-
PDF
- Split View
-
Views
-
Cite
Cite
GENNADIY NOVITSKIY, RAJANI RAVI, JAMES J. POTTER, LYNDA RENNIE-TANKERSLEY, LAN WANG, ESTEBAN MEZEY, EFFECTS OF ACETALDEHYDE AND TNFα ON THE INHIBITORY KAPPA B- α PROTEIN AND NUCLEAR FACTOR KAPPA B ACTIVATION IN HEPATIC STELLATE CELLS, Alcohol and Alcoholism, Volume 40, Issue 2, March/April 2005, Pages 96–101, https://doi.org/10.1093/alcalc/agh116
Close - Share Icon Share
Abstract
Aims: Increased plasma tumour necrosis α (TNFα) and elevated monocyte nuclear factor kappa B (NF-κB) are associated with liver injury and inflammation in models of alcoholic liver disease and are found to be elevated in monocytes of patients with alcoholic hepatitis. Acetaldehyde enhances, whereas TNFα inhibits, transcription of the type I collagen promoters and type I collagen production. NF-κB, an inhibitor of the type I collagen promoters, is increased by both acetaldehyde and TNFα. This study determined the effects of acetaldehyde in comparison to the effects of TNFα on inhibitory kappa B-α (IκB-α) protein and NF-κB activation in hepatic stellate cells. Methods: Activated rat hepatic stellate cells in culture were exposed to acetaldehyde or TNFα for short periods of time, following which the cells were harvested for the determination of IκB-α protein, IκB-α kinase activity and nuclear NF-κB. Results: Acetaldehyde increased IκB-α kinase activity and decreased IκB-α after 10 min of exposure, with recovery towards control levels at 20 min. In contrast, TNFα resulted in higher IκB-α kinase activity at 20 min than at 10 min, and similar low IκB-α at 10 and 20 min. Both acetaldehyde and TNFα enhanced nuclear NF-κB (p65), but acetaldehyde alone also increased NF-κB (p50). Conclusions: TNFα and acetaldehyde independently activate NF-κB by rapid enhancement of IκB-α kinase activity and degradation of IkB-α protein. Increased TNFα is the principal mechanism for the elevation of NF-κB in severe alcoholic hepatitis. The elevation of NF-κB due to TNFα enhance liver injury, but inhibit fibrogenesis. In contrast, the effect of acetaldehyde in activating NF-κB is associated with increases in both liver injury and fibrogenesis, indicating that the effects of acetaldehyde on fibrogenesis are mediated by cytokines and by trans-acting factors other than NF-κB.
(Received 27 July 2004; first review notified 16 September 2004; in revised form 18 October 2004; accepted 19 October 2004)
INTRODUCTION
Increased plasma tumour necrosis factor α (TNFα) correlates with the severity and mortality of alcoholic hepatitis (Bird et al., 1990; Felver et al., 1990). Activation of the transcription factor, the nuclear factor kappa B (NF-κB), is associated with liver injury and inflammation in models of alcoholic liver disease and is found to be elevated in monocytes of patients with alcoholic hepatitis (Hill et al., 2000; Mezey et al., 2004), in association with increases in TNFα mRNA (Hill et al., 2000). TNFα activates NF-κB and in turn, NF-κB increases the production of various inflammatory cytokines such as TNFα (Ghosh et al., 1998). Endotoxin plays a role in alcoholic liver disease by activation of NF-κB (Uesugi et al., 2001) and by enhancement of alcohol dehydrogenase (Potter et al., 2003), resulting in increased formation of acetaldehyde. Both NF-κB and acetaldehyde are essential factors in the formation of oxygen radicals which are a principal mechanism for alcohol-induced inflammation and fibrosis.
Hepatic stellate cells are the source of collagen and other extracellular matrix proteins that accumulate during hepatic fibrosis. Acetaldehyde, the product of alcohol oxidation, enhances α1(I) and α2(I) gene transcription and type I collagen production by hepatic stellate cells (Casini et al., 1991; Anania et al., 1996). TNFα increases hepatic inflammation, but inhibits α1(I) and α2(I) gene transcription by stellate cells (Hernandez et al., 2000; Novitskiy et al., 2004). NF-κB consists principally of homo- and heterodimers of p50 and p65 protein units, which in their inactive state are present in the cytoplasm, bound to the inhibitors of NF-κB activity (IκB). Phosphorylation of IκB by kinases results in the degradation of IκB with release of NF-κB, which translocates to the nucleus where it is active in regulation of transcription gene.
Acetaldehyde (Roman et al., 2000; Novitskiy et al., 2004) and TNFα (Nehls et al., 1991; Novitskiy et al., 2004) independently increase NF-κB (p65) and its binding to the α2(I) collagen promoter (Novitskiy et al., 2004).
The activities of both the α2(I) (Novitskiy et al., 2004) and α1(I) (Rippe et al., 1999) collagen promoters are inhibited by transfection of the NF-κB (p65) expression vector. Hence, the inhibitory effect of TNFα on the α1(I) collagen promoter may be mediated by NF-κB (p65), whereas the activating effect of acetaldehyde on the collagen promoters is unrelated to its effect on NF-κB (p65).
The aim of this study was to determine the time course and mechanism of the effects of acetaldehyde in comparison to the effects of TNFα on NF-κB activation in hepatic stellate cells.
MATERIALS AND METHODS
Materials
Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS) penicillin–streptomycin, fungizone, and trypsin–EDTA were purchased from Life Technologies Inc. (Gaithersburg, MD). Plastic 25 cm2 tissue culture flasks were purchased from Corning Inc. (Corning, NY). Pronase E, collagenase IV and phenylmethylsulfonyl fluoride (PMSF) were from Sigma Chemical Co. (St Louis, MO). Acetaldehyde was from Fisher Scientific (Pittsburgh, PA). [γ-32P] ATP was from ICN Biochemicals, Inc. (Irvine, CA).
Animals and stellate cell culture
Adult male Sprague–Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). All animals received humane care in compliance with the guidelines of the Animal Care and Use Committee of the Johns Hopkins University. Stellate cells were isolated in situ, under xylazine and ketamine anaesthesia, by perfusion of the portal vein under sterile conditions with 0.2% pronase E and 0.015% collagenase in DMEM in a sequential manner. The liver cell suspension obtained was centrifuged at 1400 g in a two-step discontinuous Nycodenz gradient (Anania et al., 1996). The isolated cells were suspended in DMEM and seeded in 25 cm2 tissue culture flasks maintained in DMEM containing 10% FBS, fungizone (2.5 mg/ml), penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37°C with a humidified atmosphere of 5% CO2 and 95% air. The medium was changed every 48 h, while the cells transform into activated cells after 10–14 days in culture. The experiments with TNFα and acetaldehyde were in the activated stellate cells at 70–80% confluence. The media was removed, the cells were washed with serum-free DMEM, and after 1 h the medium was changed to serum-free DMEM containing the following six supplemental growth factors (SGF6): epidermal growth factor (10 μg/l), transferrin (0.5 mg/l), selenous acid (5 μg/l), linoleic acid (0.5 mg/l), bovine serum albumin (0.5 mg/l) and fetuin (0.5 mg/l). TNFα was added at a concentration of 0.6 nM, while the concentration of added acetaldehyde was 200 μM. The flasks were tightly capped.
Western Blot Analysis
Cells were lysed in NP-40 lysis buffer containing 50 mM Tris–HCl, pH 8.0, 400 mM NaCl, 5 mM EDTA, 1% NP-40, 1 mM Na3VO4, 1 mM PMSF, 1 μg/ml of leupeptin, and 1 μg/ml aprotinin for 1 h at 4°C, and then centrifuged at 12 000 g for 15 min at 4°C. The cytosolic protein in the supernatant was initially stored at −80°C. Protein concentration was determined by the method of Lowry et al. (1951). The protein for western blot was separated on mini-SDS gels at 100 V for 1 h and electrotransferred to polyvinylidine difluoride membranes (Hybond-P, Amersham Biosciences, Piscataway, NJ) at 100 V for 1 h. The membranes were washed in PBS, pH 7.6, containing 0.1% Tween 20 (PBS-T), blocked with 5% (w/v) dried nonfat milk in PBS-T for 1 h, then incubated with rabbit polyclonal antibodies to NF-κB (p50), NF-κB (p65), IκB-α and phosphorylated IκB-α (Santa Cruz Biotechnology) at 4°C overnight. After repeated washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:10 000 dilution, Amersham Life Science) at room temperature for 1 h. The membranes were then subsequently washed and visualized by enhanced chemiluminescence reaction (ECL-plus, Amersham Biosciences).
IκB Kinase Assay
IκB-α kinase activity was determined by the method of Mercurio et al. (1997). The cytosolic protein was incubated with antibody to IKK-α (Santa Cruz Biotechnology) overnight. Twenty microlitres of protein A Sepharose (Rockland, Gilbertsville, PA) was then added. After 2 h of incubation, the antigen–antibody–protein A Sepharose complex was precipitated, washed and then assayed for kinase activity. The reaction mixture for the kinase activity consisted of 20 mM HEPES buffer, pH 7.4, 20 mM MgCl2, 2 mM MnCl2, 10 mM β-glycerophosphate, 10 mM NaF, 10 mM p-nitrophenyl phosphate, 300 μM Na3VO4, 1 mM benzamidine, 1 mM DTT, 2 μM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 10 μg/ml of aprotinin, 20 μCi [γ-32P] ATP, 10 μM ATP and 2 μg GST– IκB-α (Santa Cruz Biotechnology). The total volume of reaction mixture was 25 μl. After incubation at 30°C for 30 min, the reaction was terminated by boiling with 5 μl of 6× SDS sample buffer for 5 min. The protein was resolved on a 10% polyacrylamide gel under reducing conditions. The gel was dried and the radioactive bands were visualized by PhosphorImaging.
Data analysis
Data were analysed with the Student's t-test when appropriate, or by two-way analysis of variance when comparing means of more than two groups.
RESULTS
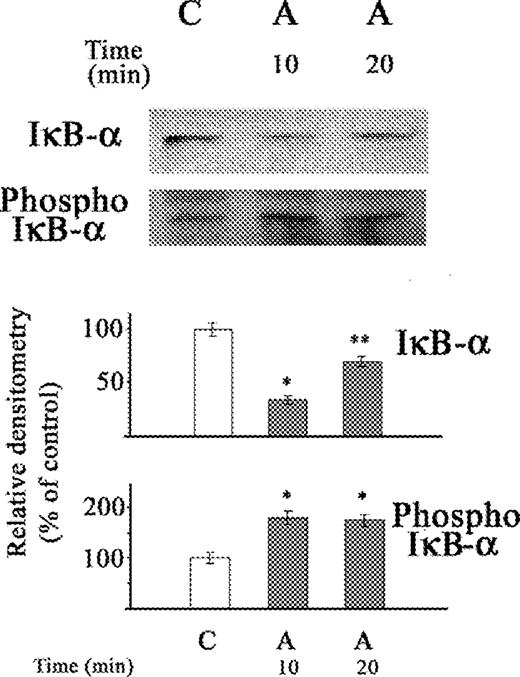
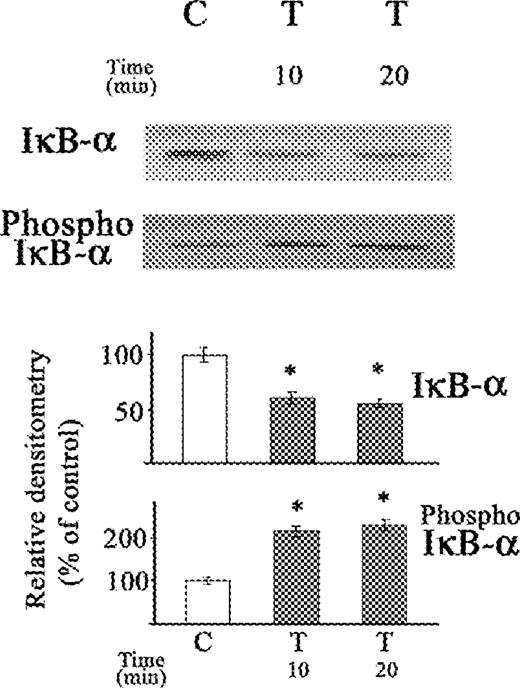
In previous studies, exposure of stellate cells to acetaldehyde for 30 min resulted in an increase of NF-κB (p65) that was accompanied by an increase in IκB-α, rather than the expected decrease in IκB-α, despite increased IκB-α kinase activity and phosphorylated IκB-α (Novitskiy et al., 2004). This study shows that IκB-α decreases markedly after a short incubation period of 10 min with acetaldehyde, with a recovery towards normal after a longer 20 min period of exposure to acetaldehyde (Fig. 1). This effect of acetaldehyde differs from that of TNFα (0.6 nM), which led to similar lower levels of IκB-α after 10 and 20 min of exposure (Fig. 2), without any recovery toward normal levels.
Western blot of the effect of acetaldehyde (A) on IκB-α protein and phosphorylated IκB-α in cytosolic extract of stellate cells. The cells were not exposed (C) or exposed to acetaldehyde (A) (200 μM) for 10 and 20 min. The relative densitometry readings (mean ± SE) from three samples for each determination are shown. *P < 0.05 vs respective control. *P < 0.05 vs respective group.
Western blot of the effect of TNFα on IκB-α protein and phosphorylated IκB-α in cytosolic extract of stellate cells. The cells were not exposed (C) or exposed to TNFα (T) (0.6 nM) for 10 and 20 min. The relative densitometry readings (mean ± SE) from three samples for each determination are shown. *P < 0.05 vs respective control.
Both acetaldehyde and TNFα resulted in similar increases in phosphorylated IκB-α, after exposure of stellate cells, for 10 and 20 min periods (Figs 1 and 2).
Inhibitory κB kinase activity, using GST-IκB-α as a substrate (Iκ-Bα kinase), was maximally increased by acetaldehyde at 10 min of exposure, with a subsequent fall at 20 min (Fig. 3A). This is in contrast to the effect of TNFα, which caused a higher IκB-α kinase activity after 20 min than after 10 min of exposure (Fig. 3B).
Effect of acetaldehyde (A) and TNFα (B) on IκB-α kinase activity in stellate cells of rat. Cytosol was precipitated with antibody to IKK-α. The kinase assay used GST-IκB-α as a substrate and [γ32-ATP]. The changes in 32P phosphorylated GST-IκB-α are shown. The cells were not exposed (C) or exposed to acetaldehyde (A) (200 μM) (A) or to TNFα (T) (0.6 nM) (B) for 10 and 20 min. The relative densitometry readings (mean ± SE) from three samples for each determination are shown. *P < 0.05 vs respective control.
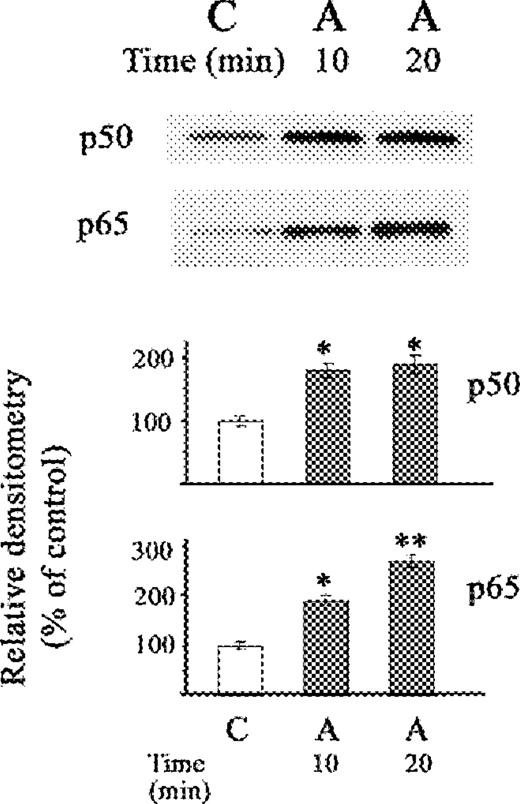
The decrease in IκB-α after exposure to acetaldehyde resulted in increases in NF-κB (p50) and in NF-κB (p65) (Fig. 4). The increase in NF-κB (p50) caused by acetaldehyde was of similar magnitude after 10 and 20 min, whereas the increase in NF-κB (p65) was higher at 20 min than at 10 min (P < 0.05). The differences in the time of maximal activation of IκB-α kinase activity at 10 min by acetaldehyde when compared to TNFα at 20 min, with respect to similar maximal increased in NF-κB (p65) at 20 min, possibly reflects differences between acetaldehyde and TNFα in the mechanism of activation of the kinase and the subsequent rate of activation of NF-κB.
Western blot of the effect of acetaldehyde (A) on nuclear fraction of NF-κB (p50) and NF-κB (p65) proteins in stellate cells. The cells were not exposed (C) or exposed to acetaldehyde (A) (200 μM) for 10 and 20 min. The relative densitometry readings (mean ± SE) from 3 samples for each determination are shown. *P < 0.05 vs respective control.
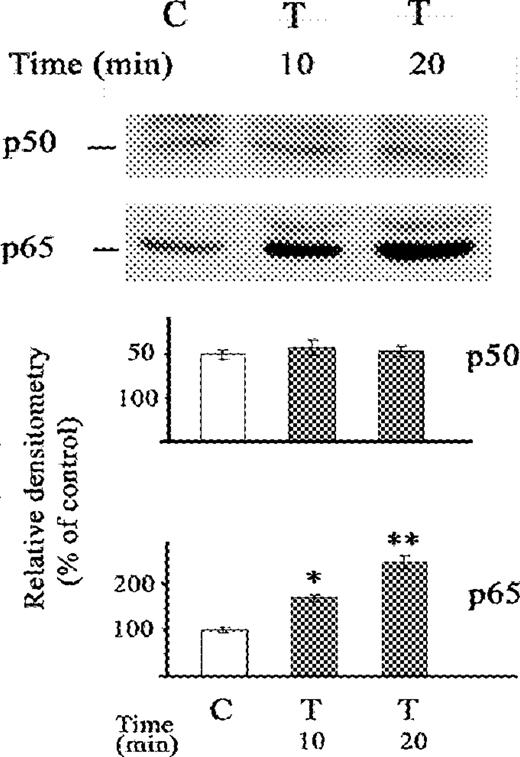
In contrast to the effects with acetaldehyde, TNFα did not change NF-κB (p50) levels after 10 and 20 min periods (Fig. 5). Moreover, no changes in NF-κB (p50) were demonstrated after 30 min of TNFα exposure in previous studies (Novitskiy et al., 2004). However, the increase of NF-κB (p65) after TNFα was similar to that observed with acetaldehyde, with gradual increase from 10 to 20 min after exposure.
Western blot of the effect of TNFα on nuclear fraction of NF-κB (p50) and NF-κB (p65) proteins in stellate cells. The cells were not exposed (C) or exposed to TNFα (T) (0.6 nM) for 10 and 20 min. The relative densitometry readings (mean ± SE) from 3 samples for each determination are shown. *P < 0.05 vs respective control.
DISCUSSION
Activation of NF-κB in Kupffer cells and hepatocytes is an important factor in the pathogenenesis of liver injury in alcoholic liver disease. Activated NF-κB injures the liver by stimulation of inflammatory cytokines and enhanced expression of the RANTES chemokine, which migrates CD14T lymphocytes to the liver (Hirano et al., 2003). NF-κB is found in the cytoplasm of cells as an inactive complex that when dissociated, releases active NF-κB, which then translocates to the nucleus of the cells (Karin and Ben-Neriah, 2000). NF-κB is usually in a complex with an IκB protein such as IκB-α and the degradation of the IκB protein, stimulated by various factors such as TNFα, frees NF-κB that then translocates to the nucleus where it activates a variety of genes. An association between elevation of TNFα mRNA and activation of NF-κB was found in alcoholic hepatitis (Hill et al., 2000). Moreover, elevation of nuclear NF-κB and TNFα mRNA was higher in the liver of female than in the liver of male rats in association with more severe liver injury in the female rats after intragastric administration of ethanol (Kono et al., 2000). TNFα is known to activate NF-κB and NF-κB in turn was found to increase TNFα (Ghosh et al., 1998). This study demonstrates that the principal initial event for the association between elevation of NF-κB, and TNFα is a very rapid and marked effect of TNFα on IκB-α kinase activity, with a decrease in IκB-α resulting in activation of NF-κB which then persists for several hours.
This study also shows that acetaldehyde results in an earlier and more transient increase in IκB-α kinase activity and decrease in IκB-α than found with TNFα indicating that the effect of acetaldehyde in decreasing IκB-α, which results in increased nuclear NF-κB, is not mediated by TNFα. Furthermore, this study explains the previous contradictory data on the effect of acetaldehyde, which either did not change (Roman et al., 2000) or increased IκB-α (Novitskiy et al., 2004) after 30 min of exposure, since those experiments missed the early decrease in IκB-α occurring at 10 min after exposure to acetaldehyde. Despite the findings that degradation of IκB-α after exposure to acetaldehyde is followed by its recovery to control or higher IκB-α levels, nuclear NF-κB(p65) remained elevated for up to 24 h (Novitskiy et al., 2004). Similarly, after stimulation of various cells by lipopolysaccaride (LPS) and cytokines such as interleukin-1b or TNFα, NF-κB (p65) remains elevated for up to 24 h although IκB-α is newly synthesized (Lee et al., 2003).
In this study, TNFα in the stellate cells results in a greater increase in IκB-α kinase after 20 min than after 10 min of exposure. This result differs to some extent from the effect of TNFα in other cell types (DiDonato et al., 1997; Kirillova et al., 1999), where maximum increase in IκB-α kinase occurred after 5 min of exposure, with subsequent decreases after 15–20 min. The decrease in IκB-α kinase is followed by a further slight decrease at 30 min, which then remains steady for at least 90 min (DiDonato et al., 1997).
The effect of acetaldehyde on NF-κB activation is possibly mediated by increased oxidative stress. Acetaldehyde increases the formation of oxygen radicals in hepatocytes (Putarulo and Cederbaum, 1989; Shaw and Jayatilleke, 1990) and in stellate cells (Novitskiy et al., 2004, and our unpublished data.). Reactive oxygen species in turn have been shown to mediate the effects of cytokines or to lead directly to NF-κB activation (Kutuk and Basaga, 2003; Ryan et al., 2004).
The effects of NF-κB differ with respect to liver injury and fibrogenesis. The activation of NF-κB increases liver injury in hepatocytes, whereas it inhibits fibrogenesis in stellate cells (Novitskiy et al., 2004). As expected, the effects of TNFα in activating NF-κB are associated with increased liver injury and decreased fibrogenesis. In contrast, the effect of acetaldehyde in activating NF-κB is associated with increases in both liver injury and fibrogenesis, indicating that the effects of acetaldehyde on fibrogenesis are mediated by trans-acting factors other than NF-κB or by cytokines that stimulate fibrogenesis such as TGFβ. The activation of murine type I collagen promoters has been shown to be mediated by NF-I (Anania et al., 1995) and C/EBPβ (Attard et al., 2001). TGFβ is a strong stimulator of fibrogenesis (Penttinen et al., 1988). A number of studies indicate that the effects of acetaldehyde on enhancing Type I collagen transcription and collagen formation are, at least in part, mediated by TGFβ (Anania et al., 1996; Chen, 2002). Acetaldehyde increases the secretion and activation of TFGβ by stellate cells and increases the expression of the type II TGF-β1 receptor (Chen, 2002). The effect of acetaldehyde in activating the rat α1(I) collagen promoter is partially blocked by neutralizing anti-TGFβ antibodies and by antisense Tβ-RII oligonucleotides (Chen, 2002), and neutralizing anti-TGFβ antibody is known to suppress the effect of acetaldehyde in increasing mouse α1(I) collagen mRNA (Anania et al., 1996).
The additional finding in this study that acetaldehyde increased both nuclear NF-κB (P65) and (p50), whereas TNFα merely increased NF-κB (p65), probably contributes to the lack of suppressive effect of acetaldehyde on fibrogenesis as well. NF-κB (p50) has been described to be inactive or less active in transcriptional regulation of genes, and in some cases suppresses the effects of NF-κB (p65) (Udalova et al., 2000). Furthermore, the NF-κB (p65), but not the NF-κB (p50) expression vector resulted in inhibition of the α2(I) collagen promoter in transfected stellate cells (Novitskiy et al., 2004). In contrast, other studies with different cell types also found that TNFα increased only NF-κB (p65) (Aoudjit et al., 1997). The activating effect of acetaldehyde on NF-κB (p50) with a lack of effect and/or suppression of an enhancing effect of NF-κB (p65) may allow other trans-activating factors to mediate the activating effect of acetaldehyde on the collagen promoter.
In conclusion, TNFα and acetaldehyde independently activate NF-κB by rapid enhancement of IκB-α kinase activity with degradation of the IκB-α protein. Increased TNFα is the principal mechanism for the elevation of NF-κB in severe alcoholic hepatitis. The elevation of NF-κB due to TNFα enhances liver injury but inhibit fibrogenesis. In contrast, the effect of acetaldehyde in activating NF-κB is associated with increases in both liver injury and fibrogenesis, indicating that the effects of acetaldehyde on fibrogenesis are mediated by cytokines and by trans-acting factors other than NF-κB.
This work was supported by Grant AA00626 from the United States Public Health Service.
REFERENCES
Anania, F. A., Potter, J. J., Rennie-Tankersley, L. and Mezey, E. (
Anania, F. A., Potter, J. J., Rennie-Tankersley, L. and Mezey, E. (
Aoudjit, F., Brochu, N., Belanger, B., Stratowa, C., Hiscott, J. and Audette, M. (
Attard, F. A., Wang, L., Potter J. J., Rennie-Tankersley, L. and Mezey, E. (
Bird, G. L., Sheron, N., Goka, A. K., Alexander, G. J. and Williams, R. S. (
Casini, A., Cunningham, M., Rojkind, M. and Lieber, C. S. (
Chen, A. (
DiDonato, J. A., Hayakawa, M., Rothwarf, D. M., Zandi, E. and Karin, M. (
Felver, M. E., Mezey, E., McGuire, M., Mitchell, M. C., Herlong, H. F., Veech, G. A. and Veech, R. L. (
Ghosh, S., May, M. J. and Kopp, E. B. (
Hernandez, I., de la Torre, P., Rey-Campos, J., Garcia, I., Sanchez, J. A., Munoz., R., Rippe, R. A., Munoz-Yague, T. and Solis-Herruzo, J. A. (
Hill, D. B., Barve, S., Joshi-Barve, S. and McClain, C. (
Hirano, F., Komura, K., Fukawa, E. and Makino, I. (
Karin, M. and Ben-Neriah, Y. (
Kirillova, I., Chaisson, M. and Fausto, N. (
Kono, H., Wheeler, M. D., Rusyn, I., Lin, M., Seabra, V., Rivera, C. A., Bradford, B. U., Forman, D. T. and Thurman, R. G. (
Kutuk, O. and Basaga, H. (
Lee, Y., Allport, V., Sykes, A., Lindstrom, T., Slater, D. and Bennett, P. (
Lowry, O. H., Rosebrough, N. J., Farr, A. L. and Randall, R. J. (
Mercurio, F., Zhu, H., Murray, B. W. et al. (
Mezey, E., Potter, J. J., Rennie-Tankerley, L., Caballeria, J. and Pares A. (
Nehls, M. C., Rippe, R. A., Veloz, L. and Brenner, D. A. (
Novitskiy, G., Potter, J. J., Rennie-Tankersley, L. and Mezey, E. (
Penttinen, R. P., Kobayashi, S. and Bornstein, P. (
Potter, J. J., Rennie-Tankersley, L. and Mezey, E. (
Putarulo, S. and Cederbaum, A. I. (
Rippe, R. A., Schrum, L. W., Stefanovic, B., Solis-Herruzo, J. A. and Brenner, D. A. (
Roman, J., Gimenez, A., Lluis, J. M., Gasso, M., Rubio, M., Caballeria, J., Pares, A., Rodes, J. and Fernandez-Checa, J. C. (
Ryan, K. A., Smith, M. F. Jr, Sanders, M. K. and Ernst, P. B. (
Shaw, S. and Jayatilleke, E. (
Udalova, I. A., Richardson, A., Denys, A., Smith, C., Ackerman, H., Foxwell, B. and Kwiatkowski, D. (
Author notes
1Department of Medicine
2Department of Oncology and Immunology, The Johns Hopkins University School of Medicine, Baltimore, MD 21205-2195, USA




![Effect of acetaldehyde (A) and TNFα (B) on IκB-α kinase activity in stellate cells of rat. Cytosol was precipitated with antibody to IKK-α. The kinase assay used GST-IκB-α as a substrate and [γ32-ATP]. The changes in 32P phosphorylated GST-IκB-α are shown. The cells were not exposed (C) or exposed to acetaldehyde (A) (200 μM) (A) or to TNFα (T) (0.6 nM) (B) for 10 and 20 min. The relative densitometry readings (mean ± SE) from three samples for each determination are shown. *P < 0.05 vs respective control.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/alcalc/40/2/10.1093/alcalc/agh116/2/m_agh116f3.jpeg?Expires=1716484278&Signature=LJwjt0~pLb9y5xxDstCU80aZOb5b02e4IDxfqGBY7C~5TiyfJcztuCMAfDnzxRYg57kUFNN9S0zlpFObet~WjMFsOpi6-Lly5epa9ngLzZtKQB91K3Dx1pASRd9hZOnvWFLI8TqQfkZpq6cPun1vmW50ndlr~iFGOcAd36ZpsFByaeLbo-tmyon9Dv9Oo-Kpu1sFcwNNTlQh73U6HGrZmgde7nNmM~6VaM118OZYAO3e95jS59CKtgT-I6KLRfsgBscmdVL38UV8-XuJ4smVL8k3wLguzEhLuB0eflSgffYiK3wD-C8r09AA1nzaRG7grh5pY5d2FAr70lUaHLRKmw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)