-
PDF
- Split View
-
Views
-
Cite
Cite
Matthias Alexander Hellmann, Thomas Michael Gerhardt, Christian Rabe, Susanne Haas, Tilman Sauerbruch, Rainer Peter Woitas, Goodpasture's syndrome with massive pulmonary haemorrhage in the absence of circulating anti-GBM antibodies?, Nephrology Dialysis Transplantation, Volume 21, Issue 2, February 2006, Pages 526–529, https://doi.org/10.1093/ndt/gfi279
Close - Share Icon Share
Introduction
Clinical manifestation of Goodpasture's syndrome is usually characterized by the combination of glomerulonephritis and diffuse alveolar haemorrhage accompanied by anti-glomerular basement membrane (GBM) antibodies in serum or tissue. In >90% of patients with Goodpasture's syndrome, anti-GBM antibodies can be detected in the serum [1]. Only 2% of the patients develop diffuse alveolar haemorrhage without clinically evident renal disease. Typically, young male smokers are affected by Goodpasture's syndrome [2,3]. Here, we report the case of a 27-year-old Polish man who developed massive pulmonary haemorrhage without any clinical signs of renal dysfunction. Moreover, no circulating anti-GBM antibodies were detectable in his serum using common laboratory methods.
Case
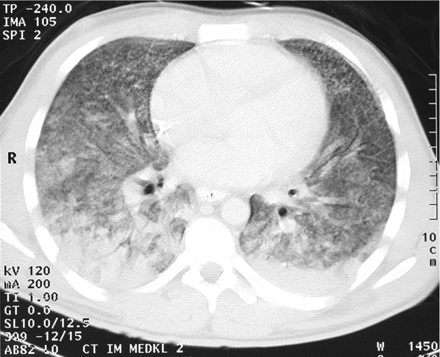
A 27-year-old male patient who had a history of heavy smoking (15 pack-years) was referred to a local hospital after 1 week of dyspnoea and haemoptysis. He did not use any drugs nor any prescribed medication, in particular he did not take antithyroid drugs and antiplatelet agents. He was a migrant worker from Poland, who worked in a nursery and was not in contact with any toxic liquids or gases. Severe respiratory failure required intubation and mechanical ventilation. With suspected acute respiratory distress syndrome as a result of sepsis, the patient was then transferred to our centre. Upon admission, the patient's haemoglobin was 5.1 g/dl. After blood transfusion, there was no further decrease in haemoglobin. The patient had no history of renal or pulmonary disease. A thoracic computed tomography (CT) scan showed distinct bilateral infiltrates sparing only the right upper lobe segment of the lung (Figure 1). On bronchoscopy, diffuse bleeding was detectable.
The CT scan of the upper lobes shows diffuse, bilateral, ground-glass opacities consistent with alveolar haemorrhage.
Bacterial cultures obtained during bronchoscopy and virus serology were repeatedly negative. In particular, mycobacteria, toxoplasmosa, mycoplasma, legionella, influenza, parainfluenza and Pneumocystis jiroveci were excluded in repeated investigations. Immunological laboratory tests repeatedly showed negative titres for anti-GBM (QUANTA LITE™ GBM Inova Diagnostics, San Diego, CA), indirect immunofluorescence (IFT) for antinuclear cytoplasmic antibody (ANCA; Inova Diagnostics, San Diego, CA) as well as antinuclear antibody (ANA; IFT, Biorad, München, Germany), antithyroid antibodies (enzyme immunoassay, Biorad, München, Germany), cryoglobulins and antistreptolysin antibodies. All tests were repeated three times within a period of 8 weeks. Importantly, renal function seemed to be completely unaffected. Serum creatinine was normal (0.89 mg/dl) and urine sediment not active. However, calculated glomerular filtration rate (GFR; MDRD4) was 43 ml/min/1.73 m2 indicating renal impairment. The urine analysis repeatedly showed no blood or protein. Quantitative urine protein analysis was negative for protein (cut-off <116 mg/l).
In view of a suspected immunological disease, we decided to start a 6 day course of intravenous methylprednisolone (500 mg/day) that led to slight improvement. The patient relapsed with pulmonary haemorrhage when steroid medication was reduced to 100 mg/day. At this time, a lung biopsy was obtained thoracoscopically. Besides diffuse intra-alveolar haemorrhage (Figure 2A and B), histology showed faint but linear deposition of IgG antibodies along the alveolar basement membrane and the alveolar capillary walls (Figure 2C and D). There were no signs of necrotizing vasculitis.
(A) Histological features of pulmonary haemosiderosis with diffuse patchy intra-alveolar haemorrhage. Prussian blue stain reveals haemosiderosis both within the alveolar septa and in numerous haemosiderin-laden macrophages within the pulmonary alveoli (Prussian blue staining, 1:40). (B) Focal fibrous thickening of the alveolar septa as well as nodular mesenchymal proliferates can be seen. There is prominent hyperplasia of the alveolar lining cells. (haematoxylin–eosin, 1:100). (C and D) Focal linear deposition of IgG antibodies is seen along the alveolar basement membrane and alveolar capillary walls as indicated by arrows [immunohistological staining for IgG, (C) 1:100, (D) 1:200].
The immunohistological findings were not definitively specific, but were, however, compatible with Goodpasture's syndrome. One may speculate that Goodpasture's syndrome was anticipated, diagnosed and treated at a very early time point of the disease process so that only focal linear deposition of the antibodies could take place in the alveolar basement membrane. In view of this histology, we consequently started plasmapheresis treatment (six sessions against 4000 ml of fresh frozen plasma within a period of 14 days). Additionally, intravenous cyclophosphamide (750 mg) pulses were given every 18 days starting on day 10 after admission. In total, the patient received three cyclophosphamide courses as an in-patient.
During treatment with steroids, cyclophosphamide and plasmapheresis, pulmonary haemorrhage decreased. Subsequently the patient improved, weaning was successful and no further artificial respiration was necessary. Mechanical ventilation was required until the 20th hospital day. In the following weeks, his condition ameliorated and a 99mTc-DTPA clearance to determine GFR by means of a gold standard procedure showed a considerably reduced GFR of 55.7 ml/min/1.73 m2.
Discussion
Here we describe the case of a 27-year-old male patient who developed a GBM antibody-negative Goodpasture's syndrome with massive pulmonary haemorrhage without evident renal failure although calculated GFR was considerably reduced. More than 90% of patients with Goodpasture's syndrome have circulating anti-GBM antibodies in the serum [1]. Circulating antibodies against the NC1 domain of the α3 chain of type IV collagen react with the basement membrane of pulmonary alveoli and the GBM [1,4]. The disease occurs twice as often in men than in women. Usually, patients at the age of 20–30 are affected [4–6]. However, a second peak of incidence is well known to occur in the fifth and sixth decade. The aetiology of anti-GBM antibody production is not completely understood, but environmental factors such as cigarette smoking, drug use and virus infections have been considered as causative agents [4,7–10]. Smoking is not involved directly in the production of antibodies but may precipitate haemorrhage in the presence of autoantibodies.
Our patient showed a severe pulmonary haemorrhage with a decrease in haemoglobin to 5.1 g/dl but normal creatinine and urine sediment. Further, no anti-GBM antibodies were detectable in the serum. For these cases, some authors propose antibody detection using a highly sensitive biosensor system [11]. After exclusion of other differential diagnoses such as drugs, toxins, viral and bacterial infections, or exacerbation of tuberculosis, an immunological disease was likely. To confirm diagnosis in this very critical situation, it was necessary to perform a lung biopsy. In patients in whom the aetiology of pulmonary haemorrhage is still in question after thorough clinical evaluation, including bronchoscopy, or in whom the underlying cause of pulmonary haemorrhage is still unclear after appropriate serological testing, a surgical biopsy should be performed [1,12,13]. Some authors postulate that surgical lung biopsies are useful to confirm pulmonary haemorrhage but are unable to identify the underlying course [1,12,13]. They prefer renal biopsy to confirm a diagnosis. In contrast to this concept, we took a surgical lung biopsy, keeping in mind the virtually unaffected renal function.
To authors who prefer renal biopsy, we have to concede that lung biopsy of this patient did not show a bright and perfect linear staining of the alveolar basement membrane. In contrast, although faint, we found focal linear IgG depositions that were compatible with Goodpasture's syndrome. It is conceivable that the patient presented at a very early stage of the disease process so that especially after initial methylprednisolone therapy only focal linear deposition of the antibodies could take place in the alveolar basement membrane. This also would be in line with the negative anti-GBM antibody results. In this context, it should be considered that these anti-GBM antibodies may be of high affinity and therefore exist solely in a tissue-bound form and hence are not detectable in the circulation. Nevertheless, all findings together with the patient's clinical presentation provided strong evidence to start an aggressive treatment regimen comprising corticosteroids, cyclophosphamide and plasmapheresis [1,14,15].
Since Goodpasture's syndrome in the absence of anti-GBM antibodies is a rare differential diagnosis of pulmonary haemorrhage that may be very intricate to prove, a note of caution is mandatory before Goodpasture's syndrome may be diagnosed and aggressive treatment is initiated. Despite the suggestive context of all clinical features and the full remission under typical immunosuppressive treatment, the truth may still be elusive without decisive histology.
Conflict of interest statement. None declared.
References
Leatherman JW, Davies SF, Hoidal JR. Alveolar hemorrhage syndromes: diffuse microvascular lung hemorrhage in immune and idiopathic disorders.
Garcia-Rostan y Perez GM, Garcia Bragado F, Puras Gil AM. Pulmonary hemorrhage and antiglomerular basement membrane antibody-mediated glomerulonephritis after exposure to smoked cocaine (crack): a case report and review of the literature.
Colby TV, Fukuoka J, Ewaskow SP, Helmers R, Leslie KO. Pathologic approach to pulmonary hemorrhage.
Escolar Castellon JD, Roche PA, Escolar Castellon A, Minana Amada C. The modifications produced in allergic alveolitis and in Goodpasture's syndrome due to exposure to cigarette smoke.
Levy JB, Turner AN, Rees AJ, Pusey CD. Long-term outcome of anti-glomerular basement membrane antibody disease treated with plasma exchange and immunosuppression.
Savage CO, Pusey CD, Bowman C, Rees AJ, Lockwood CM. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980–4.
Wilson CB and Dixon FJ, Anti-glomerular basement membrane antibody-induced glomerulonephritis.
Fort J, Espinel E, Rogriquez JA, Curull V, Madrenas J, Piera L. Partial recovery of renal function in an oligoanuric patient affected with Goodpasture's syndrome after treatment with steroids, immunosuppressives and plasmapheresis.
Salama AD, Dougan T, Levy JB et al. Goodpasture's disease in the absence of circulating anti-glomerular basement membrane antibodies as detected by standard techniques.
Tsai CC, Kissane JM, Germuth FG Jr. Paramyxovirus- like structures in Goodpasture's syndrome.
Travis WD, Colby TV, Lombard C, Carpenter HA. A clinicopathologic study of 34 cases of diffuse pulmonary hemorrhage with lung biopsy confirmation.
Kim YO, Choi JY, Park JI et al. A case of Goodpasture's syndrome with massive pulmonary hemorrhage.
Author notes
1Medizinische Klinik und Poliklinik I and 2Zentrum für Pathologie, Universitätsklinikum Bonn, Germany



![(A) Histological features of pulmonary haemosiderosis with diffuse patchy intra-alveolar haemorrhage. Prussian blue stain reveals haemosiderosis both within the alveolar septa and in numerous haemosiderin-laden macrophages within the pulmonary alveoli (Prussian blue staining, 1:40). (B) Focal fibrous thickening of the alveolar septa as well as nodular mesenchymal proliferates can be seen. There is prominent hyperplasia of the alveolar lining cells. (haematoxylin–eosin, 1:100). (C and D) Focal linear deposition of IgG antibodies is seen along the alveolar basement membrane and alveolar capillary walls as indicated by arrows [immunohistological staining for IgG, (C) 1:100, (D) 1:200].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ndt/21/2/10.1093/ndt/gfi279/2/m_gfi279f2.gif?Expires=1716342063&Signature=Y~t9jsX~q6nPRMixdeMTA2NXYXwoRcb7QkQccs3m~9w7ERZSn9Z85gjNbtbKeuU8xetW32L6ZxbTS-Db9oD0FH6Ni5OZ2pJLt62d2GxrV1IMek2htTlnsnEXs5vl8MQ-tD70he~~m4lXLpPaKpl9NLMMNfxTdOHNIBNQ~cIbnZIssIcqmkRPkwRkdLHeRq82qP9ryVCvqOW6QNSaPNOKAxjZSGa50wTkypIFQdmX4H-S9Lx0wh3wApyJ-HLTxSzixfGwgcXpwTioYKI2hXTSt9yeRfeNHlfJgKmOLNn5AWT-lgYzJJhAqEaDq7U2A1HaSb8YGW9XzVHtp92YTtVRNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


Comments