Smith RJF,
Chivers DP, Smith RJF,
Crowl TA, Covich AP,
Dondale CD, Redner JH,
Edgar WD,
Gore RH,
Grostal P, Dicke M,
Hagstrum D,
Hoffmeister TS, Roitberg BD,
Holomuzki JR, Short TM,
Jakob EM, Marshall SD, Uetz GW,
Kats LB, Dill LM,
Kats LB, Petranka JW, Sih A,
Koskela E, Ylönen H,
Lima SL,
Lima SL, Dill LM,
Lowrie DC,
Malmqvist B,
Marshall SD, Rypstra AL,
Persons MH, Uetz GW,
Persons MH, Rypstra AL,
Persons MH, Walker SE, Rypstra AL, Marshall,
Punzo F,
Punzo F, Kukoyi O,
Repka S, Ketola M, Walls M,
Rovner JS, Knost SJ,
Spitze K,
Stokes M, Davis C, Koch G,
Sweitzer RA, Berger J,
Venzon M, Janssen A, Pallini A, Sabelis MW,
Wahle RA,
Ylönen H, Ronkainen H,