-
PDF
- Split View
-
Views
-
Cite
Cite
Lara H. Heflin, Beth E. Meyerowitz, Per Hall, Paul Lichtenstein, Boo Johansson, Nancy L. Pedersen, Margaret Gatz, Cancer as a Risk Factor for Long-Term Cognitive Deficits and Dementia, JNCI: Journal of the National Cancer Institute, Volume 97, Issue 11, 1 June 2005, Pages 854–856, https://doi.org/10.1093/jnci/dji137
Close - Share Icon Share
Abstract
Previous studies have shown that cancer survivors frequently experience short-term cognitive deficits, but it is unknown how long these deficits last or whether they worsen over time. Using a co-twin control design, the cognitive function of 702 cancer survivors aged 65 years and older was compared with that of their cancer-free twins. Dementia rates were also compared in 486 of the twin pairs discordant for cancer. Cancer survivors overall, as well as individuals who had survived cancer for 5 or more years before cognitive testing, were more likely than their co-twins to have cognitive dysfunction (odds ratio [OR] = 2.10, 95% confidence interval [CI] = 1.36 to 3.24; P <.001; and OR = 2.71, 95% CI = 1.47 to 5.01; P <.001, respectively). Cancer survivors were also twice as likely to be diagnosed with dementia as their co-twins, but this odds ratio did not reach statistical significance (OR = 2.0, 95% CI = 0.86 to 4.67; P = .10). These results suggest that cancer patients are at increased risk for long-term cognitive dysfunction compared with individuals who have never had cancer, even after controlling for the influence of genetic factors and rearing environment.
Progress in the treatment of cancer has led to extended survival for many patients, making the understanding of long-term and late effects essential. Research has documented that patients exhibit cognitive deficits persisting up to 5 years post-treatment ( 1 – 12 ) . Most studies have focused on short-term cognitive sequelae of treatments ( 1 – 14 ) ; however, evidence of neurologic changes in cancer survivors ( 15 ) suggests that some treatments may act as neurologic insults that decrease cognitive reserve or initiate pathologic processes of dementia ( 16 ) . Subtle cognitive deficits have been identified in long-term survivors treated with chemotherapy, relative to those treated locally, suggesting that treatment-related deficits may indeed persist ( 17 ) . It is possible that these cognitive deficits worsen and become most apparent in older age, when risk for cognitive dysfunction is increased.
The present study, approved by the University of Southern California and Karolinska Institutet internal review boards, investigated whether older cancer survivors exhibited long-term cognitive deficits and increased risk for dementia compared with co-twins without a cancer history. The twin design allowed the effects of cancer and its treatments to be considered while controlling for genetic and early environmental influences. Such control is important because estimates of heritability of cognitive functioning in older adults range from 32% to 79% ( 18 – 20 ) , and dementia heritability is estimated at 43% to 60% ( 21 , 22 ) .
Participants were twin pairs from the Swedish Twin Registry, a population-based registry of all twins residing in Sweden, who completed a telephone cognitive screening at age 65 years and older ( 23 ) . The validated cognitive screening assessed orientation, short-term memory, working memory, general knowledge, verbal recall, and verbal abstract reasoning ( 24 , 25 ) . If a twin performed poorly or was unable to be interviewed, an informant completed the Blessed Dementia Rating scale ( 26 ) . Using cognitive screening scores and informant reports, an algorithm was used to assign cognitive functioning scores: 0 = cognitively intact, 1 = minor errors, 2 = poor performance, and 3 = cognitive dysfunction sufficient to interfere with managing everyday life demands.
Linkage between the Swedish Twin Registry and the Swedish Cancer Registry yielded 702 twin pairs in which one twin had been diagnosed with malignant cancer, excluding brain cancer due to its direct effect on cognition. The average age of participants was 74.9 years (standard deviation = 6.3 years). Analysis of variance showed no statistically significant age differences between long-term (≥5 years post-diagnosis of most recent cancer at the time of the cognitive screening), short-term (1–5 years post-diagnosis), and immediate (<1 year post-diagnosis) cancer survivors at the time of cognitive screening ( P = .98). The mean time between cancer diagnosis and cognitive screening was 14.06 years for long-term survivors, 2.98 years for short-term survivors, and 0.53 years for immediate survivors. Table 1 shows time from diagnosis by cancer site.
Number of patients and time span (in years) between cancer diagnosis and cognitive functioning assessment for the 14 most common cancers in the sample and for all sites combined *
| Cancer . | N . | Mean (SD) . | Range . |
|---|---|---|---|
| Breast | 140 | 9.21 (8.39) | 0.04–40.26 |
| Prostate | 115 | 4.63 (4.47) | 0.05–23.53 |
| Colorectal | 72 | 7.85 (7.26) | 0.07–35.91 |
| Skin | 44 | 7.56 (6.94) | 0.08–23.91 |
| Bladder | 44 | 7.54 (7.11) | 0.03–34.33 |
| Corpus uteri | 41 | 13.39 (8.95) | 0.33–37.28 |
| Melanoma | 34 | 14.25 (10.00) | 0.82–33.43 |
| Endocrine gland | 25 | 9.69 (8.41) | 0.67–40.36 |
| Ovary | 22 | 14.44 (10.01) | 2.04–37.59 |
| Cervical | 18 | 22.46 (14.14) | 0.17–40.75 |
| Non-Hodgkin's | 18 | 6.17 (4.50) | 0.24–15.18 |
| Kidney | 14 | 9.90 (9.84) | 0.42–37.59 |
| Leukemia | 14 | 7.67 (6.37) | 0.96–22.63 |
| Thyroid | 14 | 16.65 (11.31) | 3.89–40.39 |
| All sites | 702 | 9.11 (8.72) | 0.03–40.75 |
| Cancer . | N . | Mean (SD) . | Range . |
|---|---|---|---|
| Breast | 140 | 9.21 (8.39) | 0.04–40.26 |
| Prostate | 115 | 4.63 (4.47) | 0.05–23.53 |
| Colorectal | 72 | 7.85 (7.26) | 0.07–35.91 |
| Skin | 44 | 7.56 (6.94) | 0.08–23.91 |
| Bladder | 44 | 7.54 (7.11) | 0.03–34.33 |
| Corpus uteri | 41 | 13.39 (8.95) | 0.33–37.28 |
| Melanoma | 34 | 14.25 (10.00) | 0.82–33.43 |
| Endocrine gland | 25 | 9.69 (8.41) | 0.67–40.36 |
| Ovary | 22 | 14.44 (10.01) | 2.04–37.59 |
| Cervical | 18 | 22.46 (14.14) | 0.17–40.75 |
| Non-Hodgkin's | 18 | 6.17 (4.50) | 0.24–15.18 |
| Kidney | 14 | 9.90 (9.84) | 0.42–37.59 |
| Leukemia | 14 | 7.67 (6.37) | 0.96–22.63 |
| Thyroid | 14 | 16.65 (11.31) | 3.89–40.39 |
| All sites | 702 | 9.11 (8.72) | 0.03–40.75 |
Patients aged 65 years and older who were identified as having cancer and as having a twin without cancer from linkage between the Swedish Twin Registry and the Swedish Cancer Registry. Patients with brain cancer were excluded due to the direct effect on cognition. SD = standard deviation.
Number of patients and time span (in years) between cancer diagnosis and cognitive functioning assessment for the 14 most common cancers in the sample and for all sites combined *
| Cancer . | N . | Mean (SD) . | Range . |
|---|---|---|---|
| Breast | 140 | 9.21 (8.39) | 0.04–40.26 |
| Prostate | 115 | 4.63 (4.47) | 0.05–23.53 |
| Colorectal | 72 | 7.85 (7.26) | 0.07–35.91 |
| Skin | 44 | 7.56 (6.94) | 0.08–23.91 |
| Bladder | 44 | 7.54 (7.11) | 0.03–34.33 |
| Corpus uteri | 41 | 13.39 (8.95) | 0.33–37.28 |
| Melanoma | 34 | 14.25 (10.00) | 0.82–33.43 |
| Endocrine gland | 25 | 9.69 (8.41) | 0.67–40.36 |
| Ovary | 22 | 14.44 (10.01) | 2.04–37.59 |
| Cervical | 18 | 22.46 (14.14) | 0.17–40.75 |
| Non-Hodgkin's | 18 | 6.17 (4.50) | 0.24–15.18 |
| Kidney | 14 | 9.90 (9.84) | 0.42–37.59 |
| Leukemia | 14 | 7.67 (6.37) | 0.96–22.63 |
| Thyroid | 14 | 16.65 (11.31) | 3.89–40.39 |
| All sites | 702 | 9.11 (8.72) | 0.03–40.75 |
| Cancer . | N . | Mean (SD) . | Range . |
|---|---|---|---|
| Breast | 140 | 9.21 (8.39) | 0.04–40.26 |
| Prostate | 115 | 4.63 (4.47) | 0.05–23.53 |
| Colorectal | 72 | 7.85 (7.26) | 0.07–35.91 |
| Skin | 44 | 7.56 (6.94) | 0.08–23.91 |
| Bladder | 44 | 7.54 (7.11) | 0.03–34.33 |
| Corpus uteri | 41 | 13.39 (8.95) | 0.33–37.28 |
| Melanoma | 34 | 14.25 (10.00) | 0.82–33.43 |
| Endocrine gland | 25 | 9.69 (8.41) | 0.67–40.36 |
| Ovary | 22 | 14.44 (10.01) | 2.04–37.59 |
| Cervical | 18 | 22.46 (14.14) | 0.17–40.75 |
| Non-Hodgkin's | 18 | 6.17 (4.50) | 0.24–15.18 |
| Kidney | 14 | 9.90 (9.84) | 0.42–37.59 |
| Leukemia | 14 | 7.67 (6.37) | 0.96–22.63 |
| Thyroid | 14 | 16.65 (11.31) | 3.89–40.39 |
| All sites | 702 | 9.11 (8.72) | 0.03–40.75 |
Patients aged 65 years and older who were identified as having cancer and as having a twin without cancer from linkage between the Swedish Twin Registry and the Swedish Cancer Registry. Patients with brain cancer were excluded due to the direct effect on cognition. SD = standard deviation.
Individuals obtaining a cognitive score of 3 upon screening, and their co-twins, received complete dementia work-ups. A neurologist and psychologist diagnosed dementia using Diagnostic and Statistical Manual-IV criteria ( 27 ) and classified non-demented individuals as intact or having questionable dementia. After ascertaining that cancer was diagnosed before dementia onset and excluding pairs containing individuals with questionable dementia, a total of 486 twin pairs discordant for cancer were available for the dementia analysis.
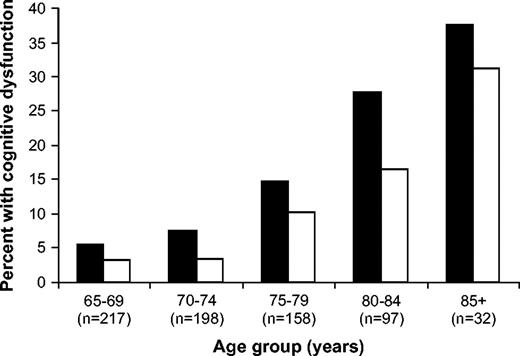
The prevalence of cognitive dysfunction by age of twins with and without cancer diagnoses is shown ( Fig. 1 ). Overall, 14.5% of cancer-surviving twins had cognitive dysfunction (i.e., scoring 3), compared with 8.7% of their cancer-free twins. Two-sided McNemar's chi-square analyses and odds ratios (ORs) were used to determine whether cancer was a statistically significant risk factor for cognitive difficulties and dementia. Cancer history was statistically significantly associated with cognitive dysfunction overall and in long-term cancer survivors compared with their cancer-free co-twins (OR = 2.10, 95% confidence interval [CI] = 1.36 to 3.24; P <.001; and OR = 2.71, 95% CI = 1.47 to 5.01; P <.001, respectively, Table 2 ). A statistically significant difference between long-term cancer survivors and their co-twins was maintained when individuals with poor performance were grouped with those found to have more serious dysfunction (OR = 1.95, 95% CI = 1.33 to 2.85; P <.001). Short-term survivors did not have increased risk, however. These findings are consistent with the conceptualization that cancer or its treatments decrease cognitive reserve, making survivors more susceptible over time to reaching the threshold for measurable cognitive deficits.
Percentage of cancer-surviving and cancer-free twins classified by cognitive screening as having cognitive dysfunction. Patients and co-twins are grouped in 5-year age bands to illustrate the comparative increase in rate of cognitive dysfunction by age in cancer survivors relative to co-twins. The percentage of cancer survivors in each age band classified as having cognitive dysfunction ( solid bars ) and the percentage of cancer-free co-twins in each age band classified as having cognitive dysfunction ( open bars ) are shown.
Association of cognitive functioning scale scores with cancer diagnosis in twin pairs discordant for cancer, collapsed across cancer sites, stratified by time since cancer diagnosis *
| . | Cognitive functioning scale dichotomization 3 versus 0, 1, and 2 . | . | |
|---|---|---|---|
| Time stratification . | OR (95% CI) . | P . | |
| All pairs (N = 702) | 2.10 (1.36 to 3.24) | <.001 | |
| Long-term pairs (n = 405) † | 2.71 (1.47 to 5.01) | <.001 | |
| Short-term pairs (n = 220) | 1.23 (0.59 to 2.56) | .578 | |
| Immediate pairs (n = 77) | 3.00 (0.81 to 11.09) | .083 | |
| . | Cognitive functioning scale dichotomization 3 versus 0, 1, and 2 . | . | |
|---|---|---|---|
| Time stratification . | OR (95% CI) . | P . | |
| All pairs (N = 702) | 2.10 (1.36 to 3.24) | <.001 | |
| Long-term pairs (n = 405) † | 2.71 (1.47 to 5.01) | <.001 | |
| Short-term pairs (n = 220) | 1.23 (0.59 to 2.56) | .578 | |
| Immediate pairs (n = 77) | 3.00 (0.81 to 11.09) | .083 | |
0 = cognitively intact, 1 = minor errors, 2 = poor performance, 3 = cognitive dysfunction. OR = odds ratio; CI = confidence interval; P values (two-sided) were calculated using McNemar's chi-square analysis.
Long-term cancer survivors are ≥ 5 years post-diagnosis of most recent cancer at the time of the cognitive screening; short-term survivors are 1–5 years post-diagnosis; immediate survivors are < 1 year post-diagnosis.
Association of cognitive functioning scale scores with cancer diagnosis in twin pairs discordant for cancer, collapsed across cancer sites, stratified by time since cancer diagnosis *
| . | Cognitive functioning scale dichotomization 3 versus 0, 1, and 2 . | . | |
|---|---|---|---|
| Time stratification . | OR (95% CI) . | P . | |
| All pairs (N = 702) | 2.10 (1.36 to 3.24) | <.001 | |
| Long-term pairs (n = 405) † | 2.71 (1.47 to 5.01) | <.001 | |
| Short-term pairs (n = 220) | 1.23 (0.59 to 2.56) | .578 | |
| Immediate pairs (n = 77) | 3.00 (0.81 to 11.09) | .083 | |
| . | Cognitive functioning scale dichotomization 3 versus 0, 1, and 2 . | . | |
|---|---|---|---|
| Time stratification . | OR (95% CI) . | P . | |
| All pairs (N = 702) | 2.10 (1.36 to 3.24) | <.001 | |
| Long-term pairs (n = 405) † | 2.71 (1.47 to 5.01) | <.001 | |
| Short-term pairs (n = 220) | 1.23 (0.59 to 2.56) | .578 | |
| Immediate pairs (n = 77) | 3.00 (0.81 to 11.09) | .083 | |
0 = cognitively intact, 1 = minor errors, 2 = poor performance, 3 = cognitive dysfunction. OR = odds ratio; CI = confidence interval; P values (two-sided) were calculated using McNemar's chi-square analysis.
Long-term cancer survivors are ≥ 5 years post-diagnosis of most recent cancer at the time of the cognitive screening; short-term survivors are 1–5 years post-diagnosis; immediate survivors are < 1 year post-diagnosis.
Cancer was not statistically significantly associated with dementia diagnosis (OR = 2.0, 95% CI = 0.86 to 4.67). Nevertheless, the point estimate suggests that the risk was twofold, which may represent a clinically meaningful difference. Power analysis using Dupont's method ( 28 ) indicated that the power to reject the null hypothesis was only .05.
Two limitations of this study should be noted. First, we did not have access to information on cancer treatments, preventing comparison of cognitive functioning among cancer survivors who received different treatments. Thus, the cognitive risk reported here may represent an overestimate for some cancer survivors and an underestimate for others. Second, because both cancer and cognitive decline could be influenced by many of the same factors, it is possible that other risk factors—such as alcohol consumption, sedentary lifestyle, or low socioeconomic status—influenced the development of both cancer and cognitive dysfunction. Twins do tend to be more similar than do unrelated individuals on many of these background factors, however ( 29 ) .
This study has several strengths. We studied a large population-based cohort, including patients whose cancers had been diagnosed many years before cognitive assessment, unlike most studies that were smaller and assessed patients only up to 5 years post-treatment. Because an entire cohort of twins was contacted for participation in the cognitive screening, this study avoids many of the selection biases inherent in studies of hospital patients. Our outcome measures included a complete clinical dementia work-up. Furthermore, the twin design provided a control group that matched for at least 50% of genetic factors and for early environmental influences associated with growing up in the same family.
The nearly exclusive focus of prior studies on short-term cognitive function has left cancer patients and their medical teams uncertain whether cognitive deficits would persist or eventually abate. Our data suggest that cancer and its treatments may lower survivors' cognitive reserve and thus increase their long-term risk of cognitive dysfunction and dementia, a serious clinical concern for physicians treating cancer survivors. Further research should identify mechanisms that mediate the relationship between cancer and cognitive dysfunction and explore whether specific treatments are associated with long-term cognitive effects. This knowledge will help health care providers and patients make informed decisions about treatments.
This study was funded by National Institutes of Health Grant No. R01 AG 08724 (MG), a Zenith Award from the Alzheimer's Association (ZEN-02–3895; MG), and a Multidisciplinary Research Training Grant in Gerontology (T32 AG 00037) to LHH.
References
Silberfarb PM, Philibert D, Levine PM. Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients.
Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer.
Ahles TA, Tope DM, Furstenberg C, Hann D, Mills L. Psychologic and neuropsychologic impact of autologous bone marrow transplantation.
Ahles TA, Silberfarb PM, Herndon J II, Maurer LH, Kornblith AB, Aisner J, et al. Psychologic and neuropsychologic functioning of patients with limited small-cell lung cancer treated with chemotherapy and radiation therapy with or without warfarin: a study by the Cancer and Leukemia Group B.
van Dam FSAM, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, et al. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy.
Schagen SB, van Dam FSAM, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma.
Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy.
Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FSAM. Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function.
Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature.
Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer.
Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. ‘Chemobrain’ in breast carcinoma?: a prologue.
Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial.
Kaasa S, Olsnes BT, Mastekaasa A. Neuropsychological evaluation of patients with inoperable non–small-cell lung cancer treated with combination chemotherapy or radiotherapy.
Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn D, Rodenhuis S, et al. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients.
Johnson BE, Patronas N, Hayes W, Grayson J, Becker B, Gnepp D, et al. Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: further follow-up of 6- to 13-year survivors.
Stern Y. What is cognitive reserve? Theory and research application of the reserve concept.
Ahles TA, Saykin AJ, Furstenberg CT, Cole B, Matt LA, Skalla K, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma.
Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span.
McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, et al. Substantial genetic influence on cognitive abilities in twins 80 or more years old.
Swan GE, Carmelli D. Evidence for genetic mediation of executive control: a study of aging male twins.
Bergem ALM, Engedal K, Kringlen E. The role of heredity in late-onset Alzheimer disease and vascular dementia: a twin study.
Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins.
Gatz M, Fratiglioni L, Johansson B, Berg S, Mortimer JA, Reynolds CA, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study.
Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases.
Gatz M, Reynolds C, John R, Johansson B, Mortimer JA, Pedersen NL. Telephone screening to identify potential dementia cases in a population-based sample of older adults.
Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): American Psychiatric Association,
Dupont WD. Power calculations for matched case–control studies.