-
PDF
- Split View
-
Views
-
Cite
Cite
Jon Thomassen Hestetun, Gabrielle Tompkins-Macdonald, Hans Tore Rapp, A review of carnivorous sponges (Porifera: Cladorhizidae) from the Boreal North Atlantic and Arctic, Zoological Journal of the Linnean Society, Volume 181, Issue 1, September 2017, Pages 1–69, https://doi.org/10.1093/zoolinnean/zlw022
Close - Share Icon Share
Abstract
Family Cladorhizidae, comprising the carnivorous sponges, represents a unique innovation within the phylum Porifera. Rather than filter feeding, carnivorous sponges have developed the ability to passively capture small invertebrates such as crustaceans using filaments or other appendages, coupled with an adhesive surface and the ability of sponge cells to migrate to and envelop prey items. Cladorhizids are most commonly deep-sea species and are found worldwide, with around 150 species currently described. The boreal Atlantic and neighbouring Arctic areas have a species-rich cladorhizid fauna, including many of the first cladorhizids described from early marine biological investigations. While a number of records exist for parts of these areas, other areas, such as the North-west Atlantic, are less well known, and species descriptions are scattered and in many cases lacking necessary information. Using a large set of specimens from newly collected material and multiple museum collections, and integrating this with previously published records from the area, we provide an overview of the cladorhizid fauna of the boreal Atlantic and adjoining Arctic Oceans. In all, we provide updated descriptions of 25 species, of which four, Asbestopluma (Asbestopluma) ruetzleri sp. nov., Cladorhiza kenchingtonae sp. nov., Lycopodina novangliae sp. nov. and L. tendali sp. nov., are previously undescribed. We also provide an overview of the known distribution and depth ranges of each species as well as a key for identification to species level. Finally, we provide some general discussion on the boreo-Atlantic and Arctic cladorhizid fauna and its relationship to neighbouring areas.
INTRODUCTION
Carnivorous sponges are poecilosclerid demosponges belonging to family Cladorhizidae. Found worldwide, they are exceptional within phylum Porifera in their ability to entangle, capture and envelop small invertebrates such as crustaceans, rather than filtering water through an aquiferous system as in other sponges (e.g. Vacelet & Boury-Esnault, 1995; Vacelet, 2007). A recent phylogenetic analysis shows that all known carnivorous sponges constitute a monophyletic group, implying a single origin of carnivory in sponges (Hestetun et al., 2016b). Currently, some ~150 species are known belonging to nine genera, and new species are regularly described (van Soest et al., 2016). Carnivorous sponges are usually erect, in most cases single-stem, arbuscular or pedunculate, with numerous filaments or other appendages to maximize the surface area. The aquiferous system present in other sponges is either strongly reduced or absent. Prey gets entangled or stuck to the adhesive surface of the sponge; sponge cells migrate to the area of contact and envelop and digest the prey item over a period of several days (Vacelet & Duport, 2004).
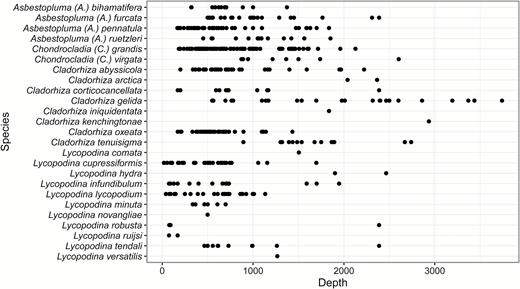
Cladorhizids are typically considered deep-sea sponges, and carnivory is believed to be an adaptation to oligotrophic conditions at lower depths, where filter-feeding strategies are less efficient due to the low amount of particulate matter in surrounding water masses. Some carnivorous sponges have been reported from very shallow waters < 20 m, often in connection with special habitats such as submarine caves (e.g. Vacelet, 1996), and while depths of 100–200 m are not uncommon, cladorhizid records become more numerous at depths of 400–500 m and below. At greater depths, they start to constitute a larger fraction of the total sponge fauna, and they represent one of the dominant sponge groups at bathyal, abyssal and hadal depths (Vacelet, 2007).
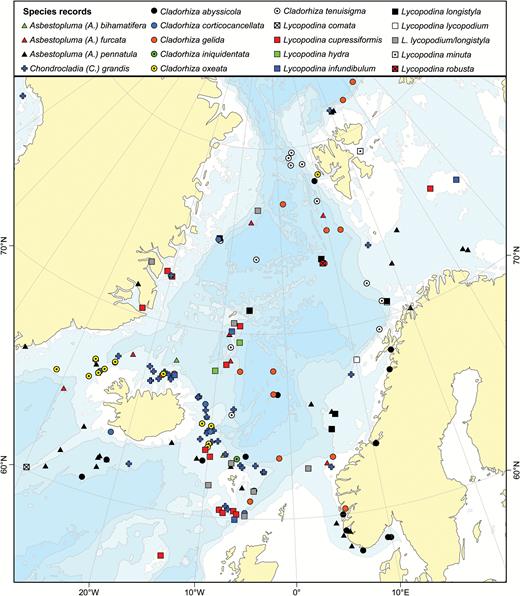
The boreal Atlantic and adjacent Arctic areas contain some 20–30 cladorhizid species. Boreal Atlantic cladorhizids show a greater affinity to and overlap with species found in Arctic areas such as the Davis Strait and Baffin Bay and the North side of the Greenland-Iceland-Faroe (GIF) Ridge including the Greenland-Icelandic-Norwegian (GIN), Barents and Kara Seas, than to the species composition farther to the south in the Atlantic: the distribution of North Atlantic cladorhizids shows a shift in species composition loosely coinciding with the North Atlantic Current (NAC), approximately 40°N in the NW and 50°N in the North-east (NE) Atlantic (Fig. 1). Some northern species such as Asbestopluma (Asbestopluma) pennatula, Cladorhiza gelida and Lycopodina infundibulum have been reported at special habitats south of the NAC such as on the Mid-Atlantic Ridge (MAR) at the Lucky Strike vent site (Desbruyères et al., 2001). However, only C.abyssicola is known to have a well-established presence on both sides of the current. To distinguish this area from the boreo-Arctic North Atlantic, we here use the term southern North Atlantic to describe the area and fauna of the Atlantic south of the NAC, but north of the equator, which roughly corresponds to the use of ‘Lusitanian’ in Cárdenas & Rapp (2015).
Known distribution of Atlantic boreo-Arctic (black circles) and southern North Atlantic only (white circles) cladorhizid species (with the majority of overlap from Cladorhiza abyssicola). The North Atlantic Current is indicated.
While the NAC is used here as a practical demarcation line, its strength decreases with depth, and the influence of the NAC and opposite deep-water flows such as Iceland-Scotland Overflow Water (ISOW) on the distribution of deep-sea sponge dispersal is poorly known (Cárdenas & Rapp, 2015). In the case of non-carnivorous deep-sea sponges, Cárdenas & Rapp (2015) found that the Charlie-Gibbs Fracture Zone (CGFZ), a 4500-m deep 300-km displacement of the MAR at around 52°N (Felley, Vecchione & Wilson, 2008), is a possible latitudinal barrier for sponge dispersal along the MAR. Other factors, such as lower sampling effort in the southern North Atlantic, could also play a partial role. Cladorhizids at lower bathyal and abyssal depths (> 3000 m) represent another distinct fauna apart from the boreo-Arctic cladorhizids. Species found at these depths for the most part belong to a separate set of species, and while collection records are sparse, some abyssal species have been reported over very large distances (Hestetun et al., 2015).
Historically, scientific cruise activity has been comparatively high in the boreal Atlantic and Arctic, especially the NE Atlantic and part of the Arctic bordering Europe, from the pioneering days of marine biology in the 1870s until more recently, and cladorhizid records exist from multiple sources (Schmidt, 1870; Sars, 1872; Carter, 1874, 1876; Levinsen, 1887; Topsent, 1892; Lundbeck, 1905; Arnesen, 1920; Hentschel, 1929; Brøndsted, 1933; Koltun, 1964). Activity in the NW Atlantic has been more limited, with all cladorhizid records from early sources (Verrill, 1879, 1885; Lambe, 1900b).
Our aim in this study was to provide an overview of the cladorhizid fauna in the boreal North Atlantic, defined here as the Atlantic north of the 40–50°N boundary set by the North Atlantic Current, and adjoining Arctic areas. To this end, we have examined or re-examined specimens from multiple museum collections and newly collected material in order to create a comprehensive list of updated species descriptions, including the comparatively little surveyed NW Atlantic. As a special case, we have also included the species Chondrocladia (Chondrocladia) virgata Thomson, 1873, found off Southern Europe and North Africa, based on the examined material from this little-known species. Adding records from previous sources to our own material, we also provide an overview of the known distribution of the different cladorhizid species and discuss the relationships between the boreo-Atlantic and Arctic fauna and neighbouring areas. Finally, we provide a key to the identification of known boreo-Atlantic and Arctic cladorhizid species.
MATERIAL AND METHODS
Cladorhizid specimens, including type material where available, were examined from multiple museum collections with most specimens referenced from the collections at the Copenhagen National History Museum (Denmark), Smithsonian Institution National History Museum (USA), National History Museum of London, Yale Peabody Museum (USA), the Bergen University Museum (Norway) and the NTNU University Museum (Trondheim, Norway). Recently collected material was obtained from the 2006–2007 BIODEEP, 2008–2014 GeoBio, 2013 MAREANO, 2013 IceAGE 2, NEREIDA 0509, 2007–2025, 2009–2030, 2010–2029 and 2013–2021/029 CCGS ‘Hudson’, LAR2012 and 2009–2015 R/V ‘Paamiut’ cruises. In all, over 400 specimens were examined or re-examined for this article.
Species were identified to species level whenever possible using spicule preparations from tissue dissolved in nitric acid and made via the standard method as described in Boury-Esnault & Rützler (1997), with 30 measurements made of each spicule type. For megascleres, measurements were made of the length and width at the widest point (excluding tyles, usually in the middle) of the spicule; for microscleres, measurements were made of the length from the top to the lower end. In the case of species where a large number of specimens were available, identification of material beyond 5–10 samples per species is based on a lower number of measurements per specimen. Measurements are reported here as minimum–mean–maximum lengths of all available measurements for the species, except where otherwise indicated. Scanning electron microscopy (SEM) images of spicules were obtained of all examined species using type material where available and using a standard gold–palladium coating. Histological thick sections (200–800 µm) were made by embedding in either low viscosity (LV) resin or araldite, with toluidine blue staining.
To provide comprehensive coverage of known biogeographic distribution, we searched existing literature for positions, depth and other relevant information from previously published records. These data were added to our specimen dataset for creation of maps and depth distributions for the different cladorhizid species.
Molecular sequencing was used to complement the morphological taxonomy. The markers, partial 28S rDNA (C1-D2, ~700–750 bp) (Chombard et al., 1997), COI (‘Folmer’ and ‘Erpenbeck’ partitions, 1216 bp) (Folmer et al., 1994; Rot et al., 2006) and ALG11 (~930 bp) (Belinky et al., 2012), were chosen in order to allow comparison with previous results (Hestetun et al., 2016b) and have proved suitable for genus- and species-level phylogeny. Phylogenetic analyses were run on a concatenated 2859 bp data set using maximum likelihood (ML) in RAxML 8.0 (Stamatakis, 2014) and Bayesian inference in MrBayes 3.2.2 (Huelsenbeck et al., 2001; Ronquist & Huelsenbeck, 2003) with a GTR+G model. Methods for both sequencing and phylogenetic analysis are identical to those used, and further described, in Hestetun et al. (2016b).
Geographical abbreviations: AMOR, Arctic Mid-Ocean Ridge; GIF Ridge, Greenland-Iceland-Faroe Ridge; GIN Seas, Greenland-Icelandic-Norwegian Seas; MAR, Mid-Atlantic Ridge; NAC, the North Atlantic Current.
Museum collection abbreviations: BMNH, Natural History Museum (UK); CMN, Canadian Museum of Nature; MNHN, Muséum Nationale d’Histoire Naturelle de Paris; MNCN, Museo Nacional de Ciencias Naturales (Spain); NIWA, National Institute of Water and Atmospheric Research (New Zealand); NTNU, NTNU University Museum (Norway); QMG, Queensland Museum (Australia); SMF, Senckenberg Naturmuseum Frankfurt (Germany); USNM, Smithsonian Institution National Museum of Natural History (USA); YPM, Yale Peabody Museum (USA); ZIN RAS, Zoological Institution of Russian Academy of Sciences, Saint-Petersburg (Russia); ZMAPOR, Naturalis Biodiversity Center, Leiden (Netherlands); ZMUC, Natural History Museum of Denmark, Zoological Museum; ZMBN, University Museum of Bergen (Norway).
RESULTS
Systematic index
Phylum Porifera Grant, 1836
Class Demospongiae Sollas, 1885
Subclass Heteroscleromorpha Cárdenas, Perez & Boury-Esnault, 2012
Order Poecilosclerida Topsent, 1928
Family Cladorhizidae Dendy, 1922
Genus Asbestopluma Topsent, 1901
Subgenus Asbestopluma Topsent, 1901
Asbestopluma (A.) bihamatifera (Carter, 1876)
Asbestopluma (A.) furcata Lundbeck, 1905
Asbestopluma (A.) pennatula (Schmidt, 1875)
Asbestopluma (A.) ruetzleri sp. nov.
Genus Chondrocladia Thomson, 1873
Subgenus Chondrocladia Thomson, 1873
Chondrocladia (C.) grandis (Verrill, 1879)
Chondrocladia (C.) virgata Thomson, 1873
Genus Cladorhiza Sars, 1872
Cladorhiza abyssicola Sars, 1872
Cladorhiza arctica Koltun, 1959
Cladorhiza corticocancellata Carter, 1876
Cladorhiza gelida Lundbeck, 1905
Cladorhiza iniquidentata Lundbeck, 1905
Cladorhiza kenchingtonae sp. nov.
Cladorhiza oxeata Lundbeck, 1905
Cladorhiza tenuisigma Lundbeck, 1905
Genus Lycopodina Lundbeck, 1905
Lycopodina comata (Lundbeck, 1905)
Lycopodina cupressiformis (Carter, 1874)
Lycopodina hydra (Lundbeck, 1905)
Lycopodina infundibulum (Levinsen, 1887)
Lycopodina lycopodium (Levinsen, 1887)
Lycopodina novangliae sp. nov.
Lycopodina minuta (Lambe, 1900b)
Lycopodina robusta (Levinsen, 1887)
Lycopodina ruijsi van Soest, 2016
Lycopodina tendali sp. nov.
Lycopodina versatilis (Topsent, 1890)
SPECIES DESCRIPTIONS
Genus Asbestopluma Topsent, 1901
Synonymy:
Cometella Schmidt, 1870: 49 (nomen oblitum); Asbestopluma Lankester, 1882: 478 (nomen nudum); Asbestopluma Topsent, 1901: 23; Helophloeina Topsent, 1929: 8. Not: Lycopodina Lundbeck, 1905: 58; Cotylina Lundbeck, 1905: 68.
Diagnosis:
Cladorhizidae with at least one small category of palmate, in a few species modified to anchorate unguiferate, anisochela. Usually with a second larger type of palmate to arcuate anisochela that may be modified to isochela, anisoplacochela, tridentate anchorate chela or diancistra. Sigmancistras and basal acanthotylostyles are also present with a few exceptions. Forceps spicules not present (modified from Hestetun et al., 2016b).
Type species:
Cladorhiza pennatula Schmidt, 1875 (by subsequent designation; Topsent, 1901).
Subgenus Asbestopluma Topsent, 1901
Diagnosis:
Asbestopluma without spear-shaped microtylostyles (from Lopes, Bravo & Hajdu, 2011).
Type species:
Cladorhiza pennatula Schmidt, 1875 (by subsequent designation; Topsent, 1901).
Remarks:
The typical spicule complement of Asbestopluma is one category of mycalostyle, one category of subtylostyle, acanthotylostyles in the basal sheath, one larger and one smaller category of palmate anisochela and finally sigmancistras. The North Atlantic species treated here do not deviate from this except for the slightly modified larger anisochelae of A. (A.) ruetzleri sp. nov. However, globally Asbestopluma species can be found where the large palmate anisochelae are missing or where either type of anisochela has been modified into different morphologies such as arcuate (Kelly & Vacelet, 2011; Hestetun, Rapp & Xavier, 2017) or anchorate unguiferate (Lopes & Hajdu, 2014). Monocrepid desmas have also been reported in some species. Subgenus Asbestopluma is defined as species within the genus that do not have spear-shaped microtylostyles and thus do not meet the definition of subgenus Helophloeina Topsent, 1929. Subgenus Helophloeina contains only three species. The type species of the subgenus, A. (H.) stylivarians (Topsent, 1929), was collected at the Canary Islands, and the subgenus is not present in the area covered here.
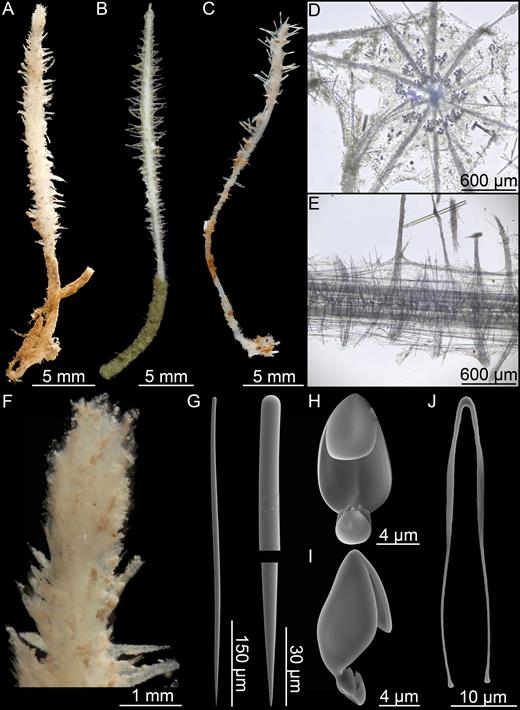
Asbestopluma (Asbestopluma) bihamatifera (Carter, 1876) (Fig. 3)
Original description:
Esperia cupressiformis var. bihamatifera Carter, 1876: 318.
Synonyms and citations:
Asbestopluma bihamatifera (Lundbeck, 1905: 51; Topsent, 1913: 50; Hentschel, 1929: 875, 933; Koltun, 1959: 74, 1964: 151, 164). Not: Cladorhiza bihamatifera (Vosmaer, 1882: 47); Esperia bihamatifera (Hansen, 1885: 15).
Material examined:
The Danish Ingolf Expedition, BIOFAR, LAR2012, IceAGE 2 (see Supporting Information).
Diagnosis:
Erect, single-axis Asbestopluma with upper part of stem with four to six filament rows. Megascleres mycalostyles, subtylostyles and acanthotylostyles; microscleres one type of palmate to arcuate anisochela c. 41–61 µm, one type of palmate anisochela c. 9–11 µm and sigmancistras c. 17–22 µm.
Description:
Erect sponge consisting of a single, hard but flexible stem bare in the lower part; upper part with four to six filament rows, equally distributed around the stem. Stem round in cross section. Most specimens 5–10 cm tall and 1–2 mm in diameter, with filament-bearing part 1–2 cm in length. Sponge attached with branching root-like processes. Lower stem light brown; upper part, including filament-bearing part, light grey to white. Processes up to 3 mm long, but often reduced to knobs, either because filaments are withdrawn or due to specimen damage (Fig. 3A, B).
Skeleton:
Skeleton composed of a main axis of tightly packed mycalostyles with points toward the apex. Lower stem with outer layer of acanthotylostyles; upper part with several rows of filaments. Filaments composed of a central stem made up of overlapping subtylostyles perpendicular to and anchored in the centre of the main axis.
Spicules:
Mycalostyles, straight or slightly curved, fusiform, 653-821-935 µm long and 17.3-23.5-31.4 µm wide (Fig. 3C).
Subtylostyles, straight, with an elongated, slightly offset tyle characteristic for the genus, slightly fusiform. Found in the filament-bearing upper part only, 465-633-754 µm long and 12.1-16.1-20.4 µm wide (Fig. 3D).
Acanthotylostyles, curved, found in the lower stem cover, 110-128-140 µm long (Fig. 3E).
Arcuate to palmate anisochelae, with a straight shaft, a central tooth and alae covering about 40% of the total length of the spicule and a lower end with two rudimentary dorsal processes and three teeth, about 15% of the total length of the spicule. Associated with the upper, filament-bearing part, 40.7-55.3-60.7 µm (Fig. 3F, G).
Palmate anisochelae, numerous, with a curved shaft, a central tooth and lateral alae covering about 75% of the total length of the spicule and a lower end with two rudimentary dorsal processes and the central part in the form of a leaf-shaped plate with an upwards-pointing central process about 1/6th of total length, 8.6-10.2-11.0 µm (Fig. 3H, I).
Sigmancistras, numerous, twisted about 90°, with a flattened internal margin, 17.1-19.7-22.2 µm (Fig. 3J).
Distribution:
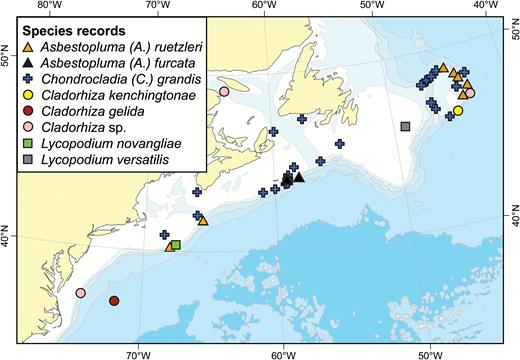
Most records of this species are in the vicinity of Iceland and the Faroes. In contrast to A. (A.) pennatula, it has not been recorded from the Norwegian Coast or the Barents Sea. However, there are two records from Svalbard, one uncertain record from the Kara Sea and one record from the Hatton Basin, Labrador Sea (Fig. 2). Recorded depth is around 500–1500 m.
A map of reported collection localities of Asbestopluma (Asbestopluma) bihamatifera, A. (A.) furcata, A. (A.) pennatula and A. (A.) ruetzleri.
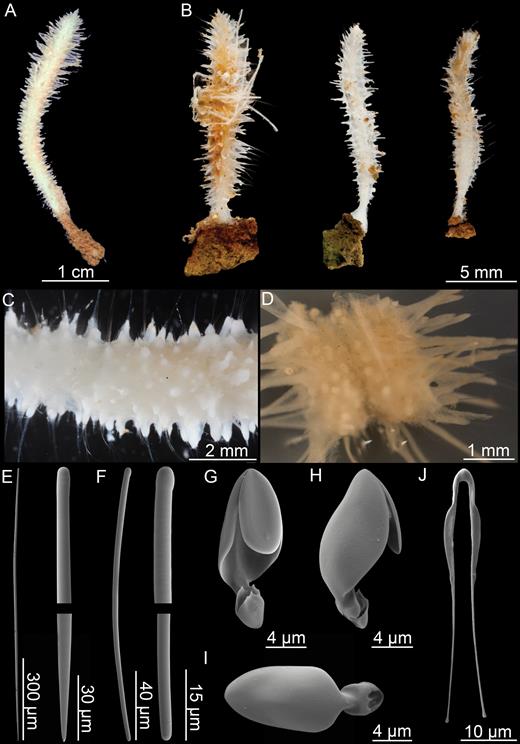
Asbestopluma (Asbestopluma) bihamatifera. (A) Specimens part of ZMBN 103447 (IceAGE 2 st. 880, Iceland-Faroe Ridge), (B) branch detail of two specimens from ZMBN 103447, (C) mycalostyle, (D) subtylostyle, (E) acanthotylostyle, (F, G) palmate/arcuate anisochela front and back view, (H, I) palmate anisochela front and side view, (J) sigmancistra.
Related species:
Asbestopluma (Asbestopluma) furcata Lundbeck, 1905; A. (A.) pennatula (Schmidt, 1875); A. (A.) ruetzleri sp. nov.
Remarks:
The spicule complement of this species is very similar to that of Asbestopluma (A.) pennatula and A. (A.) furcata, but molecular results from Hestetun et al. (2016b) confirmed that this is a separate species, and the upper alae of the larger type of palmate anisochela cover a smaller part of the total length of the spicule in A. (A.) bihamatifera (compare Fig. 3E, F and Fig. 6J, K, or see Fig. 41G–J). More easily, A. (A.) bihamatifera can be recognized by its morphology: In A. (A.) pennatula and A. (A.) furcata, filaments are placed on opposite sides of a flattened stem, while in A. (A.) bihamatifera filaments are placed in four to six rows equally distributed around the stem, which is round in cross section. The last Asbestopluma species known from the area is A. (A.) ruetzleri sp. nov. However, the long alae and curved back of the large anisochelae are clearly different from the morphology of the large anisochelae of A. (A.) bihamatifera.
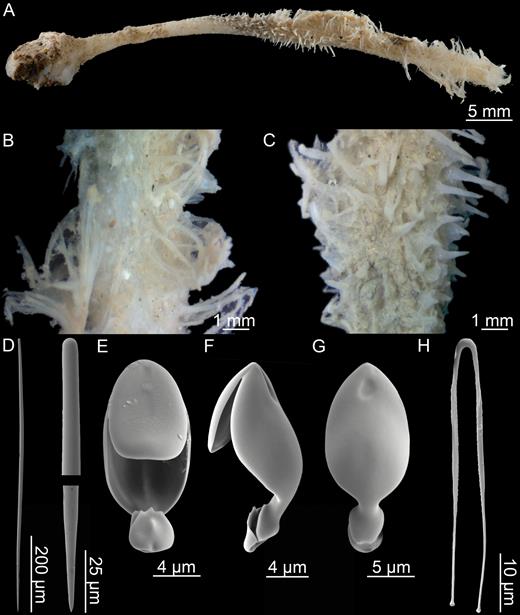
Asbestopluma (Asbestopluma) furcata Lundbeck, 1905 (Figs 4, 5)
Original description:
Asbestopluma furcata Lundbeck, 1905: 54.
Synonyms and citations:
Esperia bihamatifera in part (Hansen, 1885: 15); Asbestopluma furcata (Hentschel, 1929: 933; Koltun, 1964: 152, 163; Barthel & Tendal, 1993: 84).
Type material examined:
Lectotype: The Danish Ingolf Expedition, st. 101 ZMUC-DEM-281 (10 July 1896, 66°23ʹN, 012°05ʹW, 1011 m). Paralectotypes: M/S ‘Michael Sars’ 1902, (13 March 1902), (26 June 1902, 62°53ʹN, 004°14ʹE, 848 m).
Other material examined:
M/S ‘Johan Ruud’, ‘Polarstern’, BIOICE, BIODEEP, CCGS ‘Hudson’ 2007–2025, GeoBio 2008–2012 (see Supporting information).
Diagnosis:
Erect, dichotomously branching Asbestopluma with two rows of filaments. Megascleres mycalostyles, subtylostyles and acanthotylostyles; microscleres one type of palmate to arcuate anisochela c. 39–63 µm, one type of palmate anisochela c. 7–12 µm and sigmancistras c. 12–24 µm.
Description:
Erect sponge with repeatedly dichotomously branching solid, but flexible stem that may reach over 20 cm in length in some cases, with branches 0.5–2 mm in diameter. Colour of the lower part brown; upper part slight yellowish grey to white. Branches set with two opposite rows of fine filaments up to 4 mm in length and terminate in small bud-like enlargements. Sponge connected to the substrate with a structure composed of several branching root-like processes (Fig. 4).
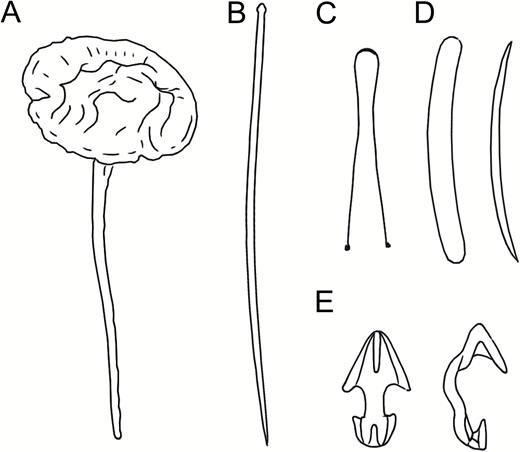
Asbestopluma (Asbestopluma) furcata. (A, B) In situ pictures from Jan Mayen, (C) Specimen ZMBN 103453 (GeoBio 2008, ROV 6), (D) lectotype ZMUC-DEM-281, (E) specimen Hud2007-025-1058, (F, G) detail of ZMBN 103453.
Skeleton:
Branch skeleton composed of hard, central core of longitudinally arranged mycalostyles. Lower stem with outer layer of acanthotylostyles. In the upper part, the main skeletal support is in the outer stem. The filament skeleton composed of subtylostyles and originates in the centre of the stem (Fig. 5A–C).
Asbestopluma (Asbestopluma) furcata. (A) Specimen ZMBN 103453, (A) cross section, (B) branch detail, (C) branch end detail, (D) mycalostyle, (E) subtylostyle, (F) acanthotylostyle, (G, H) palmate/arcuate anisochela front and back view, (I, J) palmate anisochela front and back view, (K) sigmancistra.
Spicules:
Mycalostyles, straight or very slightly curved, fusiform, 333-628-968 µm long and 6.3-15.1-23.6 µm wide (Fig. 5D).
Subtylostyles, straight, with an elongated, slightly offset tyle characteristic for the genus, slightly fusiform. Found in the filament-bearing upper part only, 279-360-475 µm long and 4.7-9.4-14.1 µm wide (Fig. 5E).
Acanthotylostyles, curved, found in the lower stem cover, 44-73-107 µm long (Fig. 5F).
Arcuate to palmate anisochelae, with a straight shaft, a central tooth and lateral alae covering about half of the total length of the spicule and a lower end with two rudimentary dorsal processes and two frontal teeth, about 15% of the total length of the spicule. Associated with the upper, filament-bearing part, 39.3-48.6-62.6 µm (Fig. 5G, H).
Palmate anisochelae, numerous, with a curved shaft, a central tooth and lateral alae covering about 75% of the total length of the spicule and a lower end with two rudimentary dorsal processes and the central part in the form of a leaf-shaped plate with an upwards-pointing central process about 20% of the total length, 7.0-9.2-12.1 µm (Fig. 5I, J).
Sigmancistras, numerous, contorted about 90°, with a flattened internal margin, 12.1-16.6-22.7 µm (Fig. 5K).
Distribution:
Asbestopluma (A.) furcata is distributed around the GIN Seas with records from the Iceland-Greenland and Iceland-Faroe Ridges, the continental slope of Norway and Greenland, along the Mid-Arctic Ridge and in the Sable Gully on the Scotian Shelf (Fig. 2). The reported depth distribution is around 500–2500 m.
Related species:
Asbestopluma (Asbestopluma) bihamatifera (Carter, 1876); A. (A.) pennatula (Schmidt, 1875); A. (A.) ruetzleri sp. nov.
Remarks:
This species is easily recognizable and set apart from other species of Asbestopluma in the North Atlantic and Arctic by its bifurcating habit. From spicule characters alone, it can be distinguished from A. (A.) bihamatifera and A. (A.) pennatula by slightly smaller subtylostyles and chelae and from A. (A.) ruetzleri by the fact that the larger anisochelae of the latter species has a different morphology.
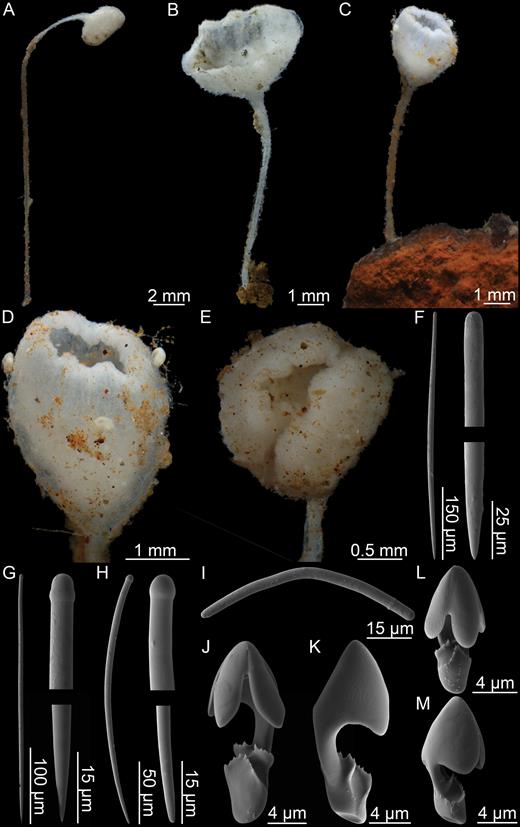
Asbestopluma (Asbestopluma) pennatula (Schmidt, 1875) (Figs 6, 7)
Original description:
Cladorhiza pennatula Schmidt, 1875: 119.
Asbestopluma (Asbestopluma) pennatula. (A) Specimen ZMBN 103467 (MAREANO 2013, st. R1186), (B) specimen ZMBN 103468 (MAREANO 2013, st. 1174), (C) specimen 70/23-21-1 (unknown cruise), (D) two specimens from CCGS ‘Hudson’ 2013–029 (con: 50), (E) two specimens from CCGS ‘Hudson’ 2013–029 (con: 108).
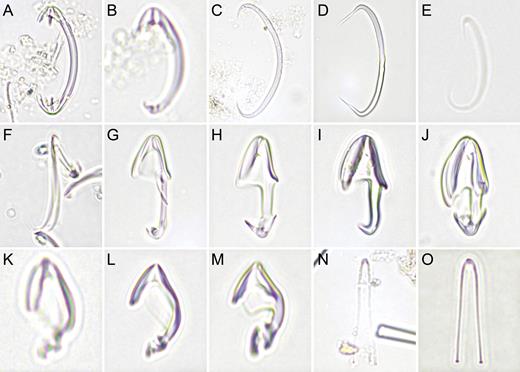
Asbestopluma (Asbestopluma) pennatula. (A, B) branch and root detail of specimen ZMBN 103468, (C) detail of specimen ZMBN 103467 showing embryos, (D) mycalostyle, (E) subtylostyle, (F) acanthotylostyle, (G, H) palmate/arcuate anisochela front and back view, (I, J) palmate anisochela front and side view, (K) sigmancistra.
Synonyms and citations:
Cladorhiza abyssicola in part (Thomson, 1873: 113); C. pennatula (Thiele, 1903: 385); C. bihamatifera (Vosmaer, 1882: 47); Esperia bihamatifera in part (Hansen, 1885: 15); C. Nordenskiöldii (Fristedt, 1887: 455); Esperella plumosa (Arnesen, 1903: 11). Asbestopluma pennatula (Topsent, 1901: 24, 28; Lundbeck, 1905: 44; Topsent, 1913: 49; Arnesen, 1920: 17; Rezvoj, 1928: 84; Hentschel, 1929: 874, 933; Burton, 1930; Koltun, 1959: 73; Koltun, 1964: 151, 163; Brunel, Bossé & Lamarche, 1998: 61; Desbruyères et al., 2001: 1334; van Soest et al., 2007: 130; Hestetun et al., 2015). Possibly: C. Nordenskiöldii (Lambe, 1896: 189, 1900a: 158).
Material examined:
The Norwegian North-Atlantic Expedition, the Danish Ingolf Expedition, M/S ‘Michael Sars’ 1900, 1910, BIOFAR, BIOICE, Caracole, GeoBio 2012, MAREANO 2013, LAR2012, CCGS ‘Hudson’ 2013–2029 (see Supporting information).
Diagnosis:
Erect, single-axis, pennate Asbestopluma with upper part of the stem with two to four rows of filaments: either one or two rows on opposite sides of the stem. Megascleres mycalostyles, subtylostyles and acanthotylostyles; microscleres one type of palmate to arcuate anisochela c. 39–74 µm, one type palmate anisochela c. 7–12 µm and sigmancistras c. 17–25 µm.
Description:
Erect Asbestopluma composed of a single hard but flexible stem, up to 1–3 mm in diameter. Large specimens may reach 25 cm in length. Stem clearly separated into a smooth, light brown, lower part and an upper, light grey to white upper part, the top of which bears filaments on each side. Smaller specimens with two filament rows, one on each side of the stem; in larger specimens, filaments organized into two parallel rows on each side for a total of four rows. Filaments may be reduced to short stubs, either because they are retracted or due to damage. Embryos, if present, in lower end of the upper part, which may appear enlarged. Filaments up to 1 cm long, occasionally slightly longer. Connected to the substrate with a branching structure of root-like processes, anchoring it into sediment or small pebbles (Figs 6A–E, 7A–C).
Skeleton:
Skeleton composed of main axis of tightly packed mycalostyles with their points towards the apex. Lower stem with outer layer of acanthotylostyles. In smaller specimens, the upper part contains two rows of filaments, but in some larger specimens the filaments are more staggered and sometimes arranged in two rows on either side of the stem for a total of four filament rows. The filaments are composed of a central stem made up of overlapping subtylostyles, anchored in the centre of the main axis.
Spicules:
Mycalostyles, straight or very slightly curved, fusiform, 500-760-1010 µm long and 9.4-18.9-31.4 µm wide (Fig. 7D).
Subtylostyles, straight, with an elongated, slightly offset tyle characteristic for the genus, slightly fusiform. Found in the filament-bearing upper part only, 372-602-810 µm long and 6.8-12.4-22.0 µm wide (Fig. 7E).
Acanthotylostyles, curved, found in the lower stem cover, 75-117-159 µm long (Fig. 7F).
Arcuate to palmate anisochelae, with a straight shaft, a central tooth and alae covering about half of the total length of the spicule and a lower end with two rudimentary dorsal processes and two frontal teeth, about 20% of the total length of the spicule. Associated with the upper, filament-bearing part, 38.8-53.1-73.5 µm (Fig. 7G, H).
Palmate anisochelae, numerous, with a curved shaft, a central tooth and lateral alae covering about 75% of the total length of the spicule and a lower end with two rudimentary dorsal processes and the central part in the form of a leaf-shaped plate with an upwards-pointing central process about 20% of the total length, 6.8-10.0-11.7 µm (Fig. 7I, J).
Sigmancistras, numerous, twisted about 90°, with a flattened internal margin, 17.3-21.2-25.1 µm (Fig. 7K).
Distribution:
This species has a wide distribution including the Arctic Kara and Barents Seas, the Labrador Sea, the GIN Seas, on the Iceland-Greenland and Iceland-Faroe Ridges, south of Iceland, the Rockall Bank and Porcupine Banks and the coast of Norway (Fig. 2). Two records deserve further comment: The species was recorded from St. Lawrence Bay by Lambe (1896); however, given the lack of details regarding the chela morphology, it is possible that this is actually A. (A.) ruetzleri sp. nov. It was also reported from the Mid-Atlantic Ridge at the Lucky Strike Vent site (Desbruyères et al., 2001). The lack of other records from this far south may be from the lack of sampling. Reported depth range is 150–1800 m.
Related species:
Asbestopluma (Asbestopluma) bihamatifera (Carter, 1876); A. (A.) furcata Lundbeck, 1905; A. (A.) ruetzleri sp. nov.
Remarks:
The distribution of this species overlaps that of Asbestopluma (A.) bihamatifera, from which it can be recognized by its clear pennate habit with filaments placed on opposite sides of the stem and by the fact that the upper alae cover a greater portion of the larger type of chela; A. (A.) furcata, from which it can be distinguished most easily by its lack of bifurcating habit and to a lesser degree the Western Atlantic species A. (A.) ruetzleri sp. nov., that has a very similar habit, but from which it can be distinguished by the morphology of the larger anisochelae.
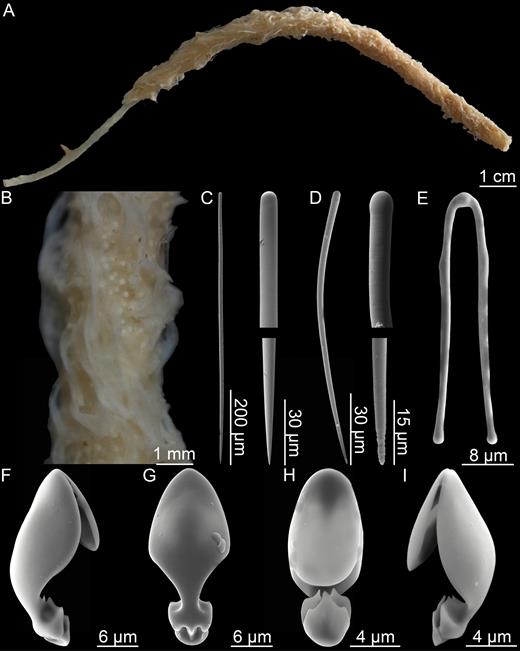
Asbestopluma (Asbestopluma) Ruetzleri sp. nov. (Fig. 8; Table 1)
urn:lsid:zoobank.org:act:0F63150D-5C0B- 41EC-B235-00C798107DEA
Type material:
Holotype: Bureau of Land Management., st. 35984, USNM 32295 (16 May 1979, 40°15.6ʹN, 068°05.5ʹW, 780–1040 m). Paratype: Sch. ‘Albatross’ 1885, st. 2528, YPM 6826 (13 July 1885, 41°47ʹN, 065°37.5ʹW, 1421 m).
Individual spicule measurements from the holotype and paratype of A. (A.) ruetzleri sp. nov.
| Mycalostyles | Subtylostyles | Acantho- tylostyles | Arcuate chelae | Palmate chelae | Sigmancistras | |
| Holotype USNM 32295 | 581-690-838 × 16.0-25.0-34.2 | 514-602-661 × 14.1-17.4-20.3 | 95-117-142 | 45.0-51.1-57.2 | 8.9-10.3-11.7 | 20.3-22.8-25.3 |
| Paratype YPM 6826 | 610-735-890 × 17.3-23.4-31.4 | 570-667-810 × 11.0-15.0-18.8 | 75-98-126 | 45.5-49.0-53.4 | 9.4-10.5-11.0 | 20.4-22.6-25.1 |
| Mycalostyles | Subtylostyles | Acantho- tylostyles | Arcuate chelae | Palmate chelae | Sigmancistras | |
| Holotype USNM 32295 | 581-690-838 × 16.0-25.0-34.2 | 514-602-661 × 14.1-17.4-20.3 | 95-117-142 | 45.0-51.1-57.2 | 8.9-10.3-11.7 | 20.3-22.8-25.3 |
| Paratype YPM 6826 | 610-735-890 × 17.3-23.4-31.4 | 570-667-810 × 11.0-15.0-18.8 | 75-98-126 | 45.5-49.0-53.4 | 9.4-10.5-11.0 | 20.4-22.6-25.1 |
Individual spicule measurements from the holotype and paratype of A. (A.) ruetzleri sp. nov.
| Mycalostyles | Subtylostyles | Acantho- tylostyles | Arcuate chelae | Palmate chelae | Sigmancistras | |
| Holotype USNM 32295 | 581-690-838 × 16.0-25.0-34.2 | 514-602-661 × 14.1-17.4-20.3 | 95-117-142 | 45.0-51.1-57.2 | 8.9-10.3-11.7 | 20.3-22.8-25.3 |
| Paratype YPM 6826 | 610-735-890 × 17.3-23.4-31.4 | 570-667-810 × 11.0-15.0-18.8 | 75-98-126 | 45.5-49.0-53.4 | 9.4-10.5-11.0 | 20.4-22.6-25.1 |
| Mycalostyles | Subtylostyles | Acantho- tylostyles | Arcuate chelae | Palmate chelae | Sigmancistras | |
| Holotype USNM 32295 | 581-690-838 × 16.0-25.0-34.2 | 514-602-661 × 14.1-17.4-20.3 | 95-117-142 | 45.0-51.1-57.2 | 8.9-10.3-11.7 | 20.3-22.8-25.3 |
| Paratype YPM 6826 | 610-735-890 × 17.3-23.4-31.4 | 570-667-810 × 11.0-15.0-18.8 | 75-98-126 | 45.5-49.0-53.4 | 9.4-10.5-11.0 | 20.4-22.6-25.1 |
Asbestopluma (Asbestopluma) ruetzleri sp. nov. (A) Holotype USNM 32295, (B) specimens from Lar2012-003 (con: 145), (C) specimens from Lar2012-003 (con: 141), (D) branch detail of holotype USNM 32295, (E) mycalostyle, (F) subtylostyle, (G) acanthotylostyle, (H, I) arcuate anisochela front and back view, (J, K) palmate anisochela front and back view, (L) sigmancistra.
Additional material examined:
Hud2007, dive 1058 (11 July 2011, 43°58.08ʹN, 059°01.10ʹW), NEREIDA 0509, DR04 (31 May 2009, 48°28.47ʹN, 044°07.37ʹW, 1852 m), DR07 (2 June 2009, 48°15.38ʹN, 044°01.85ʹW, 1339 m), BC32 (14 June 2009, 47°05.10ʹN, 043°36.43ʹW, 811 m), BC34 (15 June 2009, 47°07.26ʹN, 043°27.29ʹW, 1163 m); Lar2012-003, DS1_S con: 141–145 (9 October 2012, 61°09.08ʹN, 060°46.00ʹW, 1051 m); Hud2013-021, VV2 (48°51.14ʹN, 045°29.15ʹW 1564 m); Hud2013-029, DS1_S_VV6 (23 August 2013, 61°16.37ʹN, 060°52.42ʹW, 554 m), DS1_T_VV1 (24 August 2013, 61°32.28ʹN, 060°29.29ʹW, 1065 m), DS1_T_VV3 (24 August 2013, 61°32.34ʹN, 060°29.33ʹW, 1060 m), DS1_T_VV5 (24 August 2013, 61°32.34ʹN, 060°29.34ʹW, 1065 m), DS1_I_VV4 (25 August 2013, 61°20.56ʹN, 061°07.33ʹW, 554 m), DS1_I_VV12 (24 August 2013, 61°20.76ʹN, 061°07.52ʹW, 563 m), DS1_T_VV9 (10 September 2013, 61°32.31ʹN, 060°28.87ʹW, 1057 m), DS1_Q_QIn (12 September 2013, 60°37.19ʹN, 061°20.87ʹW, 451 m).
Diagnosis:
Erect, pennate, single-axis Asbestopluma with upper part of stem containing four rows of filaments, paired on opposite sides of the stem. Megascleres mycalostyles, subtylostyles and acanthotylostyles; microscleres one type of characteristic arcuate anisochela with upper and lower frontal teeth touching or nearly touching, c. 45–57 µm, one type palmate anisochela c. 9–12 µm and sigmancistras c. 20–25 µm.
Description:
Erect Asbestopluma consisting of a single axis, solid, but flexible stem. Holotype is c. 14 cm long and 2–3 mm in diameter. Paratype in the form of several 1- to 1.5-cm-long stem fragments. The lower part of the stem is bare, with light brown colour; the upper part is light grey to white, with four rows of filaments. Filament rows are paired, with two parallel, staggered rows projecting from each side of the stem; filaments up to 8 mm long. In some specimens, filaments reduced to short stubs, either because they are retracted or due to damage. As basal part is missing for both holotype and paratype, the manner of attachment to the substrate is unknown (Fig. 8A–C).
Skeleton:
Skeleton is composed of a main axis of tightly packed mycalostyles with points toward the apex. Lower stem has an outer layer containing acanthotylostyles. Upper part contains four rows of filaments, two in each opposite direction. The skeleton of the filaments is composed of subtylostyles and originate in the centre of the stem.
Spicules:
Mycalostyles, straight or very slightly curved, fusiform, 581-731-918 µm long and 16.0-23.8-34.2 µm wide (Fig. 8E).
Subtylostyles, straight, with an elongated, slightly offset tyle characteristic for the genus, slightly fusiform. Found in the filament-bearing upper part only, 514-638-810 µm long and 11.0-15.8-20.3 µm wide (Fig. 8F).
Acanthotylostyles, curved, found in the lower stem cover, 75-111-142 µm long (Fig. 8G).
Arcuate anisochelae, with a slightly curved shaft, a central tooth and alae covering about 70% of the total length of the spicule and a lower end with two dorsal processes and two well-developed frontal teeth about 30% of the total length of the spicule. The central upper tooth and lateral alae reach down to touch or almost touch the lower end, and fit into the spaces between the lower processes. Associated with the upper, filament-bearing part, 42.7-49.3-57.2 µm (Fig. 8H, I).
Palmate anisochelae, numerous, with a curved shaft, a central tooth and lateral alae covering about 75% of the total length of the spicule and a lower end with two rudimentary dorsal processes and the central part in the form of a leaf-shaped plate with an upwards-pointing central process about 1/6th of the total length, 8.6-10.6-12.6 µm (Fig. 8J, K).
Sigmancistras, numerous, contorted about 90°, with a flattened internal margin, 20.3-23.0-31.6 µm (Fig. 8L).
Distribution:
The holotype and paratype were both collected on the New England slope off Georges Bank (Fig. 2). Although not specifically mentioned, it is possible that it is found in the submarine canyons in the area. Additional specimens were recovered from the Flemish Cap (NEREIDA 0509 and CCGS ‘Hudson’ 2009–2030) and Labrador Sea (LAR2012, CCGS ‘Hudson’ 2013–2029). Based on geographic proximity, a record of A. (A.) pennatula from the bay of St. Lawrence is also possibly this species, although the lack of further description in the source (Lambe, 1896) does not allow any precise identification. The holotype was collected at 780–1040 m depth, while the paratype was collected at 1421 m depth. Additional specimens were collected at 451–1852 m depth.
Related species:
Asbestopluma (Asbestopluma) bihamatifera (Carter, 1876); A. (A.) furcata Lundbeck, 1905; A. (A.) pennatula (Schmidt, 1875).
Etymology:
Named after the curator of sponges at the Smithsonian Institution, Klaus Rützler, for his numerous scientific contributions to sponge taxonomy as well as his preliminary examination of the holotype recognizing its unusual anisochelae.
Remarks:
The habit of this species is similar to the other Asbestopluma species from the North Atlantic and Arctic, especially A. (A.) pennatula. However, it can be distinguished from related species by the shape of the large anisochelae, where both the upper and lower teeth and alae are more developed than in other Asbestopluma species treated here. From A. (A.) furcata, it can also be distinguished by its lack of a bifurcating habit, and from A. (A.) bihamatifera by the flattened stem with opposite rows of filaments, rather than filament rows equally placed around the stem.
Genus Chondrocladia Thomson, 1873
Synonymy:
Chondrocladia Thomson, 1873: 188; Crinorhiza Schmidt, 1880: 83; Meliiderma Ridley & Dendy, 1887: 102. Not: Neocladia Koltun, 1970: 193 (Vacelet, Kelly & Schlacher-Hoenlinger, 2009).
Diagnosis:
Cladorhizidae with anchorate isochelae (from Lee et al., 2012).
Type species:
Chondrocladia virgata Thomson, 1873 (by monotypy).
Subgenus Chondrocladia Thomson, 1873
Diagnosis:
Chondrocladia without a layer of special spicules (subtrochirhabds or trochirhabds), lacking special rostriform (snoutlike) subtylostyles in filaments or terminal balls, and without planar vanes formed of evenly spaced upright branches (from Lee et al., 2012).
Type species:
Chondrocladia virgata Thomson, 1873 (by monotypy).
Remarks:
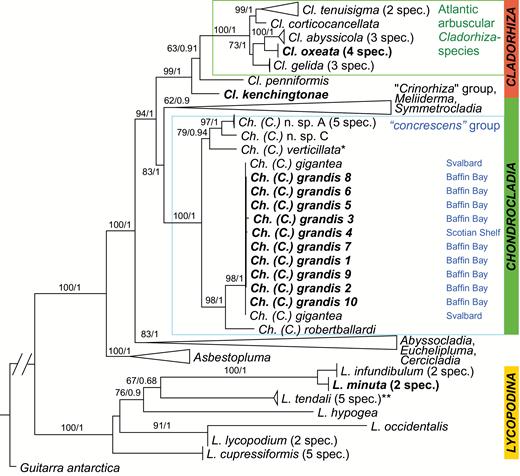
Genus Chondrocladia is diagnosed as Cladorhizidae with anchorate isochelae and currently contains the subgenera Chondrocladia, Meliiderma Ridley & Dendy, 1887 and Symmetrocladia Lee et al., 2012. Only subgenus Chondrocladia is present in the North Atlantic and Arctic. Molecular evidence (Hestetun et al., 2016b) suggests that subgenus Chondrocladia is polyphyletic with regards to the two other subgenera in the genus roughly corresponding to the informal grouping into ‘concrescens’ and ‘Crinorhiza’ type morphologies (e.g. Ridley & Dendy, 1886; Topsent, 1930; Tendal, 1973), the former being stem- or club-shaped while species of the latter type are typically stalked with a spherical or subspherical body. The two species treated here belong to the ‘concrescens’ type.
Chondrocladia (Chondrocladia) Grandis (Verrill, 1879) (Figs 10, 11; Table 2)
Original description:
Cladorhiza grandis Verrill, 1879: 204.
Spicule measurements of Chondrocladia (Chondrocladia) grandis, including average for all examined specimens, regional averages for the NW Atlantic and GIN Seas, as well as measurements of selected single specimens
| Mycalostyles I | Mycalostyles II | Acantho- subtylostyles | Anchorate isochelae I | Anchorate isochelae II | Sigmancistras | |
| C. (C.) grandis lectotype USNM 8462 | 1257-1960-2458 × 27-33-39 | 860-1025-1188 × 17-20-22 | 202-249-312 | 68-72-75 | 24-26-30 | 39-44-46 |
| C. (C.) grandis paralectotype YPM 6987 | 1210-1881-2543 × 15-26-37 | 650-980-1200 × 11-16-19 | 209-270-435 | 66-70-75 | 19-23-28 | 35-41-47 |
| C. (C.) gigantea paralectotype ZMBN 121 | 1203-1677-2439 × 14-20-35 | 693-938-1186 × 12-16-21 | 166-216-263 | 66-70-76 | 19-22-25 | 42-48-59 |
| NW Atlantic | 1210-1801-2543 × 15-29-50 | 309-934-1360 × 7-18-31 | 190-252-435 | 52-70-81 | 16-23-34 | 31-42-58 |
| NE Atlantic/GIN Seas | 1203-1718-2439 × 14-24-42 | 563-883-1190 × 9-16-28 | 129-213-263 | 52-67-78 | 17-23-33 | 36-45-59 |
| Total | 1203-1785-2543 × 14-28-50 | 309-919-1360 × 7-18-31 | 129-246-435 | 52-69-81 | 16-23-34 | 31-42-59 |
| Mycalostyles I | Mycalostyles II | Acantho- subtylostyles | Anchorate isochelae I | Anchorate isochelae II | Sigmancistras | |
| C. (C.) grandis lectotype USNM 8462 | 1257-1960-2458 × 27-33-39 | 860-1025-1188 × 17-20-22 | 202-249-312 | 68-72-75 | 24-26-30 | 39-44-46 |
| C. (C.) grandis paralectotype YPM 6987 | 1210-1881-2543 × 15-26-37 | 650-980-1200 × 11-16-19 | 209-270-435 | 66-70-75 | 19-23-28 | 35-41-47 |
| C. (C.) gigantea paralectotype ZMBN 121 | 1203-1677-2439 × 14-20-35 | 693-938-1186 × 12-16-21 | 166-216-263 | 66-70-76 | 19-22-25 | 42-48-59 |
| NW Atlantic | 1210-1801-2543 × 15-29-50 | 309-934-1360 × 7-18-31 | 190-252-435 | 52-70-81 | 16-23-34 | 31-42-58 |
| NE Atlantic/GIN Seas | 1203-1718-2439 × 14-24-42 | 563-883-1190 × 9-16-28 | 129-213-263 | 52-67-78 | 17-23-33 | 36-45-59 |
| Total | 1203-1785-2543 × 14-28-50 | 309-919-1360 × 7-18-31 | 129-246-435 | 52-69-81 | 16-23-34 | 31-42-59 |
Spicule measurements of Chondrocladia (Chondrocladia) grandis, including average for all examined specimens, regional averages for the NW Atlantic and GIN Seas, as well as measurements of selected single specimens
| Mycalostyles I | Mycalostyles II | Acantho- subtylostyles | Anchorate isochelae I | Anchorate isochelae II | Sigmancistras | |
| C. (C.) grandis lectotype USNM 8462 | 1257-1960-2458 × 27-33-39 | 860-1025-1188 × 17-20-22 | 202-249-312 | 68-72-75 | 24-26-30 | 39-44-46 |
| C. (C.) grandis paralectotype YPM 6987 | 1210-1881-2543 × 15-26-37 | 650-980-1200 × 11-16-19 | 209-270-435 | 66-70-75 | 19-23-28 | 35-41-47 |
| C. (C.) gigantea paralectotype ZMBN 121 | 1203-1677-2439 × 14-20-35 | 693-938-1186 × 12-16-21 | 166-216-263 | 66-70-76 | 19-22-25 | 42-48-59 |
| NW Atlantic | 1210-1801-2543 × 15-29-50 | 309-934-1360 × 7-18-31 | 190-252-435 | 52-70-81 | 16-23-34 | 31-42-58 |
| NE Atlantic/GIN Seas | 1203-1718-2439 × 14-24-42 | 563-883-1190 × 9-16-28 | 129-213-263 | 52-67-78 | 17-23-33 | 36-45-59 |
| Total | 1203-1785-2543 × 14-28-50 | 309-919-1360 × 7-18-31 | 129-246-435 | 52-69-81 | 16-23-34 | 31-42-59 |
| Mycalostyles I | Mycalostyles II | Acantho- subtylostyles | Anchorate isochelae I | Anchorate isochelae II | Sigmancistras | |
| C. (C.) grandis lectotype USNM 8462 | 1257-1960-2458 × 27-33-39 | 860-1025-1188 × 17-20-22 | 202-249-312 | 68-72-75 | 24-26-30 | 39-44-46 |
| C. (C.) grandis paralectotype YPM 6987 | 1210-1881-2543 × 15-26-37 | 650-980-1200 × 11-16-19 | 209-270-435 | 66-70-75 | 19-23-28 | 35-41-47 |
| C. (C.) gigantea paralectotype ZMBN 121 | 1203-1677-2439 × 14-20-35 | 693-938-1186 × 12-16-21 | 166-216-263 | 66-70-76 | 19-22-25 | 42-48-59 |
| NW Atlantic | 1210-1801-2543 × 15-29-50 | 309-934-1360 × 7-18-31 | 190-252-435 | 52-70-81 | 16-23-34 | 31-42-58 |
| NE Atlantic/GIN Seas | 1203-1718-2439 × 14-24-42 | 563-883-1190 × 9-16-28 | 129-213-263 | 52-67-78 | 17-23-33 | 36-45-59 |
| Total | 1203-1785-2543 × 14-28-50 | 309-919-1360 × 7-18-31 | 129-246-435 | 52-69-81 | 16-23-34 | 31-42-59 |
A map of reported collection localities of Chondrocladia (Chondrocladia) grandis, C. (C.) virgata, C. (C.) robertballardi and Cladorhiza abyssicola.
Habit of Chondrocladia (C.) grandis. (A) Chondrocladia (C.) grandis lectotype USNM8463, (B) C. (C.) grandis paralectotype YPM6987, (C) C. (C.) gigantea paralectotype ZMBN 121, (D) in situ habit of specimen HUD2007-025-1058-32, (E) specimen HUD2007-025-1058-32, (F) specimen YPM 6874, (G) specimen NTNU 15204.
Chondrocladia (C.) grandis. (A) Cross section of upper stem, (B) cross section of process, (C) longitudinal section of process including swelling, (D) Mycalostyle I, (E) mycalostyle II, (F) acanthostyle, (G, H) anchorate isochela I, (I) anchorate isochela II, (J) anchorate isochela II detail, (K) sigmancistra.
Synonyms and citations:
Desmacidon arcticum, D. clavatum, D. giganteum, D. nucleus Hansen, 1885: 14; Cladorhiza nobilis Fristedt, 1887: 456; Chondrocladia gigantea (Lundbeck, 1905: 102; Topsent, 1913: 48; Hentschel, 1929: 936; Burton, 1930: 492; Topsent, 1930: 28; Brøndsted, 1933: 11; Koltun, 1959: 83; Tendal & Barthel, 1993: 12; Tendal, Barthel & Tabachnick, 1993: 12; Tendal & Sahling, 1997: 16; Kübler & Barthel, 1999: 290); C. concrescens in part (Koltun, 1970a: 190). Not: C. gigantea (Boury-Esnault, Pansini & Uriz, 1994: 100).
Type material examined:
Lectotype: Sch. ‘Wachusett’, USNM 8462 (Gloucester fisheries, 1879, Western Bank, 43°17ʹN, 060°58ʹW, 329 m). Paralectotypes: Gloucester Fisheries, Sch. ‘Marion’, YPM 6987 (1879, Banquereau, 375–550 m); the Norwegian North-Atlantic Expedition, st. 48 (6 August 1876, 64°36ʹN, 010°22ʹW, 547 m), st. 57, st. 58 ZMBN 121, st. 137 (21 June 1877, 67°24ʹN, 008°58ʹE, 827 m).
Other material examined:
Gloucester Fisheries 1879–1880, Sch. ‘Albatross’ 1884, the Danish Ingolf Expedition, M/S ‘Michael Sars’ 1902, M/S ‘Thor’ 1903, ‘Tjalfe’ Expedition, Godthaab Expedition, ‘Sotra’, R/V ‘Johan Ruud’, BIOFAR, R/V ‘Paamiut’ 2004–2013, EU Flemish Cap 2007, CCGS ‘Hudson’ 2007, LAR2012 (see Supporting information).
Comparative material examined:
Chondrocladia (Chondrocladia) verticillata Topsent, 1920 (USNM 975, 31180).
Diagnosis:
Erect, club-shaped sponge with branched root, basal stem and elongated upper part with numerous secondary processes terminating in inflatable spherical structures. Megascleres two categories of mycalostyles and acanthostyles. Microscleres anchorate unguiferous isochelae of two size classes with six and six to nine teeth, respectively, and sigmancistras.
Description:
Large, erect, club-shaped sponge with well-developed branching root-like structure, basal stem and elongated, enlarged upper part set with numerous short or branch-like processes projecting in all directions from the stem. May reach a length of at least 60 cm (Tendal & Barthel, 1993). Basal stem part is usually partly covered in sediment giving a slightly coarse, dark brown appearance, while the upper part is lighter in colour, with a slightly larger diameter. Surface slightly hispid. Processes typically 1–7 cm long and 2–4 mm in diameter, sometimes reduced to wart-like knobs, and terminate in translucent inflatable spheres that deflate during collection. Spheres are maintained by a remnant aquiferous system with canals inside the main stem and are used both for prey capture and reproduction (Kübler & Barthel, 1999). Colour of the upper part is white to very lightly brown in situ or in freshly collected specimens and yellowish to light brown in ethanol.
Numerous specimens of this species were examined, including two specimens specifically mentioned by Verrill (1879): USNM 8462, here designated as the lectotype, 18.5 cm tall with almost all of the root missing; an 8.5 cm long and 1 cm in diameter stem part and a 10 cm long and 1.5–2 cm in diameter top part torn off at the apex. Branches have detached showing insertion points into the main body (Fig. 10A). The specimen is in a fragile state, with the outer layer starting to detach from the stem core. Paralectotype YPM 6987 a 10.5-cm-tall specimen with an incomplete root system, a 6 cm long and around 1.5 cm in diameter stem part, and a 4.5-cm-long and 2 cm in diameter damaged upper part. Several branches have detached from the specimen after preservation (Fig. 10B). The types of Desmacidon giganteum, D. arcticum, D. Clavatum and D. nucleus erected by Hansen (1885) and later synonymized as Chondrocladia gigantea by Lundbeck were also re-examined. Paralectotype ZMBN 121, corresponding to Hansen (1885) Pl. VII, Fig. 8, is re-described here: The specimen is 22 cm tall. Lower stem 11 cm long and 1 cm in diameter; upper part is 11 cm long and 2 cm in diameter. Projections are shorter, almost wart-like, and spaced farther apart than in the examined specimens from the NW Atlantic (Fig. 10C). In situ images were obtained of a specimen collected on the Nova Scotia shelf by the 2007 CCGS ‘Hudson’ cruise: 14.5 cm tall, with a 7.5-cm-long and 1 cm in diameter stem part and a 7-cm-long and 1.5 cm in diameter upper part. The root system, with rhizoids up to 10 cm in length, was recovered (Fig. 10D, E). Two additional specimens, YPM6874 (NW Atlantic) and NTNU15204 (Svalbard), are also pictured (Fig. 10F, G).
Skeleton:
Main stem consists of a central, dense core of closely packed bundles of mycalostyles wound in a rope-like spiral pattern around the axis that becomes less pronounced in the upper part of the stem. Around the central core is a clearly separated outer tissue layer supported by mycalostyles and containing numerous isochelae. The stem and outer layer are loosely connected with lacunose soft tissue containing canals and choanocyte chambers. The outer layer of the basal stem and roots contains acanthosubtylostyles mixed with a fine layer of sediment.
Processes are composed of a rigid central stem composed of overlapping mycalostyles, and a tough outer layer of longitudinally arranged mycalostyles with a lacunose layer of less dense tissue in between. This middle layer contains a radial spoke-like supporting skeleton except in the swelling, where mycalostyles are fewer and more confusedly arranged, allowing inflation. The central stem goes through the inflatable swelling and terminates the process in a small brush-like point. Longitudinal canals are found in both the internal, less dense tissue and in the outer skeleton. Mycalostyles are set perpendicular to the longitudinally arranged mycalostyles in the outer layer and project slightly from the surface. Both types of isochela are present, with large isochelae most common at the surface. Bundles of sigmas are found in the swelling (Fig. 11A–C).
Spicules:
Mycalostyles I, fusiform, straight or slightly curving, 1203-1785-2543 µm long, 13.9-28.0-50.4 µm wide, length partly overlapping mycalostyles II. Especially common in the branches rather than in central stem (Fig. 11D).
Mycalostyles II, fusiform, most often slightly curving, 309-919-1360 µm long, 6.6-17.7-30.9 µm wide. Present in all parts of sponge with no clear size distinction between different parts of sponge. Larger forms overlap with mycalostyles I (Fig. 11E).
Acanthostyles to subtylostyles, curved, 129-246-435 µm long, 1–3 µm wide, found in the lower stem cover tissue and in the roots only. With a slight tyle in some spicules (Fig. 11F).
Anchorate isochelae I, present in most parts of the sponge, but especially abundant in the inflatable spheres, with six quite long teeth, 51.9-69.1-81.5 µm long (Fig. 11G, H).
Anchorate isochelae II, not quite as common as anchorate isochelae I, and slightly more variable in size. Typically stout, with six to nine short teeth, 16.1-23.1-34.0 µm long (Fig. 11I, J).
Sigmancistras I, common in inflatable spheres, uncommon in rest of branch and otherwise absent from stem covering tissue or central core. Thin, most often twisted, 31.4-42.4-59.2 µm long (Fig. 11K).
Sigmancistras II, rare, only found in a few specimens (‘Sotra’ specimen, YPM 6986, a few in YPM 6533, 6875). These are seemingly associated with loose tissue surrounding the stem core, but it was not possible to associate them with any special structures such as spermatic cysts or embryos, 20.4-22.7-26.7 µm long. Possibly contamination.
Distribution:
Chondrocladia (C.) grandis has a boreo-Arctic distribution and has been reported from the coast of Norway, the GIN Seas, Svalbard and both sides of Greenland (Hansen, 1885; Lundbeck, 1905; Brøndsted, 1933; Tendal & Barthel, 1993) in addition to the shelf off New England, Nova Scotia and Newfoundland (Fig. 9). Besides records given here, video transects from the Norwegian MAREANO mapping project have confirmed that it is abundant at many localities on the Norwegian Shelf. It has also been reported at two localities in the Okhotsk Sea and Kuril Islands by Koltun (1959), who reports that specimens collected at the lower part of the species depth range seemingly have a shorter main body, longer stem and longer branches than that of the usual morphology. As Koltun did not specifically describe the specimens collected in the Northern Pacific and as he later synonymized several species considered valid today (Koltun, 1970a), the Pacific records are possibly a different species and should be regarded with some caution. A short bodied C. (C.) grandis was collected at 755 m during a R/V ‘Paamiut’ 2010 Arctic trawl, but this does not represent a particularly deep record. Previous records also exist for similarly shaped specimens (Kübler & Barthel, 1999). The reported temperature range is –1.1°C (Hansen, 1885) up to at least 2.5 °C, possibly 3.1 °C (Lundbeck, 1905). While a couple of records give a depth of 2127 m (Hansen, 1885) and 1960 m (Lundbeck, 1905), a more reliable estimate of the known bathymetric distribution is 240–1600 m (Tendal & Barthel, 1993), with the shallowest of Verrill’s specimens collected at 183 m.
The distribution of this species is discontinuous between the Davis Strait and the Flemish Cap and the bottom temperatures of the shelf off New England, Nova Scotia and Newfoundland are slightly higher than previously reported for C. (C.) gigantea. However, a similar amphi-Atlantic distribution pattern has previously been reported for several deep-sea species of Geodia and Thenea (Cárdenas & Rapp, 2015; Cárdenas et al., 2013). Additional records are expected for the Newfoundland and Labrador region in the near future from extensive trawled deep sea sponge collections maintained by Fisheries and Oceans Canada Newfoundland and Labrador Region.
Remarks:
The decision to synonymize Chondrocladia (C.) grandis and C. (C.) gigantea is based on the indistinguishable spicule complement (Table 2), morphological similarity, and virtually identical molecular sequences (~1200 bp partial COI and 848 bp partial 28S C1-D2 rDNA completely identical; two silent third codon mutations in 934 bp partial ALG11).
Verrill’s original description of this species, as Cladorhiza grandis, mentions that he had many specimens available collected by fishermen mainly from the banks off Nova Scotia. Most of these specimens have been re-examined here. Two specimens (YPM 6987, USNM 8462) were mentioned individually by Verrill (1879), and they, rather than the whole set of specimens, should be considered syntypes for the species, and USNM 8462 has been designated the lectotype here. The description of C. (C.) grandis was provided with no mention of spicule characters (Verrill, 1879; Verrill, 1885) making it virtually a nomen nudum. Thus, the species was re-described under the name Desmacidon giganteum Hansen, 1885, and C. (C.) gigantea has been used by subsequent authors for describing specimens of this species. However, the possible synonymy between C. (C.) grandis and C. (C.) gigantea was recognized by Lundbeck (1905) and Topsent (1930). As C. (C.) grandis has been generally recognized as a separate species of Chondrocladia in the literature, it does not fulfil the criteria for nomen oblitum, and this name should take precedence over the later name C. (C.) gigantea.
Specimens from the shelf off New England and Nova Scotia, where Verrill obtained his C. (C.) grandis material, have long, closely set branches, and the examined specimens are usually around 10–20 cm tall. In contrast, the morphology of many of the GIN Seas and Arctic specimens previously referred to as C. (C.) gigantea have a tendency towards slightly more swollen or club-like upper stems with shorter, almost wart-like branches set some distance apart and a size in some cases exceeding 50 cm in length, although others are more similar to the NW Atlantic shelf specimens or have an intermediate shape. In certain cases, smaller (8–13 cm) specimens have a long stem and short, spherical body. While this could point to some intraspecific variation between populations of this species, another possibility is that it represents morphological plasticity due to slightly different conditions such as higher bottom temperatures, reproductive state (more swollen specimen often contain numerous embryos) or simply nonrepresentative sampling.
Chondrocladia (C.) grandis belongs to a group of Chondrocladia species sometimes informally referred to as the ‘concrescens’ group based on morphological similarities with C. (C.) concrescens (Schmidt, 1880). Typical features include an elongated main body/branch bearing part, special spiny stem-coating spicules and branches featuring terminal or subterminal inflatable spheres (Topsent, 1930; Tendal, 1973; Lévi, 1993). The Caribbean species C. (C.) concrescens (Schmidt, 1880) and C. (C.) verticillataTopsent, 1920 are closely related to C. (C.) grandis. While there are several differences in habit and spiculation, C. (C.) verticillata can most easily be distinguished from C. (C.) grandis by its smaller sigmas, whereas C. (C.) concrescens has larger anisochelae than C. (C.) grandis. Chondrocladia (C.) virgata Thomson, 1873 can be distinguished from C. (C.) grandis by the fact that this species is bifurcated and that the branches have subterminal rather than terminal swellings, as well as differences in the spicule complement.
Chondrocladia (Chondrocladia) virgata Thomson, 1873 (Figs 12, 13; Table 3)
Original description:
Chondrocladia virgata Thomson, 1873: 187.
Chondrocladia (C.) virgata. (A) Chondrocladia (C.) virgata holotype BMNH 82.7.28.97, (B) C. (C.) michaelsarsi syntypes ZMBN 25639–641.
Chondrocladia (C.) virgata holotype BMNH 82.7.28.97: (A) Mycalostyle I, (B) mycalostyle II, (C) acanthostyle, (D) anchorate isochelae. Chondrocladia (C.) michaelsarsi syntype ZMBN25639: (E) anchorate isochela, (F) sigmancistra.
A map of reported collection localities of Cladorhiza arctica, C. kenchingtonae, C. oxeata and C. tenuisigma. Other Cladorhiza species (see Fig. 15) are indicated with smaller grey dots.
A map of reported collection localities of Cladorhiza gelida, C. corticocancellata and C. iniquidentata. Other Cladorhiza species (see Fig. 14) are indicated with smaller grey dots.
Spicule measurements of Chondrocladia (Chondrocladia) virgata with synonyms and C. (C.) robertballardi
| Specimen | Mycalostyles I | Mycalostyles II | Acanthostyles | Anchorate isochelae | Sigmancistras |
| C. (C.) virgata (Carter, 1874) | 1550 | 68 | 25 | ||
| C. (C.) virgata BMNH 82.7.28.97 (holotype)a | 1200-1562-2307 × 21-28-35 | 515-800-1190 × 15-22-33 | 249-278-299 | 56-63-68 | Not found (but Carter reports them from same spec.) |
| C. (C.) michaelsarsi (Topsent, 1930) | Mentioned | Mentioned | Mentioned | 57-60 | 35-45 |
| C. (C.) michaelsarsi ZMBN 25639 (syntype)a | 1259-1776-2296 × 20-27-32 | 580-769-1100 × 14-21-30 | 257-311-395 | 53-59-64 | 35-40-44 |
| C. (C.) gigantea (Boury-Esnault et al., 1994) | 495-1418-2308 × 8-19-34 | 150-174-300 | 48-57-76 | 22-38-40 | |
| C.robertballardi (Cristobo et al., 2015) (all spec.) | 528-1160-2181 × 6-22-37 | 53-65-70 |
| Specimen | Mycalostyles I | Mycalostyles II | Acanthostyles | Anchorate isochelae | Sigmancistras |
| C. (C.) virgata (Carter, 1874) | 1550 | 68 | 25 | ||
| C. (C.) virgata BMNH 82.7.28.97 (holotype)a | 1200-1562-2307 × 21-28-35 | 515-800-1190 × 15-22-33 | 249-278-299 | 56-63-68 | Not found (but Carter reports them from same spec.) |
| C. (C.) michaelsarsi (Topsent, 1930) | Mentioned | Mentioned | Mentioned | 57-60 | 35-45 |
| C. (C.) michaelsarsi ZMBN 25639 (syntype)a | 1259-1776-2296 × 20-27-32 | 580-769-1100 × 14-21-30 | 257-311-395 | 53-59-64 | 35-40-44 |
| C. (C.) gigantea (Boury-Esnault et al., 1994) | 495-1418-2308 × 8-19-34 | 150-174-300 | 48-57-76 | 22-38-40 | |
| C.robertballardi (Cristobo et al., 2015) (all spec.) | 528-1160-2181 × 6-22-37 | 53-65-70 |
aMeasurements based on re-examination of specimen.
Spicule measurements of Chondrocladia (Chondrocladia) virgata with synonyms and C. (C.) robertballardi
| Specimen | Mycalostyles I | Mycalostyles II | Acanthostyles | Anchorate isochelae | Sigmancistras |
| C. (C.) virgata (Carter, 1874) | 1550 | 68 | 25 | ||
| C. (C.) virgata BMNH 82.7.28.97 (holotype)a | 1200-1562-2307 × 21-28-35 | 515-800-1190 × 15-22-33 | 249-278-299 | 56-63-68 | Not found (but Carter reports them from same spec.) |
| C. (C.) michaelsarsi (Topsent, 1930) | Mentioned | Mentioned | Mentioned | 57-60 | 35-45 |
| C. (C.) michaelsarsi ZMBN 25639 (syntype)a | 1259-1776-2296 × 20-27-32 | 580-769-1100 × 14-21-30 | 257-311-395 | 53-59-64 | 35-40-44 |
| C. (C.) gigantea (Boury-Esnault et al., 1994) | 495-1418-2308 × 8-19-34 | 150-174-300 | 48-57-76 | 22-38-40 | |
| C.robertballardi (Cristobo et al., 2015) (all spec.) | 528-1160-2181 × 6-22-37 | 53-65-70 |
| Specimen | Mycalostyles I | Mycalostyles II | Acanthostyles | Anchorate isochelae | Sigmancistras |
| C. (C.) virgata (Carter, 1874) | 1550 | 68 | 25 | ||
| C. (C.) virgata BMNH 82.7.28.97 (holotype)a | 1200-1562-2307 × 21-28-35 | 515-800-1190 × 15-22-33 | 249-278-299 | 56-63-68 | Not found (but Carter reports them from same spec.) |
| C. (C.) michaelsarsi (Topsent, 1930) | Mentioned | Mentioned | Mentioned | 57-60 | 35-45 |
| C. (C.) michaelsarsi ZMBN 25639 (syntype)a | 1259-1776-2296 × 20-27-32 | 580-769-1100 × 14-21-30 | 257-311-395 | 53-59-64 | 35-40-44 |
| C. (C.) gigantea (Boury-Esnault et al., 1994) | 495-1418-2308 × 8-19-34 | 150-174-300 | 48-57-76 | 22-38-40 | |
| C.robertballardi (Cristobo et al., 2015) (all spec.) | 528-1160-2181 × 6-22-37 | 53-65-70 |
aMeasurements based on re-examination of specimen.
Synonyms and citations:
Chondrocladia virgata (Carter, 1874: 217); C.michaelsarsi Arnesen, 1920: 15 (Topsent, 1930: 429); C.gigantea (Boury-Esnault et al., 1994: 100).
Type material examined:
Holotype: HMS ‘Porcupine’, BMNH 82.7.28.97 (summer 1870, Gibraltar Strait, St. 31, 35°56ʹN, 007°06ʹW, 870 m). Syntypes (C. (C.) michaelsarsi): ‘Michael Sars’, st. 23, ZMBN 25639 (5 May 1910, 35°32ʹN, 007°07ʹW, 1215 m), st. 35, ZMBN 25640 (10 May 1910, 27°27ʹN, 014°52ʹW, 2603 m), st. 41, ZMBN 25641 (23 May 1910, 28°08ʹN, 013°35ʹW, 1365 m).
Other material examined:
BALGIM st. CP 91-I1 (1984, 34°22.3ʹN, 007°25.1ʹW, 945 m), st. CP 97-57 (1984, 34°25.4ʹN, 007°41.1ʹW, 1498–1532 m).
Comparative material examined:
Chondrocladia (Chondrocladia) verticillata Topsent, 1920 (USNM 975, 31180).
Diagnosis:
Erect, large sponge with branched root, short lower stem and elongated, dichotomously branching main upper part with large number of branch processes projecting in all directions. Processes with inflatable spherical structures halfway along their length. Megascleres two categories of mycalostyles and acanthostyles. Microscleres one size class of anchorate unguiferous isochelae and scattered sigmancistras.
Description:
Large, stalked, 20–50 cm tall, with a main, cylindrical, rigid stem 4–20 mm in diameter divided into a short basal part partly covered with sediment, and an elongated upper part covered with numerous perpendicularly arranged secondary branches or processes. The upper part may split dichotomously into two to three continuations of the stem. The processes on the upper parts are in various stages of articulation, between 3 and 40 mm long and up to 2 mm in diameter at the base, each with a central inflatable sphere and pointed end. Processes tightly set, arranged in irregular whorls and in some cases anastomosing. Longitudinal or spiral trenches or tunnels containing polynoid polychaetes are frequently found. The colour varies from white to light greyish brown in live specimens to a slightly darker shade of greyish or greenish brown in older specimens in ethanol, with exposed parts of the stem core a darker brown (Fig. 12).
Skeleton:
Main, branching stem consisting of a central core made up of thick bundles of mycalostyles. Bundles are spirally arranged in the lower main stem, becoming less twisted and more parallel in the upper stem and branches. Stem core covered by a clearly separated lighter outer tissue layer consisting of more confusedly arranged mycalostyles as well as containing anchorate isochelae. In the basal stem, this layer also contains the acanthostyles in addition to mycalostyles and anchorate isochelae and is covered in a fine layer of sediment.
Spicules:
Mycalostyles I, straight and fusiform, 1200-1646-2321 µm long, 17.2-27.5-35.5 µm wide (Fig. 13A).
Mycalostyles II, straight and fusiform, 505-800-1277 µm long, 14.1-22.0-33.0 µm wide (Fig. 13B).
Acanthostyles, 249-307-395 µm long, 1–3 µm wide (Fig. 13C).
Arcuate isochelae, with six to eight teeth and short fimbriae, in the body tissue and covering the filaments, 51.1-62.1-71.4 µm (Fig. 13D, E, G, H).
Sigmancistras, thin, typically contort, found in secondary branches, 31.2-40.4-45.8 µm (Fig. 13F, I).
The two types of mycalostyles overlap in size, and a cut-off-point of around 1200 µm was decided from a histogram of length frequencies of the megascleres.
Distribution:
Chondrocladia (C.) virgata has been collected from the Ibero-Moroccan Gulf to the Canary Islands (27°–36°N, 007°–015°W), with a reported depth range of 872–2603 m (Fig. 9). If C. (C.) robertballardi proves to be the same species (see remarks), the geographic range also includes the Galicia and Gorringe Banks (Cristobo et al., 2015). Bottom temperatures are given for two of the collection localities as 10.3 °C (Thomson, 1873) and 10.2 °C (Arnesen, 1920).
Remarks:
Chondrocladia (Chondrocladia) virgata, the type species of the genus, has been recovered several times from the same general area: the Ibero-Moroccan Gulf to the Canary Islands. However, owing to several reasons, it has been subsequently misidentified: Firstly, the original figure given of C. (C.) virgata in Thomson (1873) (Fig. 36, p188) is an idealized, not entirely accurate representation of the morphology of the sponge. Secondly, as noted by Lundbeck (1905), Carter (1874) incorrectly attributed C. (C.) virgata to a locality south of the Faroe Islands rather than its actual collection locality off Gibraltar. Thirdly, Arnesen (1920) made erroneous measurements of the spicules from the specimens collected by the ‘Michael Sars’ 1910 expedition and based the description of C. (C.) michaelsarsi on these incorrect measurements, a fact also pointed out by Topsent (1930). The specimens used for the C. (C.) michaelsarsi and Cladorhiza gelida descriptions in Arnesen (1920) were re-examined for this article, and measurements for both species are in most cases too large by a factor of between 1.3 and 2. Thus, any measurements given in that work should be used with caution. Finally, no figures were provided of either the habit or the spicules of C. (C.) michaelsarsi in the original work, and it is only by the re-examination of the types of both C. (C.) virgata and C. (C.) michaelsarsi that their identity could be established.
The lack of complete species descriptions for C. (C.) virgata and C. (C.) michaelsarsi led to the C. (C.) virgata specimens collected during the 1984 ‘Balgim’ expedition to be identified as C. (C.) gigantea (Boury-Esnault et al., 1994). It is also possible that the recently described species C. (C.) robertballardi Cristobo et al., 2015 is actually C. (C.) virgata, although as a complete specimen of that species has not been re-examined for this article, C. (C.) robertballardi is considered valid for the time being. Compared to the closely related boreal species C. (C.) grandis, C. (C.) virgata can be distinguished by its longer, branching and more slender stem and by the fact that the inflatable spheres are centrally located on the secondary branch processes rather than terminally. Furthermore, C. (C.) virgata has only one type of anchorate chela and one type of sigmancistra.
Genus Cladorhiza Sars, 1872
Synonymy:
Cladorhiza Sars, 1872: 65; Trochoderma Ridley & Dendy, 1886: 344 (preoccupied); Axoniderma Ridley & Dendy, 1886: 493; Exaxinata de Laubenfels, 1936: 122; Raoa de Laubenfels, 1936: 123.
Diagnosis:
Cladorhizidae with only anchorate/unguiferate anisochelae (from Lopes & Hajdu, 2014).
Type species:
Cladorhiza abyssicola Sars, 1872 (by monotypy).
Remarks:
Genus Cladorhiza is defined as cladorhizids with anchorate anisochelae. Most of the Cladorhiza species from the Northern North Atlantic and Arctic are closely related arbuscular forms similar to the type species of the genus, Cladorhiza abyssicola Sars, 1872. Many of these were originally described by Lundbeck (1905), most of whose material has been re-examined here. The two exceptions are the small, pedunculate species C. arctica (Koltun, 1959) and the curious long, threadlike C. kenchingtonae sp. nov.
Cladorhiza abyssicola Sars, 1872 (Figs 16, 17; Table 4)
Original description:
Cladorhiza abyssicola Sars, 1872: 65.
Cladorhiza abyssicola. (A, B) Two in situ images of Skagerrak specimens from BIOSKAG III, (C) specimen ‘Dannevig’ 1952 (st. 59, Lindesnes), (D) specimen ZMBN 103470, (E) specimen NEREIDA 0509 BC34 (Flemish Cap).
Cladorhiza abyssicola. (A) Specimen ‘Armauer Hansen’ 1917, (st. 3, Sognefjord), (B) branch detail of specimen Caracole T-875.2 (Rockall Bank), (C) ZMBN 103470 cross section, (D) ZMBN 103470 longitudinal section, (E) mycalostyle, (F–H) anchorate chela front, side and back view, (I) sigma, (J) sigmancistra.
Spicule size comparison between Cladorhiza abyssicola specimens from the GIN Seas and North Sea, and the Rockall Bank
| Mycalostyles | Anchorate chelae | Sigmas | Sigmancistras | |
| Norw. Sea, North Sea, Skagerrak | 310-549-930 × 5.2-13.6-25.1 | 18.2-22.2-28.6 | 65.0-92.3-127.4 | 34.5-40.5-52.8 |
| Rockall Bank | 270-353-430 × 7.5-10.7-14.1 | 15.0-17.2-22.0 | 55.0-66.4-81.6 | 31.4-38.7-44.0 |
| Mycalostyles | Anchorate chelae | Sigmas | Sigmancistras | |
| Norw. Sea, North Sea, Skagerrak | 310-549-930 × 5.2-13.6-25.1 | 18.2-22.2-28.6 | 65.0-92.3-127.4 | 34.5-40.5-52.8 |
| Rockall Bank | 270-353-430 × 7.5-10.7-14.1 | 15.0-17.2-22.0 | 55.0-66.4-81.6 | 31.4-38.7-44.0 |
Spicule size comparison between Cladorhiza abyssicola specimens from the GIN Seas and North Sea, and the Rockall Bank
| Mycalostyles | Anchorate chelae | Sigmas | Sigmancistras | |
| Norw. Sea, North Sea, Skagerrak | 310-549-930 × 5.2-13.6-25.1 | 18.2-22.2-28.6 | 65.0-92.3-127.4 | 34.5-40.5-52.8 |
| Rockall Bank | 270-353-430 × 7.5-10.7-14.1 | 15.0-17.2-22.0 | 55.0-66.4-81.6 | 31.4-38.7-44.0 |
| Mycalostyles | Anchorate chelae | Sigmas | Sigmancistras | |
| Norw. Sea, North Sea, Skagerrak | 310-549-930 × 5.2-13.6-25.1 | 18.2-22.2-28.6 | 65.0-92.3-127.4 | 34.5-40.5-52.8 |
| Rockall Bank | 270-353-430 × 7.5-10.7-14.1 | 15.0-17.2-22.0 | 55.0-66.4-81.6 | 31.4-38.7-44.0 |
Synonyms and citations:
Cladorhiza abyssicola (Schmidt, 1875: 119; Lambe, 1896: 188; Lundbeck, 1905: 79; Topsent, 1909: 2; Babić, 1922: 263; Hentschel, 1929: 935; Burton, 1930: 492; Vacelet, 1969: 191; van Soest, 1993: 210; Boury-Esnault et al., 1994: 101; Brunel et al., 1998: 61; van Soest et al., 2007: 130; Hestetun et al., 2015: 1330). Uncertain: C. abyssicola (von Marenzeller, 1878: 358, 371; Verrill, 1885: 531; Fristedt, 1887: 455). These early records are without spicule measurements and could be other Cladorhiza spp. Not: C. abyssicola (Thomson, 1873: 112; Carter, 1876: 319) (is actually C. gelida, see description of that species here) (Hansen, 1885: 16) (cf. Lundbeck, 1905).
Material examined:
The Norwegian North-Atlantic Expedition, the Danish Ingolf Expedition, M/S ‘Michael Sars’ 1902, M/S ‘Armauer Hansen’ 1917, M/S ‘Dannevig’ 1952, DEPRO96, BIOICE, CARACOLE, BIOSYS/HERMES 2005, BIOSKAG III, NEREIDA 0509, LAR2012-002 (see Supporting information).
Comparative material examined:
C. methanophila Vacelet & Boury-Esnault, 2002, ‘Atlantis’ AT 21-02, J633-5.
Diagnosis:
Erect Cladorhiza with arbuscular morphology consisting of branching central stem with numerous side branches in several planes directed outwards and slightly upwards. Stem and branches covered in a layer of softer tissue containing a large number of filaments projecting in all directions. Branch ends often slightly swollen. Lower stem connected to the substrate with rhizoids. Megascleres mycalostyles; microscleres one type of chela c. 15–29 µm, sigmas c. 55–127 µm and sigmancistras c. 35–52 µm.
Description:
Arbuscular species consisting of a central stem, branching in larger specimens, with side branches in multiple planes directed outwards and often slightly upwards. Specimens up to 15 cm tall with the main stem and branches 1–3 mm wide. Branches normally terminate in small swellings. Usually with a somewhat finer morphology than other closely related Cladorhiza species. Filaments typically 1–2 mm long, and project in all directions from the stem and branches. Lower stem is bare and connects to the substrate with a number of threadlike rhizoid processes. The colour in ethanol is whitish grey to light beige (Figs 16A–E, 17A, B).
Skeleton:
Skeleton of the main stem and branches composed of spicule bundles forming a solid core surrounded by looser tissue at the surface. Skeleton of each filament originating in the centre of the main skeleton and made up of overlapping mycalostyles with a slight, softer surface layer (Fig. 17C, D).
Spicules:
Mycalostyles, straight or slightly curved, fusiform, 270-486-930 µm long, 5.2-12.6-25.1 µm wide. In most specimens in the range of 300–600 µm while in a couple of specimens up to around 900 µm (Fig. 17E).
Anchorate anisochelae, numerous, with a curved shaft, five teeth in each end and fimbriae in the upper end. The upper end is about a fourth of the total length 15.0-20.5-28.6 µm (Fig. 17F–H).
Sigmas, common, solid, not contorted, 55.0-84.2-127.4 µm (Fig. 17I).
Sigmancistras, uncommon, usually found in the branch ends, 31.4-40.4-52.8 µm (Fig. 17J).
Distribution:
This species has a wide distribution including the Eastern Coast of Canada, the Norwegian Sea, the coast and fjords of Norway, the North Sea, Skagerrak, Rockall Bank, Ibero-Moroccan Gulf, Madeira, Azores, Cape Verde and the Mediterranean (Fig. 9). Most records in the northern range of the distribution are less than or around 1000 m. However, some northern and most southern specimens have been collected at greater depths (1500–2500 m).
Related species:
C. corticocancellata Carter, 1876; C. gelida Lundbeck, 1905; C. iniquidentata Lundbeck, 1905; C. methanophila Vacelet & Boury-Esnault, 2002; C. oxeata Lundbeck, 1905; C. tenuisigma Lundbeck, 1905.
Remarks:
Cladorhiza abyssicola is the type species of genus Cladorhiza and the earliest described cladorhizid (Sars, 1872). We were unable to find the type specimen of this species; however, it is possible that it is located as an uncatalogued specimen at the Natural History Museum in Oslo. The Cladorhiza species in the North Atlantic and Arctic are all closely related, which can make identification difficult. This is especially the case with C. gelida, from which C. abyssicola can be distinguished by its multiple-plane branching pattern, finer habit and smaller chelae, secondarily by slightly smaller sigma and sigmancistra sizes, as well as distribution (C. gelida is typically found deeper (> 1000 m) and is more common in the Arctic).
Cladorhiza abyssicola has the largest known distribution of all Cladorhiza spp. treated here, from the Arctic and Norwegian Sea to the Mediterranean and Eastern Mid-Atlantic. However, some early records (von Marenzeller, 1878; Verrill, 1885; Fristedt, 1887) previous to the examination of the genus by Lundbeck (1905) might be other species. Four specimens were examined from the Rockall Bank: two from the collections of Naturalis Biodiversity Center included in the phylogeny of Hestetun et al. (2016b) and two previously described in Hestetun et al. (2015). These were smaller in size, with smaller spicule sizes (Table 4). Phylogenetic results place them within C. Abyssicola, however (Hestetun et al., 2016b).
Cladorhiza arctica Koltun, 1959 (Fig. 18)
Original description:
Cladorhiza arctica Koltun, 1959: 79.
Synonyms and citations:
Cladorhiza arctica (Gorbunov, 1946: 37) (nomen nudum); C. arctica (Koltun, 1964: 163, 1970b: 289).
Diagnosis:
Small, pedunculate arctic Cladorhiza with two categories of mycalostyle, acanthoxeas and anchorate anisochelae.
Description:
No specimens were re-examined for this article, so the following description is based on Koltun (1959): Pedunculate, up to 4 cm tall sponge with body tapering downwards into a slender stem fastened to the substrate with root-like structures. The upper body of the sponge has a few projections or filaments. Colour light beige to yellow (Fig. 18A, B).
Cladorhiza arctica, redrawn from Koltun (1959) Pl. V, 4–5 and fig. 34. (A, B) Habit of two specimens, (C) mycalostyle, (D) acanthoxea, (E) anchorate anisochela.
Skeleton:
Skeleton composed of longitudinally arranged mycalostyles in the stem, which spread, following the tapering surface, into the body of the sponge.
Spicules:
Mycalostyles I, straight, fusiform, 728–1508 µm long, 12–36 µm wide (from Koltun, 1959) (Fig. 18C).
Mycalostyles II/strongyles, slightly curved, fusiform, 364–873 µm long, 6–11 µm wide. In most specimens, they are in the range of 300–600 µm while in a couple of specimens they are up to around 900 µm (from Koltun, 1959).
Acanthoxeas, nodose, 135–239 µm long, 6–8 µm wide (from Koltun, 1959) (Fig. 18D).
Anchorate anisochelae, tridentate, 35–40 µm (from Koltun, 1959) (Fig. 18E).
Distribution:
Gorbunov (1946): St. 59, 82°42ʹN, 087°03ʹE, 2365 m. Koltun (1959): Kara Sea near Ushakov Island, central Arctic Ocean (Fig. 14). Depth 2040–2365 m.
Related species:
C. longipinna Ridley & Dendy, 1886; C. corona Lehnert, Watling & Stone, 2005; C. bathycrinoides Koltun, 1955.
Remarks:
The first mention of this species is in Gorbunov (1946) as a sp. nov. identified by Burton. However, this source is an overview of distribution only, and C. arctica remained a nomen nudum until described by Koltun (1959). While Koltun refers to the species as C. arctica Gorbunov, 1946, the correct authority is Koltun, 1959.
With the exception of Cladorhiza kenchingtonae sp. nov., the other Cladorhiza species in the area are arbuscular, and all, including C. kenchingtonae, have anisochelae with five teeth (or more in the case of C. iniquidentata). Cladorhiza arctica seems more related to a group of pedunculate Cladorhiza species with tridentate anisochelae including, for instance, the North Pacific species C. longipinna Ridley & Dendy, 1886, C. corona Lehnert, Watling & Stone, 2005 and C. bathycrinoides Koltun, 1955; however, none of which have acanthoxeas. Even though it shares acanthoxeas with C. kenchingtonae, other characters suggest that these two species are not closely related. The tridentate, pedunculate Cladorhiza species tend to be more commonly found at abyssal and lower bathyal depths (~2000–5000 m), and the reported depth of 2040–2365 m and negative temperature (–0.86°C) of C. arctica is in line with this.
Cladorhiza corticocancellata Carter, 1876 (Figs 19, 20)
Original description:
Cladorhiza abyssicola var. corticocancellata Carter, 1876: 320.
Cladorhiza corticocancellata. (A) Specimen ZMBN 103471 in situ view, (B, C) specimen GeoBio 2011 ROV 6-1, (D) specimen ZMBN 103471, (E) fragments of paralectotype BMNH 82.7.28.67.
Cladorhiza corticocancellata. (A) Branch tip of paralectotype BMNH 82.7.28.67, (B) ZMBN 103471 cross section, (C) ZMBN 103471 longitudinal section of branch tip, (D) mycalostyle, (E, F) anchorate chela front and side view, (G) sigma, (H) sigmancistra.
Synonyms and citations:
Cladorhiza abyssicola in part (Hansen, 1885: 16); C. abyssicola var. corticocancellata in part (Arnesen, 1903: 12); C. corticocancellata (Lundbeck, 1905: 93; Burton, 1934: 9, 24; Koltun, 1964: 163).
Type material examined:
HMS ‘Porcupine’ 1869. Lectotype: BMNH 82.7.28.19, st. 52 (west of Shetland, 60°25ʹN, 008°10ʹW, 702 m). Paralectotypes: BMNH 82.7.28.67 and BMNH 82.7.28.82, st. 57 (60°14ʹN, 006°17ʹW, 1156 m).
Other material examined:
The Danish Ingolf Expedition, M/S ‘Michael Sars’ 1900–1902, M/S ‘Thor’ 1903, BIOICE, R/V ‘G.O. Sars’ 2008–155, GeoBio 2009–2011 (see Supporting information).
Comparative material examined:
C. methanophila Vacelet & Boury-Esnault, 2002, ‘Atlantis’ AT 21-02, J633-5.
Diagnosis:
Erect Cladorhiza with a branching morphology. Stem covered in a thick layer of soft tissue containing a large number of partly coalesced filaments creating a characteristic surface with numerous cavities. Megascleres mycalostyles; microscleres one type of chela c. 25–38 µm, characteristic sigmas with points turned outwards c. 112–182 µm and sigmancistras c. 47–96 µm.
Description:
Compared to closely related species, this is a large and solid Cladorhiza species. The examined specimens are branching, up to 20 cm in length, with branches consisting of a hard, central stem covered in a thick outer layer with a very characteristic lacunose surface stemming from partly coalesced filaments, which are very numerous and issue in all directions. The branches are typically in the range of 5–10 mm in diameter, but in some cases coalesce into thicker structures. The species is connected to the substrate with an amorphous growth or plate. The soft outer layer tends to disassociate from the hard, central stem after storage in ethanol. The colour in ethanol is whitish grey to light beige (Figs 19A–E, 20A).
Skeleton:
The skeleton of the stem core is made up of mycalostyle spicule bundles forming a solid core surrounded by looser tissue at the surface. The skeleton of the filaments originate in the centre of the main skeleton and is made up of tightly packed mycalostyles, with a slight, softer outer layer (Fig. 20B, C).
Spicules:
Mycalostyles, straight or slightly curved, fusiform, 475-670-799 µm long, 9.4-17.5-25.1 µm wide (Fig. 20D).
Anchorate anisochelae, numerous, with a curved shaft, five teeth in each end and fimbriae in the upper end. The upper end is about 20% of the total length, 25.3-32.7-37.7 µm (Fig. 20E, F).
Sigmas, common, solid, not contorted. Compared to closely related species the ends are more sharply angled, with sharper points and points pointed slightly outwards, 112-155-182 µm (Fig. 20G).
Sigmancistras, uncommon, usually found in the branch ends, 46.6-61.3-95.5 µm (Fig. 20H).
Distribution:
This species has been reported from the shelf around Iceland, off Norway, on the Arctic Mid-Ocean Ridge and on the Greenland Shelf (Fig. 15). Typical depth is 600–1200 m, but there is also one record each from 171 to 2387 m, respectively.
Related species:
Cladorhiza abyssicola Sars, 1872; C.gelida Lundbeck, 1905; C.iniquidentata Lundbeck, 1905; C. methanophila Vacelet & Boury-Esnault, 2002; C. oxeata Lundbeck, 1905; C. tenuisigma Lundbeck, 1905.
Remarks:
Cladorhiza corticocancellata is related to the other arbuscular Cladorhiza species treated in this article. From its morphology, it can most easily be recognized by the characteristic lacunose, partly coalesced surface. From its spicule complement, it can be recognized by the comparatively large anchorate anisochelae and by the morphology of the sigmas, in which the ends are sharply pointed and project partly outwards from the centre of the spicule.
Cladorhiza gelida Lundbeck, 1905 (Figs 21, 22)
Original description:
Cladorhiza gelida Lundbeck, 1905: 65.
Cladorhiza gelida. (A) Specimen ZMBN 103472 in situ view, (B) specimen GeoBio ROV 8-1, (C) specimen ZMBN 103472, (D, E) detail of specimen ZMBN 103472.
Cladorhiza gelida. (A) ZMBN 103472 cross section, (B) ZMBN 103472 longitudinal section, (C) mycalostyle, (D–F) anchorate chela front, side and back view, (G) sigma, (H) sigmancistra.
Synonyms and citations:
Cladorhiza abyssicola (Thomson, 1873: 112; Carter, 1876: 319). Cladorhiza abyssicola in part (Hansen, 1885: 16). Cladorhiza abyssicola var. corticocancellata in part (Arnesen, 1903: 12). Cladorhiza gelida (Lundbeck, 1905: 83; Topsent, 1909: 6, 1913: 48; Arnesen, 1920: 25; Hentschel, 1929: 935; Burton, 1930: 492; Koltun, 1959: 81, 1964: 152, 163; Barthel & Tendal, 1993: 8, 90). Possibly: C. aff. gelida (Hestetun et al., 2015).
Type material examined:
Lectotype: The Norwegian North-Atlantic Expedition, st. 35 (5 July 1876, 63°17ʹN, 001°27ʹW, 1977 m). Paralectotypes: ZMUC-DEM-31, the Danish Ingolf Expedition st. 113 (21 July 1896, 69°31ʹN, 007°06ʹW, 2465 m); ZMUC-DEM-283, M/S ‘Michael Sars’ 1902 (10 August 1902, 60°19ʹN, 005°39ʹE, 1169 m).
Other material examined:
The Norwegian North-Atlantic Expedition, Sch. ‘Albatross’ 1884, M/S ‘Michael Sars’ 1910, NORBI, YMER80, BIOICE, R/V ‘Polarstern’ Ark X-31-9, GeoBio 2008-2012, Faranaut.
Comparative material examined:
Cladorhiza methanophila Vacelet & Boury-Esnault, 2002, ‘Atlantis’ AT 21-02, J633-5.
Diagnosis:
Erect Cladorhiza with arbuscular morphology consisting of one or several main stems with numerous branches pointing outwards and slightly upwards. Stem and branches covered in a layer of softer tissue containing a large number of filaments projecting in all directions. Lower stem connected to the substrate with a small basal plate or with root-like processes. Megascleres mycalostyles; microscleres one type of anchorate anisochela c. 23–42 µm, sigmas c. 86–174 µm and sigmancistras c. 38–86 µm.
Description:
Specimens arbuscular, up to 25 cm tall with main stem and branches 2–4 mm wide. Branches mostly oriented in one plane from the main stem, but often quite irregular. Filaments project in all directions from the stem and branches, typically 2 mm long, but can reach over 5 mm. In specimens with more than one main stem, the stems diverge close to the base, which is connected to the substrate with a plate-like basal structure made up of a small number of solid root-like processes. Morphology similar to that of C. abyssicola, but generally larger and more solid. Colour white to light beige in ethanol (Fig. 21A–E).
Skeleton:
Skeleton of main stem and branches composed of mycalostyle spicule bundles forming a solid core surrounded by looser tissue at the surface. Filament skeleton originates in the centre of the main skeleton and is made up of tightly packed mycalostyles, with a slight, softer outer layer (Fig. 22A, B).
Spicules:
Mycalostyles, straight or slightly curved, fusiform, 380-622-1000 µm long, 7.0-16.6-25.1 µm wide (Fig. 22C).
Anchorate anisochelae, numerous, with a curved shaft, five teeth in each end and fimbriae in the upper end. The upper end is about a fifth of the total length, 22.8-31.2-42.3 µm (Fig. 22D–F).
Sigmas, common, solid, not contorted, 86-139-174 µm (Fig. 22G).
Sigmancistras, uncommon, usually found in the branch ends, 37.7-52.3-85.8 µm (Fig. 22H).
Distribution:
Most records of this species are in the GIN Seas and Faroe Ridge at 1000–2500 m, with a couple of scattered reports from shallower waters off Norway (Fig. 15). A specimen collected by the ‘Albatross’ on the lower shelf of the Eastern coast of the US (3740 m) was also identified as this species, and a small specimen with a similar spicule complement has been reported from further south on the Mid-Atlantic Ridge (3436 m) as C. aff. gelida (Hestetun et al., 2015). These are not easily distinguishable from other specimens, but both have slightly longer mycalostyles than usual for the GIN Seas. Either one or both of these records represent different, indistinguishable species, or they represent a southern continuation of the range of C. gelida at lower bathyal to abyssal depths, giving this species a wide depth range.
Related species:
Cladorhiza abyssicola Sars, 1872; C. iniquidentata Lundbeck, 1905; C. methanophila Vacelet & Boury-Esnault, 2002; C. oxeata Lundbeck, 1905; C. tenuisigma Lundbeck, 1905.
Remarks:
This species is closely related to Cladorhiza abyssicola, but it usually has a thicker stem and branches, and the swellings at the branch ends are usually less clear, which together with its often larger size gives it an overall more solid appearance. It is generally found in deeper waters than C. abyssicola, and while it is reported as shallow as 300 m off the Norwegian coast, the typical collection locality is at a depth of 1000–2500 m. Furthermore, it seems to have a more northern distribution than C. abyssicola and has been reported from Jan Mayen and the Polar Basin. The records given as C. abyssicola by von Marenzeller (1878) from Jan Mayen could very well be this species. From its spicule complement, it can be distinguished from C. abyssicola most easily by its larger anisochelae and sigmas. Other closely related species are C. tenuisigma, in which the sigmas are much smaller, C. corticocancellata, where the sigmas have a special pointed morphology, C. iniquidentata, in which the anisochelae have seven rather than five teeth and C. oxeata, which has oxeas rather than mycalostyles as megascleres.
Cladorhiza Iniquidentata Lundbeck, 1905 (Fig. 23)
Original description:
Cladorhiza iniquidentata Lundbeck, 1905: 91.
Type material examined:
Holotype: M/S ‘Michael Sars’ 1902 (29 August 1902, 63°13ʹN, 006°32ʹW, 1836 m).
Comparative material examined:
Cladorhiza methanophila Vacelet & Boury-Esnault, 2002, ‘Atlantis’ AT 21-02, J633-5.
Diagnosis:
Erect Cladorhiza with central axis and side branches covered in a layer of softer tissue with filaments projecting in all directions. Megascleres mycalostyles; microscleres one type of chela with seven teeth in the upper end, 20–29 µm.
Description:
Holotype a stem fragment 43 mm long and 1–2 mm wide with four side branches in the same plane, on the same side of the specimen. Side branches up to 8 mm long and possibly damaged. Stem and branches consist of a central core covered in a looser surface layer and carry numerous filaments issuing in all directions. Colour in ethanol white to light beige (Fig. 23A).
Cladorhiza iniquidentata. (A) Holotype, (B) mycalostyle, (C, D) anchorate chela side and back view.
Skeleton:
The central skeleton of the stem and branches is composed of bundles of longitudinally arranged mycalostyles. The skeleton of each filament is made up of overlapping mycalostyles, anchored into the main stem.
Spicules:
Mycalostyles, straight or slightly curved, fusiform, 487-600-688 µm long, 18.9-22.4-26.2 µm wide (Fig. 23B).
Anchorate anisochelae, numerous, with a curved shaft, seven teeth and fimbriae in the upper end and five teeth in the lower end. The upper end is about 20% of the total length, 19.6-23.6-28.8 µm (Fig. 23C, D).
Distribution:
The holotype location is on the Iceland-Faroe Ridge (Fig. 15).
Related species:
Cladorhiza abyssicola Sars, 1872; C. gelida Lundbeck, 1905; C. methanophila Vacelet & Boury-Esnault, 2002; C. oxeata Lundbeck, 1905; C. tenuisigma Lundbeck, 1905.
Remarks:
This species is known only from the holotype. It is similar to C. abyssicola, C. tenuisigma and C. gelida, but can be distinguished by the fact that the anchorate anisochelae have seven rather than five teeth in the larger, upper end. Unusually, the holotype also lacks sigmas and sigmancistras.
Cladorhiza Kenchingtonae sp. nov. (Figs 24, 25)
urn:lsid:zoobank.org:act:E9AB8B52-AAD8-4478- BC49-685D74132D2B
Type material:
Holotype: CMNI 2016-0014, CCGS ‘Hudson’ cruise HUD2010-029 (13 July 2010, The Flemish Cap, ROPOS dive 1336-16, 46°19.03ʹN, 044°33.46ʹW, 2935 m). The holotype was recovered using the ROPOS ROV system.
Cladorhiza kenchingtonae sp. nov. (A) In situ habit, (B) branching point, (C) branch detail, (D) recovered specimen fragment, (E, F) specimen parts, (G) stem and filaments detail.
Cladorhiza kenchingtonae sp. nov. (A) Longitudinal section showing stem and filament, (B) cross section showing stem and two filaments, (C) longitudinal section of the distal part of the recovered fragment, (D) mycalostyle, (E) style, (F) acanthoxea, (G, H) anchorate anisochela side and back views, (I) sigmancistra microscope picture.
Diagnosis:
Erect, fine, arching Cladorhiza with long, thin branches carrying filamentous projections on one side of main axis only. Megascleres mycalostyles, styles and acanthoxeas. Microscleres five-toothed anchorate anisochelae.
Description:
Large and very fine, c. 2-m-long erect, arching sponge consisting of c. 1-m-long and 5-mm-wide stem branching off into three around 1-m-long and < 5-mm-wide fine branches (Fig. 24A, B). Each branch supported by a main axis with filaments up to several centimetre long set into two rows angled c. 90° to each other and facing downwards at oblique angles (Fig. 24C). A couple of planktonic crustaceans were observed stuck to filaments. Recovered part consists of a branch fragment 16.5 cm long and 1.2–3.5 mm wide. Tissue is markedly concentrated on one side of axis only, between filaments and filament rows. Filaments are reduced or retracted into two series of distally oriented short projections (Fig. 24D, E). Filaments become more reduced towards the distal end of the collected fragment and become slightly offset with clockwise torsion. Colour white freshly collected and off-white in ethanol.
Skeleton:
The main axis consists of closely packed mycalostyles. The skeleton of the filaments are inserted into the main stem in an alternating pattern forming two oblique rows. The main part of the tissue, containing scattered mycalostyles and closely spaced microscleres, is found on the underside of the main stem and at the base of filaments. A thin surface layer, which appears to be continuous with the main tissue, also envelopes the entire branch, including the main stem, and is densely packed with chelae with their free ends pointing outwards. Small prey items were observed adhered to or partly embedded in the outer layer. The acanthoxeas are interspersed with chelae in the main and outer layer of tissue and are especially numerous at the base of the filaments (Fig. 25A–C).
Spicules:
Styles to mycalostyles, straight and more or less fusiform, rarely with tylote swellings, 262-1369-2381 µm long, 14.5-26.6-42.0 µm (Fig. 25D, E). The length distribution is continuous, however smaller styles are typically less fusiform and sometimes bent near the middle.
Acanthoxeas, with large spines, 139-175-218 µm long, 11.6-20.1-26.5 µm wide (5.8-9.0-11.0 µm without spines) (Fig. 25F).
Anchorate anisochelae, with five teeth and fimbriae on the larger end about twice as long as the teeth, in the body tissue and covering the filaments, 28.1-31.5-35.2 µm long, 2.4-3.0-3.6 µm wide (Fig. 25G, H).
Sigmancistras, slightly contorted, 29.9-33.4-36.0 µm long by 2 µm; uncommon (Fig. 25I).
Distribution:
This species is known only from the holotype locality on the lower southern slope of the Flemish Cap, north-east of the Grand Banks, a fish rich relatively shallow submarine plateau with rocky slopes extending into much deeper water. The specimen was collected at 2935 m depth (Fig. 24).
Etymology:
Named after Dr. Ellen Kenchington at Fisheries and Oceans Canada to recognize her many contributions to Arctic and North Atlantic deep sea benthic ecology, biodiversity monitoring and protection. Her expertise on Vulnerable Marine Ecosystems and Sensitive Benthic Areas, her work delineating deep-sea habitat and her participation in expert national and international panels have led to establishment of over a dozen closures to bottom fishing, protecting important benthic habitats in the high seas. Dr. Kenchington was the chief scientist of the benthic monitoring survey in which the specimen was collected.
Remarks:
Other Cladorhiza species known from the North Atlantic and Arctic are C. abyssicola Sars, 1872, C. corticocancellata Lundbeck, 1905, C. gelida Lundbeck, 1905, C. iniquidentata Lundbeck, 1905, C. oxeata Lundbeck, 1905 and C. tenuisigma Lundbeck, 1905. These are all closely related to each other, with an arbuscular morphology set with numerous filaments, lacking mycalostyles longer than 1 mm, and can thus easily be distinguished from C. kenchingtonae.
The presence of acanthoxeas is unusual in the genus, known only from C. arctica Koltun, 1959 and C. acanthoxea Hestetun et al., 2015 from the continental shelf off Angola. In the case of C. arctica, C. kenchingtonae can be distinguished by the small, clavate habit, tridentate anisochelae and less developed acanthoxeas of C. arctica. Cladorhiza acanthoxea is the currently known species most closely resembling C. kenchingtonae, but can be distinguished from the new species by having more than two rows of filaments projecting in all directions from the main axis, geographical distance, as well as differences in spicule size.
Cladorhiza oxeata Lundbeck, 1905 (Figs 26, 27)
Original description:
Cladorhiza oxeata Lundbeck, 1905: 97.
Cladorhiza oxeata. (A) In situ image from the Canadian ArcticNet project, (B) lectotype ZMUC-DEM-79, (C) specimen ‘Paamiut’ 2010-009-147.
Cladorhiza oxeata. (A) Specimen ‘Paamiut’ 2013-008-57, (B) paralectotype ZMUC-DEM-295, (C) specimen ‘Paamiut’ 2010-009-75, (D) detail of ‘Paamiut’ specimen. Spicules from lectotype ZMUC-DEM-79: (E) oxea, (F, G) anchorate chela side and back view, (H) sigma, (I) sigmancistra.
Synonyms and citations:
Cladorhiza abyssicola in part (Hansen, 1885: 16); C. abyssicola (Fristedt, 1887: 455); C. oxeata (Hentschel, 1929: 935).
Type material examined:
The Danish Ingolf Expedition. Lectotype: ZMUC-DEM-79, st. 15 (4 June 1895, 66°18ʹN, 025°59ʹW, 621 m). Paralectotypes: ZMUC-DEM-295, st. 143 (11 August 1896, 62°58ʹN, 007°09ʹW, 731 m), st. 3 (12 May 1896, 63°35ʹN, 010°24ʹW, 512 m); ‘Wandel’ Expedition, ZMUC-DEM-296 (19 September 1891, 66°57ʹN, 027°00ʹW, 633 m).
Other material examined:
‘Ingegerd’ & ‘Gladan’ Greenland Expedition 1870–1871, M/S ‘Michael Sars’ 1902, HMS ‘Porcupine’ 1869, M/S ‘Michael Sars’, ‘Tjalfe’ Expedition, Godthaab Expedition, Degerbøl Kangerlussuaq 1932, Heimland Expedition, Norw. Fisheries Investigations, BIOICE, R/V ‘Paamiut’ 2010–2014 (see Supporting information).
Comparative material examined:
Cladorhiza methanophila Vacelet & Boury-Esnault, 2002, ‘Atlantis’ AT 21-02, J633-5.
Diagnosis:
Erect Cladorhiza with arbuscular morphology consisting of a solid, branching main stem with side branches. Stem and branches set with large number of filaments. Megascleres oxeas; microscleres one type of chela c. 23–41 µm, sigmas c. 100–153 µm and sigmancistras, sometimes rare, c. 27–55 µm.
Description:
Large, erect, arbuscular Cladorhiza species, commonly over 30 cm tall, with thick branching main stem carrying numerous slightly thinner side branches. Stem typically 5–10 mm wide, thicker towards the base. Side branches 2–5 mm wide. Stem and branches covered in a thick softer, outer layer with 2- to 10-mm-long filaments issuing in all directions, but lower and central part of the stem often bare. The outer layer has a tendency to disassociate from the stem after storage in ethanol. Lower stem is bare and connects to the substrate with a basal plate. Colour white to beige, light brown, pink or reddish brown, with the stem a darker colour than the outer layer (Figs 26A–C, 27A–D).
Skeleton:
The core of the main stem and branches is composed of tightly connected spicule bundles, which creates a rigid, smooth central skeleton. This is surrounded by an outer layer of loose tissue which easily disassociates from the central skeleton.
Spicules:
Oxeas, straight or slightly curved, fusiform. They display some morphological variation, and it is often possible to see that one of the ends is slightly blunter than the other. Some are closer to styles, others blunt, to strongyles, 390-615-845 µm long, 4.9-22.0-38.9 µm wide (Fig. 27E).
Anchorate anisochelae, numerous, with a curved shaft, five teeth in each end and fimbriae in the upper end. The upper end is about a fourth of the total length, 22.6-33.3-42.0 µm (Fig. 27F, G).
Sigmas, common, solid, not contorted, 100-125-153 µm (Fig. 27H).
Sigmancistras, uncommon, sometimes absent, found in the branch ends, 26.7-43.4-55.0 µm (Fig. 27I).
Distribution:
This species has an amphi-Atlantic, boreo-Arctic distribution and has been reported from the Iceland-Faroe Ridge and between Iceland and Greenland. It also seems to be a common Cladorhiza species in the Davis Strait and Baffin Bay (Fig. 14). North American Arctic specimens were collected at 184–1433 m, while the depth range in the GIN Seas is approximately 100–800 m (Fig. 46).
Related species:
Cladorhiza abyssicola Sars, 1872; C.gelida Lundbeck, 1905; C.iniquidentata Lundbeck, 1905; C. methanophila Vacelet & Boury-Esnault, 2002; C. tenuisigma Lundbeck, 1905.
Remarks:
Cladorhiza oxeata is easily the largest of the North Atlantic and Arctic Cladorhiza species. Its large size, reddish hue, and the fact that megascleres are oxeas (blunt or style-like in some cases) instead of mycalostyles make it comparatively easy to distinguish from related species. Specimens from the GIN Seas and Baffin Bay/Davis Strait are similar in appearance and spicule characters, but no sigmancistras were observed in western Arctic specimens.
Cladorhiza Tenuisigma Lundbeck, 1905 (Fig. 28)
Original description:
Cladorhiza tenuisigmaLundbeck, 1905: 87.
Synonyms and citations:
Cladorhiza abyssicola in part (Hansen, 1885: 16); C. tenuisigma (Topsent, 1909: 7, 1913: 49; Hentschel, 1929: 935; Burton, 1930: 492; Koltun, 1959: 82, 1964: 163); C. cf. tenuisigma (Janussen, 2009: 50).
Type material examined:
Lectotype: ZMUC-DEM-161, the Danish Ingolf Expedition, st. 117 (24 July 1896, 69°13ʹN, 008°23ʹW, 1890 m), st. 105 (11 July 1896, 65°34ʹN, 007°31ʹW, 1435 m).
Other material examined:
The Danish Ingolf Expedition, R/V ‘Johan Ruud’, R/V ‘Polarstern’ Ark X-31-9, AB321/05, GeoBio 2009, IceAGE 2 (see Supporting information).
Comparative material examined:
Cladorhiza methanophila Vacelet & Boury-Esnault, 2002, ‘Atlantis’ AT 21-02, J633-5.
Diagnosis:
Erect Cladorhiza with arbuscular morphology consisting of branching stem with side branches. Stem and branches covered in a layer of softer tissue with filaments projecting in all directions. Branches typically end in a clearly visible swelling. Lower stem connected to the substrate with a root-like structure. Megascleres mycalostyles; microscleres one type of chela c. 16–32 µm, sigmas c. 31–54 µm and sigmancistras c. 42–58 µm.
Description:
Specimens erect, up to 15 cm in length, with branching main stem, typically 2–5 mm in diameter. Numerous side branches with the same thickness as the main stem, and both stem and branches enclosed in soft tissue covered with thin, 1- to 5-mm-long filaments in all directions (Fig. 28A, B). Branches usually end in a clearly visible swelling (Fig. 28C). Lower stem is bare and connects to the substrate with a number of rhizoid processes. Colour in ethanol whitish grey to light beige, with specimens kept in ethanol for a long time darker in colour, approaching reddish brown.
Skeleton:
Skeleton of main stem and branches composed of spicule bundles forming a solid core surrounded by looser tissue at the surface. Filament skeleton originates in the centre of the main stem skeleton and is made up of overlapping mycalostyles, with a slight, softer outer layer (Fig. 28D, E).
Spicules:
Mycalostyles, straight or slightly curved, fusiform, 430-704-918 µm long, 6.8-17.8-28.3 µm wide (Fig. 28F).
Anchorate anisochelae, numerous, with a curved shaft, five teeth in each end and fimbriae in the upper end. The upper end is about a fourth of the total length, 16.0-23.4-32.0 µm (Fig. 28G, H).
Sigmas, common, smaller than in related species, fine, most often not contorted, 31.4-41.7-53.6 µm (Fig. 28I).
Sigmancistras, uncommon, usually found in the swollen branch ends, 41.8-49.5-58.1 µm (Fig. 28J).
Distribution:
This species is distributed around the GIN Seas ranging from the Iceland-Faroe Ridge up to Svalbard (Fig. 14). Depth distribution of reported specimens is 900–1900 m, with two records from Svalbard at ~2700 m (Janussen, 2009).
Related species:
Cladorhiza abyssicola Sars, 1872; C. gelida Lundbeck, 1905; C.iniquidentata Lundbeck, 1905; C. methanophila Vacelet & Boury-Esnault, 2002; C. oxeata Lundbeck, 1905.
Remarks:
As indicated in the species names, the sigmas in this species are small and can easily be confused with the sigmancistras as they are roughly the same size. However, they can be recognized by the fact that they are typically not contort and are somewhat finer than the sigmancistras. The sigmas are significantly smaller than in other Cladorhiza species from the area, which together with the swollen branch ends are the clearest way of identifying this species.
Genus Lycopodina Lundbeck, 1905
Synonymy:
Lycopodina Lundbeck, 1905: 58, de Laubenfels, 1936: 122; Cotylina Lundbeck, 1905: 68.
Diagnosis:
Cladorhizidae pedunculate with body either in the form of an erect stem or sphere with filaments in all directions, or cup-shaped. Megascleres are mycalostyles and commonly shorter (tylo)styles. Microscleres are one type of arcuate or palmate anisochela where the smaller end is in the shape of a central plate and two rudimentary, flat, lateral teeth, all with serrated edges towards the middle. To this forceps spicules are often added, but may be rare or absent in particular species or specimens of a single species. Sigmas or sigmancistras are not present (from Hestetun et al., 2016b).
Type species:
Esperella cupressiformis var. lycopodium Levinsen, 1887 (by subsequent designation de Laubenfels, 1936).
Remarks:
Originally a subgenus of Asbestopluma erected by Lundbeck (1905) and raised to genus rank by de Laubenfels (1936), this genus was abandoned and more recently re-erected by Hestetun et al. (2016b) based on a combination of molecular and morphological results. It is the only cladorhizid genus that has forceps spicules. Sigmas or sigmancistras, present in all other cladorhizid genera, are absent.
The base spicule complement present in all Lycopodina species is megascleres such as styles, mycalostyles, subtylostyles, tylostyles or a combination, and palmate anisochelae. The morphology of the palmate anisochelae falls into two main categories, either similar to that of L. lycopodium (Levinsen, 1887), that is, clearly palmate with upper alae covering almost the whole shaft and a large, upper central tooth; or similar to L. infundibulum (Levinsen, 1887), that is arcuate to palmate, with the upper about half of the total length. In both cases, the lower end is modified into a characteristic set of a central raised and two lateral plates, each with one or several tips pointing upwards. This seems to be a further articulation of the similar structure common in the smaller anisochelae in Asbestopluma. Variations on this shape have been observed in a couple of species such as L. rastrichela (Hestetun et al., 2015).
The genus is challenging from a taxonomic perspective since several of the spicules associated with it are associated with reproductive structures and thus can be rare or absent in individual specimens. Conversely, published species descriptions may lack some of the spicules sometimes found in specimens of a particular species, and for many species in the genus, species descriptions only contain megascleres and anisochelae. Forceps spicules are associated with spermatic cysts (Riesgo, Taylor & Leys, 2007) and are often rare or absent. Similarly, embryos can contain distinct spicules, typically a combination of smaller megascleres such as oxeas, strongyles and tylostyles not found in the rest of the sponge, but also anisochelae of a similar shape and slightly smaller size, than in the rest of the sponge (e.g. Lundbeck, 1905).
Finally, there are sometimes very subtle differences between certain species of the genus, and different species can have a more or less indistinguishable spicule complement, for example L. lycopodium, L. occidentalis (Lambe, 1893), L. novangliae sp. nov. and L. tendali sp. nov. described here are all quite similar. For several species, such as the four cup-shaped species described in this article, and L. cupressiformis (Carter, 1874) and closely related species such as L. robusta (Levinsen, 1887) and L. ruijsi (van Soest, 2016), it is possible that the current accepted species are not accurate. However, increasing the amount of molecular data on this group may pertly remedy this.
Lycopodina comata (Lundbeck, 1905) (Fig. 31; Table 5)
Original description:
Asbestopluma comata Lundbeck, 1905: 72.
Comparison of reported spicule measurements of cup-shaped Lycopodina found in the northern Atlantic and Arctic and the North Pacific species L. infundibulum var. orientalis (Koltun, 1970)
| Mycalostyles/subtylostyles | Tapering, tortuous subtylostyles | Subtylostyles | Anisochelae | Forceps | Embryonic megascleres | Embryonic anisochelae | |
| L. infundibulum | |||||||
| (Levinsen, 1887) | 320-640 | 140-180 | 10-22 | Mentioned, crossed | |||
| (Lundbeck, 1905) | 170-830 × 6-11 | 440-600 × <7 | 149-220 × 6 | 18-27 | 75, crossed | Strongyles, styles, oxeas | 14 |
| (Hentschel, 1929) | 170-830 | 440-600 | 10-19 | 82, 104 crossed and not crossed | Styles, strongyles, 42-88 | ||
| (Boury-Esnault, 1994) | 168-1034 × 1-12 | 14-25 | 42-48, not crossed | Tylostyles, oxeas, 60-85 × 2-6 | |||
| (Hestetun et al., 2015) | 280-570 × 3-6 | 450-910 × 3-6 | 132-274 × 3-6 | 16-20 (25) | |||
| (van Soest, 2016) | 1000-1340 × 15-20, 300-600 × 5.5-20 | 300-600 | 155-420 × 2-5 | 16-23 | 65-70, crossed | ||
| This article | 171-445-675 × 4.7-7.4-9.3 | 371-463-561 × 2.3-4.7-7.0 | 133-263-466 × 4.7-5.7-9.3 | 16.3-20.4-23.3 | |||
| ‘var. orientalis’ (Koltun, 1970a) | 440-930 × 8-18 | 440-930 × 8-18 | 220-260 × 6-11 | 21-27 | 55-87, not crossed | Tylostyles, strongyles 88-121 × 5-8 | |
| L. comata | |||||||
| (Lundbeck, 1905) | 238-510 | 700-1400, polytylote | 149-230 | 17-19 | |||
| L. minuta | |||||||
| (Lambe, 1900b) | 327-548 × 6-8 | 196-294 × 5-6 | 14-21 | ||||
| This article | 271-425-565 × 6-7-10 and 197-328-442 × 4-6-8 (sub.) | 142-165-216 × 3.6-6.2-7.4 | 15.7-18.1-23.0 | Strongyles: 46-68-87 × 2-4-6 | 10.5-13.6-15.3 | ||
| L. versatilis (Topsent, 1890) | 580 | 27 | 76, not crossed | Oxeas, strongyles |
| Mycalostyles/subtylostyles | Tapering, tortuous subtylostyles | Subtylostyles | Anisochelae | Forceps | Embryonic megascleres | Embryonic anisochelae | |
| L. infundibulum | |||||||
| (Levinsen, 1887) | 320-640 | 140-180 | 10-22 | Mentioned, crossed | |||
| (Lundbeck, 1905) | 170-830 × 6-11 | 440-600 × <7 | 149-220 × 6 | 18-27 | 75, crossed | Strongyles, styles, oxeas | 14 |
| (Hentschel, 1929) | 170-830 | 440-600 | 10-19 | 82, 104 crossed and not crossed | Styles, strongyles, 42-88 | ||
| (Boury-Esnault, 1994) | 168-1034 × 1-12 | 14-25 | 42-48, not crossed | Tylostyles, oxeas, 60-85 × 2-6 | |||
| (Hestetun et al., 2015) | 280-570 × 3-6 | 450-910 × 3-6 | 132-274 × 3-6 | 16-20 (25) | |||
| (van Soest, 2016) | 1000-1340 × 15-20, 300-600 × 5.5-20 | 300-600 | 155-420 × 2-5 | 16-23 | 65-70, crossed | ||
| This article | 171-445-675 × 4.7-7.4-9.3 | 371-463-561 × 2.3-4.7-7.0 | 133-263-466 × 4.7-5.7-9.3 | 16.3-20.4-23.3 | |||
| ‘var. orientalis’ (Koltun, 1970a) | 440-930 × 8-18 | 440-930 × 8-18 | 220-260 × 6-11 | 21-27 | 55-87, not crossed | Tylostyles, strongyles 88-121 × 5-8 | |
| L. comata | |||||||
| (Lundbeck, 1905) | 238-510 | 700-1400, polytylote | 149-230 | 17-19 | |||
| L. minuta | |||||||
| (Lambe, 1900b) | 327-548 × 6-8 | 196-294 × 5-6 | 14-21 | ||||
| This article | 271-425-565 × 6-7-10 and 197-328-442 × 4-6-8 (sub.) | 142-165-216 × 3.6-6.2-7.4 | 15.7-18.1-23.0 | Strongyles: 46-68-87 × 2-4-6 | 10.5-13.6-15.3 | ||
| L. versatilis (Topsent, 1890) | 580 | 27 | 76, not crossed | Oxeas, strongyles |
Comparison of reported spicule measurements of cup-shaped Lycopodina found in the northern Atlantic and Arctic and the North Pacific species L. infundibulum var. orientalis (Koltun, 1970)
| Mycalostyles/subtylostyles | Tapering, tortuous subtylostyles | Subtylostyles | Anisochelae | Forceps | Embryonic megascleres | Embryonic anisochelae | |
| L. infundibulum | |||||||
| (Levinsen, 1887) | 320-640 | 140-180 | 10-22 | Mentioned, crossed | |||
| (Lundbeck, 1905) | 170-830 × 6-11 | 440-600 × <7 | 149-220 × 6 | 18-27 | 75, crossed | Strongyles, styles, oxeas | 14 |
| (Hentschel, 1929) | 170-830 | 440-600 | 10-19 | 82, 104 crossed and not crossed | Styles, strongyles, 42-88 | ||
| (Boury-Esnault, 1994) | 168-1034 × 1-12 | 14-25 | 42-48, not crossed | Tylostyles, oxeas, 60-85 × 2-6 | |||
| (Hestetun et al., 2015) | 280-570 × 3-6 | 450-910 × 3-6 | 132-274 × 3-6 | 16-20 (25) | |||
| (van Soest, 2016) | 1000-1340 × 15-20, 300-600 × 5.5-20 | 300-600 | 155-420 × 2-5 | 16-23 | 65-70, crossed | ||
| This article | 171-445-675 × 4.7-7.4-9.3 | 371-463-561 × 2.3-4.7-7.0 | 133-263-466 × 4.7-5.7-9.3 | 16.3-20.4-23.3 | |||
| ‘var. orientalis’ (Koltun, 1970a) | 440-930 × 8-18 | 440-930 × 8-18 | 220-260 × 6-11 | 21-27 | 55-87, not crossed | Tylostyles, strongyles 88-121 × 5-8 | |
| L. comata | |||||||
| (Lundbeck, 1905) | 238-510 | 700-1400, polytylote | 149-230 | 17-19 | |||
| L. minuta | |||||||
| (Lambe, 1900b) | 327-548 × 6-8 | 196-294 × 5-6 | 14-21 | ||||
| This article | 271-425-565 × 6-7-10 and 197-328-442 × 4-6-8 (sub.) | 142-165-216 × 3.6-6.2-7.4 | 15.7-18.1-23.0 | Strongyles: 46-68-87 × 2-4-6 | 10.5-13.6-15.3 | ||
| L. versatilis (Topsent, 1890) | 580 | 27 | 76, not crossed | Oxeas, strongyles |
| Mycalostyles/subtylostyles | Tapering, tortuous subtylostyles | Subtylostyles | Anisochelae | Forceps | Embryonic megascleres | Embryonic anisochelae | |
| L. infundibulum | |||||||
| (Levinsen, 1887) | 320-640 | 140-180 | 10-22 | Mentioned, crossed | |||
| (Lundbeck, 1905) | 170-830 × 6-11 | 440-600 × <7 | 149-220 × 6 | 18-27 | 75, crossed | Strongyles, styles, oxeas | 14 |
| (Hentschel, 1929) | 170-830 | 440-600 | 10-19 | 82, 104 crossed and not crossed | Styles, strongyles, 42-88 | ||
| (Boury-Esnault, 1994) | 168-1034 × 1-12 | 14-25 | 42-48, not crossed | Tylostyles, oxeas, 60-85 × 2-6 | |||
| (Hestetun et al., 2015) | 280-570 × 3-6 | 450-910 × 3-6 | 132-274 × 3-6 | 16-20 (25) | |||
| (van Soest, 2016) | 1000-1340 × 15-20, 300-600 × 5.5-20 | 300-600 | 155-420 × 2-5 | 16-23 | 65-70, crossed | ||
| This article | 171-445-675 × 4.7-7.4-9.3 | 371-463-561 × 2.3-4.7-7.0 | 133-263-466 × 4.7-5.7-9.3 | 16.3-20.4-23.3 | |||
| ‘var. orientalis’ (Koltun, 1970a) | 440-930 × 8-18 | 440-930 × 8-18 | 220-260 × 6-11 | 21-27 | 55-87, not crossed | Tylostyles, strongyles 88-121 × 5-8 | |
| L. comata | |||||||
| (Lundbeck, 1905) | 238-510 | 700-1400, polytylote | 149-230 | 17-19 | |||
| L. minuta | |||||||
| (Lambe, 1900b) | 327-548 × 6-8 | 196-294 × 5-6 | 14-21 | ||||
| This article | 271-425-565 × 6-7-10 and 197-328-442 × 4-6-8 (sub.) | 142-165-216 × 3.6-6.2-7.4 | 15.7-18.1-23.0 | Strongyles: 46-68-87 × 2-4-6 | 10.5-13.6-15.3 | ||
| L. versatilis (Topsent, 1890) | 580 | 27 | 76, not crossed | Oxeas, strongyles |
Type material examined:
Holotype: ZMUC-DEM-285, the Danish Ingolf Expedition, st. 78 (13 June 1896, 60°37ʹN, 029°52ʹW, 1504 m).
Diagnosis:
Minute, pedunculate, cup-shaped greyish white Lycopodina with peduncle mycalostyles, body subtylostyles and smaller subtylostyles and thin, polytylote, tapering subtylostyles. Microscleres arcuate to palmate anisochelae c. 17–19 µm.
Description:
The species is known only from the holotype and is a minute, pedunculate sponge with a cup-shaped body. Body 2 × 2 mm, laterally compressed, possibly during collection, and the upper margin folds slightly inwards. Peduncle approximately 8 mm tall and less than 100 µm wide. Basal part missing. Colour in ethanol light grey (Fig. 31A).
A map of reported collection localities of Lycopodina comata, L. cupressiformis, L. hydra, L. infundibulum, L. minuta, L. novangliae, L. robusta, L. ruijsi, and L. versatilis. The Mediterranean Lycopodina species L. hypogea (Vacelet & Boury-Esnault, 1996) and the abyssal species L. parvula (Hestetun et al., 2015) and L. rastrichela (Hestetun et al., 2015) are indicated with grey dots (see Chevaldonné et al., 2015; Hestetun et al., 2015).
A map of reported collection localities of Lycopodina lycopodium (Levinsen, 1887) and L. tendali sp. nov. Light grey dots are collection records where it is not certain whether the species collected is L. tendali or L. lycopodium.
Lycopodina comata (after Lundbeck, 1905, pl. XII fig. 1A–F). (A) Holotype ZMUC-DEM-285, (B) mycalostyles and subtylostyles I and II, (C) tapering subtylostyles, (D) arcuate/palmate chela front and side view.
Skeleton:
Peduncle composed of tightly packed mycalostyles which gradually fan out in the upper part into the sponge body. Skeleton of the sponge body composed of subtylostyles, with the smaller category associated with the upper margin of the sponge. The polytylote tapering styles project from the sponge body (Lundbeck, 1905).
Spicules:
Mycalostyles, straight and fusiform, in the peduncle, 440–510 µm long, 11–12 µm wide (from Lundbeck, 1905) (Fig. 31B).
Subtylostyles I, straight or curved, slightly fusiform, in the main body, 238–476 µm long, 7–8 µm wide (from Lundbeck, 1905) (Fig. 31B).
Subtylostyles II, straight and slightly fusiform, associated with the upper margin of the body, 149–230 µm long, 5–7 µm wide (from Lundbeck, 1905) (Fig. 31B).
Tapering subtylostyles, very thin, tapering, tortuous and polytylote, 700–1400 µm long, 5 µm wide (from Lundbeck, 1905) (Fig. 31C).
Arcuate to palmate anisochelae, with central tooth and lateral alae both approximately half the length of the spicule, with arched shaft and lower end modified into one central and two side plates with serrated edges, 17–19 µm (from Lundbeck, 1905) (Fig. 31D).
Distribution:
The species is known only from its type locality, on the Eastern slope of the Reykjanes Ridge at 1504 m depth (Fig. 29).
Related species:
Lycopodina infundibulum (Levinsen, 1887); L. minuta (Lambe, 1900).
Remarks:
While the holotype of Lycopodina comata was re-examined, no new spicule preparations were made due to the minute size of the only specimen. Thus, skeleton and spicule data are based on Lundbeck’s previous examination of the species. The species is a very close relative of L. infundibulum, mainly characterized from that species by differences in megasclere size, but especially by the polytylote nature of the tapering, tortuous category of subtylostyle.
Lycopodina cupressiformis (Carter, 1874) (Figs 32, 33; Table 6)
Original description:
Esperia cupressiformis Carter, 1874: 215.
Spicule comparison between Lycopodina cupressiformis, L. robusta and L. ruijsi
| Habit | Styles | Palmate anisochelae | Forceps spicules | Embryonic tylostyles | Embryonic anisochelae | |
| L. cupressiformis | ||||||
| (Carter, 1874) | Stylized fusiform w. filaments | 917 | 23 | 38 | ||
| (Lundbeck, 1905) | Most cylind. w. apical blade | 350-840 × 8-14 | 23-25 | 38-48, straight | up to 120 × 7 | 14 |
| Faroes and Rockall Bank specimens | Pinnate or clavate | 370-769-963 × 6.5-10.0-14.6 | 21.0-23.9-26.4 | 39.3-44.6-48.7, straight | 113-133-152 × 3.1 | 12.6-13.8-14.1 |
| Jan Mayen specimens | Cylind. w. apical blade | 456-675-810 × 4.7-10.3-15.7 | 21.0-25.0-28.3 | 36.1-40.6-47.1, straight | 66-110-152 × 1.6-3.6-6.3 | 12.6-14.5-17.3 |
| Total | 370-699-963 × 4.7-10.2-15.7 | 21.0-24.7-28.3 | 36.1-40.9-48.7, straight | 66-113-152 × 1.6-3.5-6.3 | 12.6-14.5-17.3 | |
| L. robusta | ||||||
| (Levinsen, 1887) | Cylindrical | 600-860 | 25 | 22 | ||
| (van Soest, 2016) | Cylindrical | 550-910 × 10-13 | 20-27 | 31-40 | Possibly present. | |
| Schultz/LC specimen (this article) | Cylindrical | 353-618-804 × 8.1-10.4-12.1 | 20.2-23.2-24.2 | 36.4-41.3-44.4 | 89-125-156 × 4.0-4.2-0.16 | 10.1-12.1-14.1 |
| L. ruijsi | ||||||
| (van Soest, 2016) | Cylindrical w. apical blade | 390-875 × 9-11 | 23-29 | 24-45 | 63-145 × 2.5-7 | 11-21 |
| Habit | Styles | Palmate anisochelae | Forceps spicules | Embryonic tylostyles | Embryonic anisochelae | |
| L. cupressiformis | ||||||
| (Carter, 1874) | Stylized fusiform w. filaments | 917 | 23 | 38 | ||
| (Lundbeck, 1905) | Most cylind. w. apical blade | 350-840 × 8-14 | 23-25 | 38-48, straight | up to 120 × 7 | 14 |
| Faroes and Rockall Bank specimens | Pinnate or clavate | 370-769-963 × 6.5-10.0-14.6 | 21.0-23.9-26.4 | 39.3-44.6-48.7, straight | 113-133-152 × 3.1 | 12.6-13.8-14.1 |
| Jan Mayen specimens | Cylind. w. apical blade | 456-675-810 × 4.7-10.3-15.7 | 21.0-25.0-28.3 | 36.1-40.6-47.1, straight | 66-110-152 × 1.6-3.6-6.3 | 12.6-14.5-17.3 |
| Total | 370-699-963 × 4.7-10.2-15.7 | 21.0-24.7-28.3 | 36.1-40.9-48.7, straight | 66-113-152 × 1.6-3.5-6.3 | 12.6-14.5-17.3 | |
| L. robusta | ||||||
| (Levinsen, 1887) | Cylindrical | 600-860 | 25 | 22 | ||
| (van Soest, 2016) | Cylindrical | 550-910 × 10-13 | 20-27 | 31-40 | Possibly present. | |
| Schultz/LC specimen (this article) | Cylindrical | 353-618-804 × 8.1-10.4-12.1 | 20.2-23.2-24.2 | 36.4-41.3-44.4 | 89-125-156 × 4.0-4.2-0.16 | 10.1-12.1-14.1 |
| L. ruijsi | ||||||
| (van Soest, 2016) | Cylindrical w. apical blade | 390-875 × 9-11 | 23-29 | 24-45 | 63-145 × 2.5-7 | 11-21 |
Spicule comparison between Lycopodina cupressiformis, L. robusta and L. ruijsi
| Habit | Styles | Palmate anisochelae | Forceps spicules | Embryonic tylostyles | Embryonic anisochelae | |
| L. cupressiformis | ||||||
| (Carter, 1874) | Stylized fusiform w. filaments | 917 | 23 | 38 | ||
| (Lundbeck, 1905) | Most cylind. w. apical blade | 350-840 × 8-14 | 23-25 | 38-48, straight | up to 120 × 7 | 14 |
| Faroes and Rockall Bank specimens | Pinnate or clavate | 370-769-963 × 6.5-10.0-14.6 | 21.0-23.9-26.4 | 39.3-44.6-48.7, straight | 113-133-152 × 3.1 | 12.6-13.8-14.1 |
| Jan Mayen specimens | Cylind. w. apical blade | 456-675-810 × 4.7-10.3-15.7 | 21.0-25.0-28.3 | 36.1-40.6-47.1, straight | 66-110-152 × 1.6-3.6-6.3 | 12.6-14.5-17.3 |
| Total | 370-699-963 × 4.7-10.2-15.7 | 21.0-24.7-28.3 | 36.1-40.9-48.7, straight | 66-113-152 × 1.6-3.5-6.3 | 12.6-14.5-17.3 | |
| L. robusta | ||||||
| (Levinsen, 1887) | Cylindrical | 600-860 | 25 | 22 | ||
| (van Soest, 2016) | Cylindrical | 550-910 × 10-13 | 20-27 | 31-40 | Possibly present. | |
| Schultz/LC specimen (this article) | Cylindrical | 353-618-804 × 8.1-10.4-12.1 | 20.2-23.2-24.2 | 36.4-41.3-44.4 | 89-125-156 × 4.0-4.2-0.16 | 10.1-12.1-14.1 |
| L. ruijsi | ||||||
| (van Soest, 2016) | Cylindrical w. apical blade | 390-875 × 9-11 | 23-29 | 24-45 | 63-145 × 2.5-7 | 11-21 |
| Habit | Styles | Palmate anisochelae | Forceps spicules | Embryonic tylostyles | Embryonic anisochelae | |
| L. cupressiformis | ||||||
| (Carter, 1874) | Stylized fusiform w. filaments | 917 | 23 | 38 | ||
| (Lundbeck, 1905) | Most cylind. w. apical blade | 350-840 × 8-14 | 23-25 | 38-48, straight | up to 120 × 7 | 14 |
| Faroes and Rockall Bank specimens | Pinnate or clavate | 370-769-963 × 6.5-10.0-14.6 | 21.0-23.9-26.4 | 39.3-44.6-48.7, straight | 113-133-152 × 3.1 | 12.6-13.8-14.1 |
| Jan Mayen specimens | Cylind. w. apical blade | 456-675-810 × 4.7-10.3-15.7 | 21.0-25.0-28.3 | 36.1-40.6-47.1, straight | 66-110-152 × 1.6-3.6-6.3 | 12.6-14.5-17.3 |
| Total | 370-699-963 × 4.7-10.2-15.7 | 21.0-24.7-28.3 | 36.1-40.9-48.7, straight | 66-113-152 × 1.6-3.5-6.3 | 12.6-14.5-17.3 | |
| L. robusta | ||||||
| (Levinsen, 1887) | Cylindrical | 600-860 | 25 | 22 | ||
| (van Soest, 2016) | Cylindrical | 550-910 × 10-13 | 20-27 | 31-40 | Possibly present. | |
| Schultz/LC specimen (this article) | Cylindrical | 353-618-804 × 8.1-10.4-12.1 | 20.2-23.2-24.2 | 36.4-41.3-44.4 | 89-125-156 × 4.0-4.2-0.16 | 10.1-12.1-14.1 |
| L. ruijsi | ||||||
| (van Soest, 2016) | Cylindrical w. apical blade | 390-875 × 9-11 | 23-29 | 24-45 | 63-145 × 2.5-7 | 11-21 |
Lycopodina cupressiformis. (A, B) In situ ROV footage from Jan Mayen, (C) specimens from the Rockall Bank and Faroe channel: syntype BMNH 82.7.28.43, IceAGE 2 876/4 and BIOFAR 732, (D) specimens from the Jan Mayen Mid-Arctic Ridge (BIODEEP 2006; GeoBio 2011-2012) showing different sizes and morphology, (E) detail of leaf-shaped specimen, (F) detail of specimen IceAGE 2 876/4, (G) detail of GeoBio 2011 specimen.
Lycopodina cupressiformis. (A) Cross section showing flattened stem, (B) longitudinal section, (C) style, (D) tylostyle, (E–G) palmate chela front, side and back view, (H) smaller, embryonic chela, (I) forceps spicule.
Synonyms and citations:
Esperia bihamatifera in part (Hansen, 1885: 15); Asbestopluma cupressiformis in part (Lundbeck, 1905: 58; Hentschel, 1929: 934; Koltun, 1959: 77, 1964: 151, 163); A. cuppressiformis [sic] (Burton, 1934: 27). Possibly: Cladorhiza cupressiformis (Fristedt, 1887: 457), Esperella fristedtii in part Lambe, 1900a: 157. Not: Esperella cupressiformis var. robusta (Levinsen, 1887: 364, cf. van Soest, 2016).
Type material examined:
Syntype: BMNH 82.7.28.43, HMS ‘Porcupine’ 1869, st. 52 (summer 1869, 60°25ʹN, 008°10ʹW, 702 m).
Other material examined:
‘Wandel’ Expedition, the East Greenland Expedition, the Danish Ingolf Expedition, M/S ‘Michael Sars’ 1902, ‘Tjalfe’ Expedition, Godthaab Expedition, BIOFAR, BIODEEP 2006, GeoBio 2008–2012, R/V ‘Paamiut’ 2011, IceAGE 2 (see Supporting information).
Diagnosis:
Erect Lycopodina composed of cylindrical basal stem and solid club-shaped or leaf-shaped, slightly flattened body. Projections range from small knobby structures and ridges to longer, more filamentous structures. Megascleres styles c. 400–800 µm, palmate anisochelae c. 20–28 µm and straight forceps spicules c. 35–50 µm, with addition of smaller tylostyles and palmate anisochelae associated with embryos.
Description:
Erect Lycopodina composed of cylindrical stem that develops into a thicker, club-shaped, slightly flattened body. Body carries numerous projections ranging from short knobs and ridges in some specimens to developed into filaments in others and might be coalesced to varying degrees. In some of the larger specimens, the body, especially the upper part, is further developed into a flattened leaf shape. The colour in ethanol is light beige to light brown, while the colour in situ is off white to light red (Fig. 32A–G).
Skeleton:
Skeleton arranged around an erect, main spicule axis composed of longitudinally arranged megascleres. As opposed to the cylindrical cross-section common in other cladorhizids, the central axis cross section is oval or flattened in more developed specimens. The tissue around the central axis is less dense and is kept rigid by single megascleres radiating from the centre. Where ridges or filaments project from the stem, megascleres are denser than in the rest of the surrounding tissue and organized into bundles (Fig. 33A, B).
Spicules:
Styles, straight or slightly curved, sometimes slightly subtylote, very slightly fusiform, in the stem and body, 370-650-963 µm long, 4.7-10.2-15.7 µm wide (Fig. 33C).
Tylostyles, mostly curved, associated with embryos, 66-96-152 µm long and 1.6-3.5-6.3 µm wide (Fig. 33D).
Palmate anisochelae I, found in large number in all parts of the sponge body, with central upper tooth slightly less than half the total length of the spicule and lateral upper alae 80–100% of the total length of the shaft. The upper lateral alae give the spicule a kite-like shape when viewed from the front. The shaft is arched and the lower end is in the form of one central plate with a central and sometimes two lateral points, and two side plates each with a more pronounced central and less pronounced lateral point, 21.0-24.7-28.3 µm (Fig. 33E–G).
Palmate anisochelae II, found in embryos, similar in shape to the larger type, 12.6-14.5-17.3 µm (Fig. 33H).
Forceps spicules, with straight, slightly diverging legs terminating in slight swellings, 36.1-40.9-48.7 µm (Fig. 33I).
Distribution:
Given the similarity between this species, Lycopodina robusta and L. ruijsi, its distribution is uncertain. It is known from the area between Faroe and Iceland (holotype location: Thomson Ridge) and the Rockall Bank. It has also been reported from Jan Mayen and East Greenland, possibly with a slightly different morphology (see Remarks). Other records identified as L. cupressiformis include the Davis Strait, Baffin Bay, Hudson Bay and the Barents Sea. These are possibly one of the other two closely related species, however (Fig. 29). Typical depth, with a couple of exceptions, is 100–1000 m.
Related species:
Lycopodina robusta (Levinsen, 1887); L. ruijsi (van Soest, 2016); L.lycopodium (Levinsen, 1887); L. novangliae sp. nov.; L. tendali sp. nov.
Remarks:
The habit figure from the original description is an idealized, schematic representation of the sponge and shows rows of filaments similar to L. lycopodium (Carter, 1874: Pl. VII, Fig. 16A–H). Having examined a syntype from Carter’s material, the specimen actually has a quite different pinnate to clavate shape (Fig. 32C) although the other syntypes, which were not re-examined, may have a slightly different morphology. Given this possible, discrepancy, we do not designate the examined syntype as lectotype here.
Previous descriptions of L. cupressiformis range from cylindrical with some articulation of filaments to more leaf-shaped forms (e.g. Lundbeck, 1905). In the specimens examined for this article, there was a slight habit difference between southern (Rockall Bank and Faroe) and more northern (Jan Mayen) forms of L. cupressiformis. The habit of the specimens from Jan Mayen seems to be dependent on size. Smaller specimens have a cylindrical elongated body with only a slight modification at the apex. In larger specimens, the apex develops into a leaf-like upper part, although the exact shape varies between specimens (Fig. 32D). The three specimens, including the syntype, examined from the Rockall Bank and the Faroe Channel have a slightly different, more feather-like morphology, including one specimen with thin, irregular filaments (Fig. 32C).
Recently, van Soest (2016) re-erected L. robusta (Levinsen, 1887) and described L. ruijsi (van Soest, 2016) as separate species from L. cupressiformis based on material from the Kara Sea ‘Varna’ expedition. The spiculation of these three species is virtually identical, and the species are distinguished based on habitus characters. The closely related species, L. novangliae sp. nov. and the diverging molecular results for L. lycopodium (see Hestetun et al., 2016b) also suggest that there might be additional species diversity which is not easily apparent from spicule characters. Thus, it is possible that L. cupressiformis is two or more closely related species. However, all L. cupressiformis molecular data available are from specimens from Jan Mayen (Hestetun et al., 2016b), so sequencing of specimens from other areas, ideally also including L. robusta and L. ruijsi, would be needed to establish the exact relationships within and between these species.
Lycopodina hydra (Lundbeck, 1905) (Fig. 34; Table 7)
Original description:
Asbestopluma hydra Lundbeck, 1905: 66.
Spicule comparison between Lycopodina hydra, L. hypogea and L. parvula
| Subtylostyles 1 | Subtylostyles 2 | Palmate anisochelae | Forceps spicules | |
| L. hydra | ||||
| Present study | 399-561-744 × 7.3-12.7-22.8 | 214-265-339 × 5.0-6.7-7.7 | 8.6-11.2-12.1 | |
| L. hypogea | ||||
| (Vacelet & Boury-Esnault, 1996) | 350-920 × 3-7 | 385-780 × 2-7 | 9-13 | 25-34 |
| L. parvula | ||||
| (Hestetun et al. 2015) | 600-806-1030 × 4.7-7.3-7.9 | 220-358-580 × 4.7-7.7-12.6 | 11.0-14.7-20.4 |
| Subtylostyles 1 | Subtylostyles 2 | Palmate anisochelae | Forceps spicules | |
| L. hydra | ||||
| Present study | 399-561-744 × 7.3-12.7-22.8 | 214-265-339 × 5.0-6.7-7.7 | 8.6-11.2-12.1 | |
| L. hypogea | ||||
| (Vacelet & Boury-Esnault, 1996) | 350-920 × 3-7 | 385-780 × 2-7 | 9-13 | 25-34 |
| L. parvula | ||||
| (Hestetun et al. 2015) | 600-806-1030 × 4.7-7.3-7.9 | 220-358-580 × 4.7-7.7-12.6 | 11.0-14.7-20.4 |
Spicule comparison between Lycopodina hydra, L. hypogea and L. parvula
| Subtylostyles 1 | Subtylostyles 2 | Palmate anisochelae | Forceps spicules | |
| L. hydra | ||||
| Present study | 399-561-744 × 7.3-12.7-22.8 | 214-265-339 × 5.0-6.7-7.7 | 8.6-11.2-12.1 | |
| L. hypogea | ||||
| (Vacelet & Boury-Esnault, 1996) | 350-920 × 3-7 | 385-780 × 2-7 | 9-13 | 25-34 |
| L. parvula | ||||
| (Hestetun et al. 2015) | 600-806-1030 × 4.7-7.3-7.9 | 220-358-580 × 4.7-7.7-12.6 | 11.0-14.7-20.4 |
| Subtylostyles 1 | Subtylostyles 2 | Palmate anisochelae | Forceps spicules | |
| L. hydra | ||||
| Present study | 399-561-744 × 7.3-12.7-22.8 | 214-265-339 × 5.0-6.7-7.7 | 8.6-11.2-12.1 | |
| L. hypogea | ||||
| (Vacelet & Boury-Esnault, 1996) | 350-920 × 3-7 | 385-780 × 2-7 | 9-13 | 25-34 |
| L. parvula | ||||
| (Hestetun et al. 2015) | 600-806-1030 × 4.7-7.3-7.9 | 220-358-580 × 4.7-7.7-12.6 | 11.0-14.7-20.4 |
Type material examined:
Lectotype: ZMUC-DEM-35, the Danish Ingolf Expedition, st. 113 (21 July 1896, 69°31ʹN, 007°06ʹW, 2465 m). Paralectotype: ZMUC-DEM-284, st. 119 (25 July 1896, 67°53ʹN, 010°19ʹW, 1902 m)
Comparative material examined:
Lycopodina hypogea (Vacelet & Boury-Esnault, 1996), two specimens from the Mediterranean 3PP cave; L. parvula (Hestetun et al., 2015), MNHN DJV163-168.
Diagnosis:
Small, pedunculate, Lycopodina with conical, apically oriented body with numerous filaments projecting upwards and outwards. Spicules are peduncle and body subtylostyles, and slightly smaller and finer filament subtylostyles. Microscleres palmate anisochelae only, 9–14 µm.
Description:
Specimens pedunculate, with conical to ovoid bodies, most with filaments directed upwards and outwards from the sponge. Specimens attached to small pebbles with a small basal plate. Total length of the specimens c. 3–10 mm, with the peduncle 0.1–1 mm in diameter, and the body < 1–1.5 mm in diameter. Colour in ethanol is white to slightly beige (Fig. 34A).
Lycopodina hydra. (A) Lectotype ZMUC-DEM-35, (B) subtylostyle I, (C) subtylostyle II, (D, E) palmate anisochela front and back view.
Skeleton:
The peduncle is composed of tightly packed subtylostyles bundles which diverge towards in the upper end forming the skeleton of the body. Subtylostyles radiate from the centre of the sponge making up the supporting skeleton of the filaments.
Spicules:
Subtylostyles I, only slightly subtylote, straight and fusiform, in the peduncle and body, 399-561-744 µm long, 7.3-12.7-22.8 µm wide (Fig. 34B).
Subtylostyles II, only slightly subtylote, straight and fusiform, making up the filaments, 214-265-339 µm long, 5.0-6.7-7.7 µm wide (Fig. 34C).
Palmate anisochelae, with central tooth approximately half total length of the spicule and lateral alae about 80% of total length of spicule. Arched shaft and lower end modified into one central and two side plates with serrated edges, 8.6-11.2-12.1 µm (Fig. 34D, E).
Distribution:
The species was collected by the Ingolf Expedition from two localities between Jan Mayen and Iceland at 1902–2465 m depth (Fig. 29).
Related species:
Lycopodina hypogea (Vacelet & Boury-Esnault, 1996); L. parvula (Hestetun et al., 2015).
Remarks:
While superficially somewhat similar to Lycopodina infundibulum or L. minuta, L. hydra lacks the cup-like cavity and tapering subtylostyles of that species, and the morphology of the chelae is palmate rather than palmate/arcuate as is the case in, for example, L. infundibulum. The body is stalked, but not elongated as seen in for instance L. lycopodium, being roughly spherical instead. Two other species with a similar morphology and spicule complement are known the southern North Atlantic: L. hypogea (Vacelet & Boury-Esnault, 1996) from the Mediterranean and coast of Spain and France (Bakran‐Petricioli et al., 2007; Aguilar et al., 2011; Chevaldonné et al., 2015) and L. parvula (Hestetun et al., 2015) at abyssal depths at the Porcupine Abyssal Plain and off Gabon-Congo (Table 7). In addition to geographical distance, L. hydra can be distinguished from L. hypogea by having shorter subtylostyles and lacking forceps spicules and from L. parvula by its smaller subtylostyle and chela sizes.
Lycopodina infundibulum (Levinsen, 1887) (Fig. 35; Table 5)
Original description:
Esperella infundibulum Levinsen, 1887: 366.
Synonyms and citations:
Esperia cupressiformis in part (Carter, 1874: 215); E. bihamatifera in part (Hansen, 1885: 15); Asbestopluma infundibulum (Lundbeck, 1905: 68; Hentschel, 1929: 875, 935; Koltun, 1959: 77; Boury-Esnault et al., 1994: 102; Desbruyères et al., 2001: 1334; Hestetun et al., 2015: 1318). Lycopodina infundibulum (van Soest, 2016).
Material examined:
The Danish Ingolf Expedition, DIAPISUB, R/V ‘G.O. Sars’ 2008–155 (see Supporting information).
Diagnosis:
Small, pedunculate, cup-shaped greyish white Lycopodina with main mycalostyles to subtylostyles, smaller subtylostyles and thin, tortuous subtylostyles protruding from sponge surface. Microscleres arcuate to palmate anisochelae 10–25 µm and forceps spicules 42–82 µm.
Description:
Thin, cup-shaped, apically oriented main body supported by a thin peduncle. Body diameter is in the range of 3–8 mm with approximately equal height. Body often flattened upon collection. Peduncle less than 0.5 mm in diameter, up to 40 mm long and attached to hard substrate with small basal plate. Peduncle usually partly covered in sediment giving it a light brown colour, while upper peduncle and body is whitish light grey. The surface of the body is slightly hispid (Fig. 35A, B).
Lycopodina infundibulum. (A) Specimen ZMBN103460, (B) detail of ZMBN103460, (C) style, (D) subtylostyle, (E) tortuous, tapering subtylostyle, (F, G) arcuate to palmate anisochela front and back views, (H) forceps spicule [redrawn from Levinsen (1887) Pl. XXXI, Fig. 19].
Skeleton:
Peduncle skeleton composed of closely packed bundles of the main mycalostyles/subtylostyles; mostly fine, straight but become slightly shorter and more curving towards the basal plate. Spicule bundles in the upper peduncle gradually fan out into the skeleton of the main body. The thin, tapering subtylostyles are found projecting from the main body giving it its hispid appearance, while shorter subtylostyles are found at the fold of the upper body margin.
Spicules:
Mycalostyles to subtylostyles, mostly straight and slightly fusiform, with tyles sometimes faint or absent, 171-445-675 µm long, 4.7-7.4-9.3 µm wide (Fig. 35C).
Subtylostyles, slightly bent and slightly tapering, 133-263-466 µm long, 4.7-5.7-9.3 µm wide (Fig. 35D).
Tapering subtylostyles, very thin, tapering and tortuous, with faint tyle, 371-463-561 µm long, 2.3-4.7-7.0 µm wide (Fig. 35E).
Arcuate to palmate anisochelae, with central tooth and lateral alae both approximately half the length of the spicule, with arched shaft and lower end modified into one central and two side plates with serrated edges. Abundant through the body tissue, 16.3-20.4-23.3 µm (Fig. 35F, G).
Forceps spicules, not encountered in the specimens examined here. With ends curving inwards and crossing, previously reported as 42–82 µm (Lundbeck, 1905; Hentschel, 1929; Boury-Esnault et al., 1994) (Fig. 35H).
Distribution:
Boreal North Atlantic and Arctic: The GIN Seas, Faroe Ridge, Norwegian Shelf, Barents and Kara Seas (Fig. 29). Other distribution: Ibero-Moroccan gulf (Boury-Esnault et al., 1994), Azores (Desbruyères et al., 2001), Coast off Guyana (Hestetun et al., 2015).
The reported depth distribution is 91–710 m within the Northern North Atlantic and Arctic. Recordings further south have a deeper distribution of 1590–1947 m. Bottom temperatures, where recorded, are in the range of –1.45 to –0.3 °C (Carter, 1874; Lundbeck, 1905; Koltun, 1959).
Related species:
Lycopodina comata (Lundbeck, 1905); L.minuta (Lambe, 1900); L.versatilis (Topsent, 1890).
Remarks:
Some variation in spicules sizes have been reported for this species: The chela sizes of the holotype (10–22 µm) (Levinsen, 1887) are smaller than those given for specimens examined by later authors, and the length of the forceps spicules of the specimen examined by Boury-Esnault et al. (1994) is less than those of other records (Table 5). The spiculation of embryonic structures is different from the rest of the sponge. Here, megascleres are small strongyles, oxeas or styles, while anisochelae are smaller than in the rest of the sponge (Lundbeck, 1905). Embryos were not encountered in the specimens examined here.
Lycopodina infundibulum is the most common of four similar small, cup-shaped cladorhizid species from the Northern Atlantic and Arctic, the others being L. comata (Lundbeck, 1905), L. minuta (Lambe, 1900) and L. versatilis (Topsent, 1890). The forceps spicules are often rare or in some cases absent from specimens, in all likelihood as they are associated with the production of spermatic cysts (Riesgo et al., 2007), and they could be absent in part of the sponge life cycle. Thus, the presence or absence of forceps spicules (forceps spicules being absent in the descriptions of L. comata and L. minuta) should not be considered an absolute diagnostic character, as these two species have been described from only a few specimens. Similarly, smaller anisochelae and curved strongyles and oxeas are found in embryos only and are not present in the rest of the sponge.
The species L. comata can most easily be distinguished from L. infundibulum based on the presence of characteristic polytylote subtylostyles, L. minuta by its smaller, more uneven and more closed off body. Lycopodina versatilis has concave, open forceps spicules rather than with distal ends crossed. The characteristic tapering subtylostyles have also not been reported for L. minuta or L. versatilis.
Lycopodina lycopodium (Levinsen, 1887) (Fig. 36; Table 8)
Original description:
Esperella cupressiformis var. lycopodium Levinsen, 1887: 365.
Spicule comparison between Lycopodina lycopodium, L. tendali sp. nov. and L. novangliae sp. nov.
| Styles | Basal styles | Embryonic (tylo)styles | Palmate anisochelae | Forceps spicules | |
| L. lycopodium | |||||
| (Levinsen, 1887) | 740-1200 | 13 | Mentioned | ||
| (Lundbeck, 1905) | 600-1500 × 7-21 | 238-400 | Up to 150 | 14 (one spec. 17) | 50-56 |
| (van Soest & Baker, 2011) | 640-1410 × 6 | 270 × 8 | 9-11 | 18-32 | |
| (Hestetun et al., 2015) | 500-1090 × 9 | 200-427 × 7 | 132-274 × 3-6 | 8-11 | 36-44 |
| (van Soest, 2016) | 570-1170 × 2-13 | 115-180 × 2-8 | 11.5-14.5 | 28-48 | |
| Present study | 314-785-1474 × 4.7-9.9-18.2 | 248-320-410 × 4.7-7.5-11.7 | 60-146 × 2-6 | 11.4-13.6-16.5 | 25.6-47.1-55.0 |
| L. tendali sp. nov. | 428-1049-2974 × 4.7-10.7-18.6 | 96-118-163 × 2.3-4.4-6.2 | 12.4-15.2-18.2 | 37.3-49.2-56.5 | |
| L. novangliae sp. nov. | 459-841-1279 × 8.3-13.7-21.0 | 16.5-19.7-23.9 | 45.5-50.0-53.7 |
| Styles | Basal styles | Embryonic (tylo)styles | Palmate anisochelae | Forceps spicules | |
| L. lycopodium | |||||
| (Levinsen, 1887) | 740-1200 | 13 | Mentioned | ||
| (Lundbeck, 1905) | 600-1500 × 7-21 | 238-400 | Up to 150 | 14 (one spec. 17) | 50-56 |
| (van Soest & Baker, 2011) | 640-1410 × 6 | 270 × 8 | 9-11 | 18-32 | |
| (Hestetun et al., 2015) | 500-1090 × 9 | 200-427 × 7 | 132-274 × 3-6 | 8-11 | 36-44 |
| (van Soest, 2016) | 570-1170 × 2-13 | 115-180 × 2-8 | 11.5-14.5 | 28-48 | |
| Present study | 314-785-1474 × 4.7-9.9-18.2 | 248-320-410 × 4.7-7.5-11.7 | 60-146 × 2-6 | 11.4-13.6-16.5 | 25.6-47.1-55.0 |
| L. tendali sp. nov. | 428-1049-2974 × 4.7-10.7-18.6 | 96-118-163 × 2.3-4.4-6.2 | 12.4-15.2-18.2 | 37.3-49.2-56.5 | |
| L. novangliae sp. nov. | 459-841-1279 × 8.3-13.7-21.0 | 16.5-19.7-23.9 | 45.5-50.0-53.7 |
Spicule comparison between Lycopodina lycopodium, L. tendali sp. nov. and L. novangliae sp. nov.
| Styles | Basal styles | Embryonic (tylo)styles | Palmate anisochelae | Forceps spicules | |
| L. lycopodium | |||||
| (Levinsen, 1887) | 740-1200 | 13 | Mentioned | ||
| (Lundbeck, 1905) | 600-1500 × 7-21 | 238-400 | Up to 150 | 14 (one spec. 17) | 50-56 |
| (van Soest & Baker, 2011) | 640-1410 × 6 | 270 × 8 | 9-11 | 18-32 | |
| (Hestetun et al., 2015) | 500-1090 × 9 | 200-427 × 7 | 132-274 × 3-6 | 8-11 | 36-44 |
| (van Soest, 2016) | 570-1170 × 2-13 | 115-180 × 2-8 | 11.5-14.5 | 28-48 | |
| Present study | 314-785-1474 × 4.7-9.9-18.2 | 248-320-410 × 4.7-7.5-11.7 | 60-146 × 2-6 | 11.4-13.6-16.5 | 25.6-47.1-55.0 |
| L. tendali sp. nov. | 428-1049-2974 × 4.7-10.7-18.6 | 96-118-163 × 2.3-4.4-6.2 | 12.4-15.2-18.2 | 37.3-49.2-56.5 | |
| L. novangliae sp. nov. | 459-841-1279 × 8.3-13.7-21.0 | 16.5-19.7-23.9 | 45.5-50.0-53.7 |
| Styles | Basal styles | Embryonic (tylo)styles | Palmate anisochelae | Forceps spicules | |
| L. lycopodium | |||||
| (Levinsen, 1887) | 740-1200 | 13 | Mentioned | ||
| (Lundbeck, 1905) | 600-1500 × 7-21 | 238-400 | Up to 150 | 14 (one spec. 17) | 50-56 |
| (van Soest & Baker, 2011) | 640-1410 × 6 | 270 × 8 | 9-11 | 18-32 | |
| (Hestetun et al., 2015) | 500-1090 × 9 | 200-427 × 7 | 132-274 × 3-6 | 8-11 | 36-44 |
| (van Soest, 2016) | 570-1170 × 2-13 | 115-180 × 2-8 | 11.5-14.5 | 28-48 | |
| Present study | 314-785-1474 × 4.7-9.9-18.2 | 248-320-410 × 4.7-7.5-11.7 | 60-146 × 2-6 | 11.4-13.6-16.5 | 25.6-47.1-55.0 |
| L. tendali sp. nov. | 428-1049-2974 × 4.7-10.7-18.6 | 96-118-163 × 2.3-4.4-6.2 | 12.4-15.2-18.2 | 37.3-49.2-56.5 | |
| L. novangliae sp. nov. | 459-841-1279 × 8.3-13.7-21.0 | 16.5-19.7-23.9 | 45.5-50.0-53.7 |
Synonyms and citations:
Esperia bihamatifera in part (Hansen, 1885: 15), Esperella fristedtii in part Lambe, 1900a: 157, Asbestopluma lycopodium (Lundbeck, 1905: 62; Hentschel, 1929: 934; Burton, 1934: 9, 33; Koltun, 1959: 75; Koltun, 1964: 151, 163), A. (A.) lycopodium (van Soest et al., 2007: 130; Hestetun et al., 2015: 1313), L. lycopodium (van Soest, 2016).
Material examined:
The Norwegian North-Atlantic Expedition, R/V ‘Johan Ruud’ 1979-1981, CARACOLE, GeoBio 2008, R/V ‘G.O. Sars’ 2008-155, MAREANO 2013 (see Supporting information).
Diagnosis:
Erect Lycopodina composed of a short basal stem and elongated, cylindrical body with numerous, flexible filamentous processes issuing in all directions. Megascleres styles in the range of 250–1500 µm with shorter styles in basal part. Microscleres palmate anisochelae 11–17 µm and forceps spicules 26–55 µm.
Description:
Erect, from 25 to 70 mm in length, and composed of a short stem 1–2 mm in diameter and up to 25 mm in length and an elongated, cylindrical, in some cases slightly fusiform body up to 50 mm in length tightly set with filaments in all directions. Connected to hard substrate with small, roughly conical basal plate. In some specimens, the lower part of the stem is covered with a rough, brown sheath. Filaments < 1–8 mm in length, flexible, very fine, in some cases reduced to knobs, and project in all directions from the sponge giving it a brush-like appearance. In many specimens, the apex of the sponge coalesces into a grooved bud-like structure. Colour in situ white with a very slight grey-brown tinge, in ethanol whitish beige (Fig. 36A–C).
Lycopodina lycopodium. (A) Norwegian shelf specimen (Nyegga, GS08-155-32ROV), (B) specimen ZMBN103462 (Nyegga, Norwegian Shelf), (C) specimen ZMBN103465 (Skjoldryggen, Norwegian Shelf), (D) cross section, (E) longitudinal section, (F) detail of the top of specimen A, (G) style, (H, I) palmate anisochela front and back view, (J) forceps spicule.
Skeleton:
Stem composed of a central core made up of style bundles surrounded by looser tissue. The skeleton of each filament is made up of overlapping styles, and is anchored into the main stem (Fig. 36D, E).
Spicules:
Styles, straight or slightly curved, very slightly fusiform, in the stem and body, 314-785-1474 µm long, 4.7-9.9-18.2 µm wide; in the basal stem and plate, curved, 248-320-410 × 4.7-7.5-11.7 µm (Fig. 36G).
Styles, slightly curved, sometimes with a small terminal tyle, associated with embryos, 60–146 µm long, 2–6 µm wide.
Palmate anisochelae, found in large number in all parts of the sponge body, with central tooth approximately half total length of the spicule and lateral alae covering the whole shaft. The outline of the upper alae is oval when viewed from the front. The shaft is arched and the lower end is modified into one central and two side plates with serrated edges, 11.4-13.6-16.5 µm. No special anisochela type or size was observed for embryos (Fig. 36H, I).
Forceps spicules, with legs straight and slightly flattened closest to the centre, then diverging first outwards, then back inwards before terminating in small swellings. Sometimes the legs are twisted or crossed, 25.6-47.1-55.0 µm (Fig. 36J).
Distribution:
The species is reported from a wide area including the Porcupine and Rockall Banks, the GIN Seas, the Kara and Laptev Seas and possibly Baffin Bay. However, older records, especially from boreal and Arctic areas, are possibly L. tendali (Fig. 30). Reported depth is 41–1134 m. Most shallow records (41–392 m) are from the Kara and Laptev Seas, while more southern records are often in the 500–1000 m range.
Related species:
L. gracilis (Koltun, 1955); L. novangliae sp. nov.; L.occidentalis (Lambe, 1893); Lycopodina tendali sp. nov.
Remarks:
Lycopodina lycopodium has an elongated habit with filaments in all directions, which is also found in several other Lycopodina species. The slight spicule differences between L. lycopodium and species like L. occidentalis (Lambe, 1893) and L.gracilis (Koltun, 1955), in addition to the smaller spicule sizes found in specimens of L. lycopodium specimens collected at the Rockall Bank (Hestetun et al., 2015; van Soest & Baker, 2011) (Table 8), have led to the suspicion that L. lycopodium might actually consist of several species (e.g. van Soest, 2016), with one new species, L. tendali sp. nov., erected here based partly on molecular evidence.
The original description by Levinsen (1887) shows a bud-like termination at the apex of the sponge, which is also present in complete specimens identified as L. lycopodium here, like specimen GS08-155-32ROV (Fig. 36F), which clearly shows the grooved apex also described by Lundbeck (1905, pl. 2, fig. 15, 17). It is not easy to say whether this structure always is present given the fragmentary nature of many of the specimens, but it does not seem to be present to any clear degree in L. tendali (Fig. 40A, B).
Lycopodina minuta (Lambe, 1900) (Fig. 37; Table 5)
Original description:
Esperella minuta Lambe, 1900: 23.
Synonyms and citations:
Asbestopluma minuta (Hentschel, 1929: 934; Gorbunov, 1946: 37; Koltun, 1959: 78, 1964: 163).
Material examined:
GeoBio 2012, st. ROV-04 (28 July 2012, 71°17.799′N, 005°46.436′W, 501 m) st. ROV 14 (1 August 2012, 71°17.907′N, 005°46.364′W, 465 m).
Diagnosis:
Small, pedunculate, Lycopodina with laterally compressed body; mycalostyles to subtylostyles 327–543 µm, smaller tylostyles 196–294 µm; microscleres palmate/arcuate anisochelae only, 18–19 µm.
Description:
Holotype described by Lambe (1900b) as pedunculate sponge with laterally compressed body. Peduncle is 10 mm in length and 3 mm in diameter, attached to the substrate with a basal plate. The body is 3.5 mm in length, 2.75 × 1 mm in width. Several specimens with a similar morphology and spicule complement were collected at the Mid-Arctic Ridge near Jan Mayen. These are pedunculate, 8–22 mm long in total, with a body 2–3 mm in length and 2.5–5 mm in diameter which varies from solid to an uneven cup shape. The body is white to slightly grey, and the stem is either grey or coated in red clay-like sediment (Fig. 37A–E).
Lycopodina minuta. (A, B) Specimens from GeoBio 2012 ROV14-12, (C) specimen from GeoBio 2012 ROV04-16, (D, E) detail of two specimens from GeoBio 2012 ROV04-16, (F) style, (G) subtylostyle, (H) tylostyle, (I) strongyle, (J, K) arcuate to palmate anisochela front and back views, (L, M) embryonic arcuate to palmate anisochela front and back views.
Skeleton:
Peduncle composed of parallel mycalostyles with a slight spiral twisting, which become more loosely associated in the body. The shorter tylostyles project from the surface at right angles in the peduncle and slightly upwards in the main body.
Spicules:
Mycalostyles, straight and fusiform, in the peduncle and body, 271-425-565 µm long and 5.7-7.1-9.7 µm wide (Fig. 37F).
Subtylostyles, straight or slightly curved, slightly fusiform, 197-328-442 µm long and 4.2-6.5-8.3 µm wide (Fig. 37G).
Tylostyles, projecting from the surface, curved, 142-165-216 µm long and 3.6-6.2-7.4 µm wide (Fig. 37H).
Strongyles, found in embryos, bent, with a small tyle situated some distance away close to one end, 46-68-87 µm long and 2.2-4.4-6.1 µm wide (Fig. 37I).
Arcuate to palmate anisochelae I, with upper central tooth and lateral alae both approximately 40% the length of the spicule, with arched shaft and lower end modified into one central and two side plates with serrated edges. Abundant through the body tissue, 15.7-18.1-23.0 µm (Fig. 37J, K).
Arcuate to palmate anisochelae II, associated with embryos, slightly smaller, with the upper tooth and alae covering 60% of the total length of the spicule, and overlapping the lower part, 10.5-13.6-15.3 µm (Fig. 37L, M).
Distribution:
The previously reported distribution is in the Arctic. The type locality is given as the Davis Strait, 10 miles off Cape Wild, at 366 m depth. The species has also been reported from the Kara Sea at 336–698 m depth (Koltun, 1959), and the East Greenland Sea at 608 m depth (Koltun, 1964). The newly collected specimens are from the Arctic Mid-Ocean Ridge close to Jan Mayen at 465–501 m depth (Fig. 29).
Related species:
Lycopodina infundibulum (Levinsen, 1887); L.comata (Lundbeck, 1905); L.versatilis (Topsent, 1890).
Remarks:
The only description of this species based on an examination of specimen material is from the single holotype specimen examined by Lambe (1900b). The species was also described by Hentschel (1929) and Koltun (1959), but these descriptions are based on the written description by Lambe only, and so the species is previously known only from a single specimen. The species was also apparently collected by the 1935 ‘Sadko’ expedition (Gorbunov, 1946), but these specimens were subsequently lost (Koltun, 1959). The species was also recorded from Spitzbergen (‘Lena’ 1958 expedition) without any further description (Koltun, 1964).
Lambe describes the body simply as a laterally flattened bud shape, which is somewhat unusual given that closely related species such as L. infundibulum and L. comata have cup-shaped bodies. We have identified several specimens collected at the Mid-Arctic Ridge as L. minuta based on the lack of forceps spicules, a similar spicule complement and a molecular sequence different to that of L. infundibulum (Fig. 35). The specimens examined range from completely closed to cup-shaped (Fig. 37A–C), but with the margins of the cup irregular and in most cases curving inwards. As Lambe described the species from only a single specimen, it is probable that the holotype does not represent the whole range of the morphological variation within the species. The specimens examined here also have embryonic spicules: strongyles and smaller anisochelae. These are presumably absent in the holotype.
The species is closely related to L. infundibulum and L. comata, but can be distinguished most easily from those species on the absence of tapering or polytylote subtylostyles. It is also a close relative to L. versatilis, but can be distinguished from that species based on its smaller anisochelae and lack of forceps spicules (‘abundant’ in L. versatilis, cf. Topsent, 1892).
Lycopodina novangliae sp. nov. (Fig. 38; Table 8)
urn:lsid:zoobank.org:act:E6E730DC-34D4-4D5D-B162-9FE0C1E2BF9F
Type material:
Holotype: USNM 1234806, R/V ‘Eastward’, st. 36023 [25 May 1979, Lydonia Canyon (NW Atl.), 40°22.764ʹN, 067°39.33ʹW, 430–613 m].
Diagnosis:
Erect Lycopodina composed of stem slightly widening into elongated cylindrical body set with filaments; megascleres styles in the range of 450–1300 µm; microscleres palmate anisochelae 16–24 µm and forceps spicules 45–54 µm.
Description:
Holotype 60 mm tall and up to 5 mm wide erect Lycopodina composed of a solid, rigid stem which broadens slightly into a cylindrical body. Stem approximately 20 mm tall and 2 mm in diameter, very slightly hispid and gradually widens into a slightly grooved body set with numerous short filaments. Fastened to a pebble with a basal plate. Embryos visible in upper part of body. The sponge is damaged and is missing the top part. Colour in ethanol light beige to light brown (Fig. 38A–C).
Lycopodina novangliae sp. nov. (A) Holotype USNM 1234806, (B, C) detailed surface view, (D) style, (E–G) palmate anisochela front, side and back views, (H) forceps spicule.
Skeleton:
Skeleton made up of a hard, strong spicule axis composed of styles making up the filament-bearing body. Filaments each is composed of a central core of styles emerging from the central stem.
Spicules:
Styles, straight or slightly curved, very slightly fusiform, in the main axis, body and filaments, 459-841-1279 µm long, 8.3-13.7-21.0 µm wide (Fig. 38D).
Palmate anisochelae, found in large number, with central tooth slightly less than half total length of the spicule and lateral alae covering 90% of the shaft. The outline of the upper alae is oval when viewed from the front. The shaft is arched and the lower end is modified into one central and two side plates with serrated edges, 16.4-19.7-23.9 µm. No special anisochela type or size was observed in the embryos (Fig. 38E–G).
Forceps spicules, with legs straight and slightly flattened closest to the centre, and then becoming thinner, diverging slightly outwards, in some cases then back inwards before terminating in small swellings, 45.5-50.0-53.7 µm (Fig. 38H).
Distribution:
This species is only known from its type locality, Lydonia Canyon, on the southern slope of Georges Bank (430–613 m) (Fig. 29).
Related species:
Lycopodina lycopodium (Levinsen, 1887); L. occidentalis (Lambe, 1893); L. gracilis (Koltun, 1955); L. cupressiformis (Carter, 1874); L. robusta (Levinsen, 1887).
Etymology:
The holotype of this species was collected on the shelf of and is named after, New England, in the north-east USA.
Remarks:
Lycopodina novangliae sp. nov. is known only from the holotype but is clearly distinguishable from other described species from the Northern Atlantic. It is a relative to L. lycopodium but differs in having a more solid morphology and larger anisochelae. The holotype location is somewhat to the south of the range of the common boreal North Atlantic and Arctic species, and it might have a more southern distribution along the North American coast.
Lycopodina Robusta (Levinsen, 1887) (Fig. 39; Table 6)
Original description:
Esperella cupressiformis var. robusta Levinsen, 1887: 364.
Synonyms and citations:
Possibly: Esperia bihamatifera in part (Hansen, 1885: 15); Asbestopluma cupressiformis in part (Lundbeck, 1905: 58; Hentschel, 1929: 934; Koltun, 1959: 77, 1964: 151, 163); A. cuppressiformis [sic] (Burton, 1934: 27), Cladorhiza cupressiformis (Fristedt, 1887: 457); Esperella fristedtii in part Lambe, 1900a: 157. Not: Esperia cupressiformisCarter, 1874: 215.
Material examined:
GeoBio 2009, st. AGT-02 (73°35.19ʹN, 007°44.08ʹE, 2387 m, between Loki’s Castle vent field and the Schultz Massif Seamount).
Diagnosis:
Erect elongated Lycopodina with stem and cylindrical to club-shaped body with ridges or upwards directed projections. Megascleres c. 450–900 µm, palmate anisochelae c. 20–27 µm and forceps spicules c. 22–44 µm.
Description:
Single specimen 11 cm in length; a 2.5-cm-long and 1.5-mm-wide stem connected to a clearly delineated 8.5 cm long up to 5 mm wide cylindrical, ridged body. Fastened to the substrate with slightly enlarged basal plate. Surface uneven, with wispy ridges, knobs and short projection in all directions, oriented mostly upwards. White, globular embryos visible just beneath surface. Colour in ethanol is light brown (Fig. 39A, B).
Lycopodina robusta. (A) Habit, (B) detail showing surface and embryos, (C) style, (D) tylostyle, (E) forceps spicule, (F, G) palmate anisochela 1, (H, I) embryonic palmate anisochela.
Skeleton:
Stem consisting of closely packed styles which become shorter and more curved in the basal plate. In the body, styles project in an upwards pattern radiating from the central stem. Palmate anisochelae are abundant in the sponge body, forceps spicules less abundant. Tylostyles and smaller anisochelae are associated with embryos.
Spicules:
Styles, straight or slightly curved, very slightly fusiform, 599-736-804 µm long, 8.1-10.6-12.1 µm wide in the stem and body; basal styles, shorter and curved, 344-406-484 µm long, 8.1-9.9-12.1 µm wide (Fig. 39C).
Tylostyles, mostly curved, associated with embryos, 89-125-156 µm long and 4.0-4.2-6.1 µm wide (Fig. 39D).
Palmate anisochelae I, found in large number in all parts of the sponge body, with central upper tooth approximately half the total length of the spicule and lateral upper alae 80–100% of the total length of the shaft. The shaft is arched and the lower end is in the form of one central plate with a central and sometimes two lateral points, and two side plates each with two points, 20.2-23.1-24.2 µm (Fig. 39F, G).
Palmate anisochelae II, found in embryos, similar in shape to the larger type, but with central upper tooth typically slightly more than half the total length of the spicule and lateral alae usually covering the whole length of the shaft. Lower end morphology similar to the larger type of anisochela, but with points typically slightly less developed, 10.1-12.1-14.1 µm (Fig. 39H, I).
Forceps spicules, with straight, slightly diverging legs terminating in slight swellings, 36.4-41.3-44.4 µm (Fig. 39E).
Distribution:
Given its close relationship with L. cupressiformis and L. ruijsi the exact distribution is not known, but records from the Barents Sea and West Greenland could possibly be this species (Fig. 29). The specimen examined here was found at the Arctic Mid-Ocean Ridge at ~2400 m depth, but other records are shallower (mainly 100–200 m).
Related species:
Lycopodina cupressiformis (Carter, 1874); L. ruijsi van Soest, 2016; L. lycopodium (Levinsen, 1887); L. novangliae sp. nov; L. tendali sp. nov.
Remarks:
This species is differentiated from L. cupressiformis based on habit characters and has a more slender, symmetrically cylindrical body than the latter species. Based on this and previous records (Kara Sea), this species could have a more northern distribution than L. cupressiformis. The specimens that were reported by Fristedt (1887) and that Lambe later named Esperella fristedtii (Lambe, 1900), some of Lundbeck’s cupressiformis material, and the specimen reported by Koltun (1964) might also be this species. While the specimen examined here fits very well in most regards with the description of van Soest (2016), there are a few differences, the most obvious being the presence of tylostyles and smaller anisochelae. These spicules are associated with embryos and are presumably absent in specimens examined by Levinsen (1887) and van Soest (2016). The degree to which the upper alae cover the shaft is also slightly variable in our specimen, ranging from 80 to 100%, whereas in van Soest’s specimens they typically cover the whole shaft.
Lycopodina Ruijsi van Soest, 2016 (Table 6)
Original description:
Lycopodina ruijsi van Soest, 2016: 315.
Description:
This species was described by van Soest (2016) based on two specimens from the 1882/83 Varna expedition. Holotype 50 mm long with 38-mm-long stalk and lower body, and 12-mm-long blade-like upper body, while the second specimen is composed of three small fragments (cf. van Soest, 2016).
Spicules:
Styles, 390–875 µm long, 9–11 µm wide (van Soest, 2016).
Tylostyles, 63–145 µm long, 2.5–7 µm wide (van Soest, 2016).
Palmate anisochelae, with upper alae covering at least 80% of total chela length, in two clearly separated size categories 23–29 and 11–21 µm (van Soest, 2016).
Forceps spicules, with straight legs, 24–45 µm (van Soest, 2016).
Distribution:
The holotype and additional specimen are from the Kara Sea with no further locality information (Fig. 29), depth between 75 and 170 m.
Related species:
Lycopodina cupressiformis (Carter, 1874); L. robusta (Levinsen, 1887); L. lycopodium (Levinsen, 1887); L. novangliae sp. nov.; L. tendali sp. nov.
Remarks:
This is a close relative of L. cupressiformis and L. robusta with the main diagnostic character being the two-part main body. While no information is given in the original description, comparison of related species indicates that tylostyles and the smaller size category of palmate anisochelae could be associated with embryos.
Lycopodina tendali sp. nov. (Fig. 40; Table 8)
urn:lsid:zoobank.org:act:055D8E45-1C14-49AE-BA89-061BB01F5667
Lycopodina tendali sp. nov. (A) Holotype (ZMBN103463), (B) additional specimens from Jan Mayen, (C) detail from holotype, (D) longitudinal section of holotype, (E) main, very slightly tylote, style, (F) tylostyle, (G–I) palmate anisochela front, side and back views, (J) forceps spicule.
Type material:
Holotype: ZMBN 103463, ‘G.O. Sars’ 2008–155, st. 33d (5 August 2008, Pockmark Dodo, Nyegga Shelf, 64°40.12ʹN, 005°15.70ʹE, 730 m). Paratypes: ZMBN 103464, ‘G.O. Sars’ GeoBio 2011, ROV 7 (17 June 2011, Troll Wall, Jan Mayen, 71°17.82ʹN, 005°46.47ʹW, 500 m); ZMBN 103466, R/V ‘Johan Hjort’ MAREANO 2013–205, st. R1114-443 (22 June 2013, Skjoldryggen, 65°37.63ʹN, 005°35.23ʹE, 611 m).
Additional material examined:
NTNU 14723, R/V ‘Johan Ruud’, st. 1179-79 (17 August 1979, 70°46ʹN, 017°00ʹE, 990 m); R/V ‘Polarstern’ Ark X-31-16+C1 (21 September 1994, 75°00.42ʹN, 012°37.42ʹW, 994 m); BIODEP 2006, ROV 12 (2006, 71°17.98ʹN, 005°46.92ʹW, 616 m), ROV 16 (2006, 71°17.86ʹN, 005°46.29ʹW, 557 m); BIODEEP 2007, ROV 9 (2007, 73°50.23ʹN, 007°38.04ʹE, 1262 m); GeoBio 2009, AGT-2 (5 August 2009, 73°35.19ʹN, 007°44.08ʹE, 2387 m); GeoBio 2012, ROV 4–15 (28 July 2012, 71°17.80ʹN, 005°46.44ʹW, 501 m), ROV 14–8 (1 August 2012, 71°17.91ʹN, 005°46.36ʹW, 465 m).
Diagnosis:
Erect Lycopodina composed of short stem and elongated, fusiform body with a large number of rigid filamentous processes in all directions. Megascleres styles c. 400–3000 µm and microstyles 90–170 µm. Microscleres palmate anisochelae 12–18 µm and forceps spicules 37–57 µm.
Description:
Holotype 40 mm long, 3 mm wide, composed of a short stem and an elongated, fusiform body with a large number of filaments in all directions. Stem connected to the substrate with small basal plate. Paratypes and other specimens examined in the range of 15–45 mm long and typically 2–4 mm in diameter at their widest point. Filaments rigid, up to 5 mm in length, and project straight outwards from the body. The tissue of the filaments is typically somewhat retracted, possibly due to fixation, exposing the spicule skeleton. Colour is white to slightly red (Fig. 40A, B).
Skeleton:
The skeleton of the main axis, making up the stem and centre of the body, is made up of style bundles. The longest styles are found making up the supporting skeleton of the projecting filaments which radiate outwards from the centre of the sponge (Fig. 40C, D).
Spicules:
Styles, straight or slightly curved, very slightly fusiform, in the main axis, body and filaments, 428-1049-2974 µm long, 4.7-10.7-18.6 µm wide (Fig. 40E).
Tylostyles, sometimes only faintly tylote, slightly curved, associated with embryos, 96-118-163 µm long, 2.3-4.4-6.2 µm wide (Fig. 40F).
Palmate anisochelae, found in large number in all parts of the sponge body, with the central tooth slightly less than half total length of the spicule and lateral alae covering 90% of the shaft. The outline of the upper alae is ovoid when viewed from the front. The shaft is arched and the lower end is modified into one central and two side plates with serrated edges, 12.4-15.2-18.2 µm. No special anisochela type or size was observed for embryos (Fig. 40G, I).
Forceps spicules, with legs straight and slightly flattened closest to the centre, and then becoming thinner, diverging slightly outwards, in some cases then back inwards before terminating in small swellings, 37.3-49.2-56.5 µm (Fig. 40J).
Distribution:
The type locality is the methane pockmark Dodo, at the Nyegga Shelf off the coast of Norway (730 m). Paratype locations are the Troll Wall locality at the Jan Mayen vent field (500 m) and the Skjoldryggen Shelf off Norway (611 m). Additional specimens are the from Jan Mayen vent field, between the Loki’s Castle vent field and the Schultz Massif seamount, on the Norwegian Shelf off Tromsø, and the Eastern Greenland Shelf (465–2387 m) (Fig. 30).
Related species:
Lycopodina lycopodium (Levinsen, 1887); L. occidentalis (Lambe, 1900); L. gracilis (Koltun, 1955); L. novangliae sp. nov.
Etymology:
Dedicated to Ole S. Tendal, at the Natural History Museum of Denmark, for his many contributions to the knowledge of the sponge fauna of the North-eastern Atlantic and elsewhere.
Remarks:
Initial identification of this species was through the molecular phylogeny results in Hestetun et al. (2016b) showing two clearly separate clades of specimens identified as L. lycopodium. The new species is described based on specimens associated with the clade containing L. infundibulum in that phylogeny. Somewhat surprising as they are not sister taxa in the molecular phylogeny, this species is very like L. lycopodium in most respects, with a comparable habit, a similar spicule component, and overlapping distribution and habitat, with specimens of both species being recovered from the same area in at least three cases.
However, careful investigation has identified a couple of consistent differences: The main difference is the presence of styles exceeding 1500 µm, and the presence of these should be considered diagnostic for the new species. Shorter basal styles have not been observed in the new species. The habit is somewhat different: The new species is usually shorter and wider than L. lycopodium, with a shorter, in some cases almost nonexisting, lower stem. Filaments are rigid, giving it a bristly appearance, and the long styles can often be seen protruding from the filaments in preserved specimens. The grooved, bud-like apex found in L. lycopodium also seems absent or reduced in L. tendali.
Lycopodina versatilis (Topsent, 1890) (Fig. 41; Table 5)
Original description:
Forcepia versatilisTopsent, 1890: 66.
Synonyms and citations:
Forcepia versatilis (Topsent, 1892: 100).
Diagnosis:
Small, pedunculate, Lycopodina with cylindrical body, megascleres subtylostyles only, palmate/arcuate anisochelae 27 µm, noncrossing forceps spicules 76 µm and embryos, where present, containing strongyles and oxeas.
Description:
Only the holotype is known from this species. The following description is based on Topsent (1890, 1892): Small, pedunculate, Lycopodina with ovoid, cylindrical body 8 × 10 mm, damaged during collection, possibly with shallow, cup-like depression. Peduncle 15 mm in length with basal part missing. Colour in ethanol light yellow (Fig. 41A).
Lycopodina versatilis (after Topsent, 1892, pl. VI fig. 5 and pl. X fig. 9A–H), (A) habit, (B) subtylostyle, (C) forceps, (D) embryonic strongyles and oxea, (E) anisochelae.
Skeleton:
The skeleton was not described in detail by Topsent, but is presumably similar to related species, that is peduncle made up of tightly packed subtylostyles that diverge to form the main body. No shorter type or tapering subtylostyles, which are present and projecting from the sponge surface in the related species L. comata and L. infundibulum, were recorded by Topsent.
Spicules:
Subtylostyles, straight and fusiform, in the peduncle and body, 580 µm long (from Topsent, 1892) (Fig. 41B).
Oxeas, strongyles, associated with larvae or embryos (from Topsent, 1892) (Fig. 41D).
Palmate/arcuate anisochelae, with arched shaft, 27 µm (from Topsent, 1892) (Fig. 41E).
Forceps spicules, with ends slightly converging, then diverging, 76 µm (from Topsent, 1892) (Fig. 41C).
Distribution:
The species is known only from its type locality, station 161 of 1887 ‘L’Hirondelle’ cruise. The coordinates given by Topsent place the station on the Grand Banks at a depth of 1267 m. As this area has a maximum depth of some 200 m, there is an error in either the coordinates or the depth recorded, stemming from the station list of the cruise. Here, we have chosen to retain the coordinates rather than the depth (Fig. 29).
Related species:
Lycopodina infundibulum (Levinsen, 1887); L.comata (Lundbeck, 1905); L. minuta (Lambe, 1900).
Remarks:
The initial description (Topsent, 1890) mentions sigmas, but in the second description (Topsent, 1892) these are instead considered to be growth forms of the anisochelae. Like L. infundibulum there are special smaller megascleres associated with embryos, and microsclere measurements are similar. However, there is no mention of any tapering subtylostyles, and the forceps spicules are not crossed as in L. infundibulum.
Identification key
B. Chelae are palmate (Fig. 42G–M) 3
2. A. Chelae are isochelae (Fig. 42A) (genus Chondrocladia, subgenus Chondrocladia 4
B. Chelae are anisochelae (Fig. 42B) (genus Cladorhiza 5
3. A. One small and one large type of palmate anisochela (Fig. 42G–J), sigmancistras present (genus Asbestopluma, subgenus Asbestopluma) 12
B. One kind of palmate anisochela only (Fig. 42K–M), no sigmancistras. Forceps spicules may be present (Fig. 42N–O) (genus Lycopodina) 15
4. A. Two size categories of anchorate isochela, sponge is a single stem, branch processes with terminal swellings. Wide amphi-Atlantic, boreo-Arctic distribution Chondrocladia (C.) grandis
B. One type of anchorate isochela only, sponge is bifurcated, branch processes with subterminal swellings Chondrocladia (C.) virgata
5. A. The morphology is small and stipitate or long, but thin and filamentous, acanthoxeas present 6
B. The morphology is branching and bush-like, no acanthoxeas 7
6. A. Sponge composed of long but thin filament-like branches with two oblique filament rows. Acanthoxeas have large, solid spines Cladorhiza kenchingtonae
B. Sponge is small, stipitate or clavate, with a small number of filaments in all directions. Acanthoxeas have small spines or knobs Cladorhiza arctica
7. A. Megascleres are oxeas. Large, solid sponge, possibly with denuded areas of the stem and a white to red-brown colour Cladorhiza oxeata
B. Megascleres are mycalostyles 8
8. A. The softer tissue surrounding the stem is thick, partly anastomosed creating a lacunose or ‘spongy’ impression and falls easily off the central stem. Large sigmas have sharp outwards-pointing tips (Fig. 42D) Cladorhiza corticocancellata
B. Soft tissue surrounding the central stem is thin and does not easily detach, individual filaments are usually not anastomosed to a great degree 9
9. A. Sigmas and sigmancistras are roughly the same size (both ~40–50 µm), but can be distinguished by shape (Fig. 42E, F). Branch ends have clearly visible swellings Cladorhiza tenuisigma
B. Sigmas are much larger than sigmancistras, commonly 80–180 µm depending on species (Fig. 42C). Sigmancistras may be rare 10
10. A. Anchorate anisochelae have seven teeth in the larger end Cladorhiza iniquidentata
B. Anchorate anisochelae have five teeth in the larger end 11
11. A. Habitus fine, branching, often with slightly swollen branch ends. Anchorate anisochelae are usually in the range of 18–25 µm, sigmas 60–120 µm Cladorhiza abyssicola
B. Habitus is slightly coarser, no obvious swelling in branch ends, numerous filaments. Side branches often in a single plane. Anchorate anisochelae are usually 25–35 µm, sigmas 80–179 µm Cladorhiza gelida
12. A. Single-stem pennate sponge with opposite rows of filaments, large palmate anisochelae with upper and lower teeth and alae almost touching and a curved shaft (Fig. 42J) Asbestopluma (A.) ruetzleri
B. Large palmate anisochelae with straight shaft and upper alae straight perpendicular to the shaft, up to 50% of total shaft length only (Fig. 42G–I) 13
13. A. Sponge with repeatedly bifurcating stem with two opposite rows of filaments. Filaments may be reduced to stubs. Branch ends often with bifurcated swellings. Slightly small spicule sizes: subtylostyles often around 300–400 µm and larger anisochelae typically 45–55 µm (Fig. 42H) Asbestopluma (A.) furcata
B. Sponge is a single stem. Spicule sizes are slightly larger than indicated for 13A 14
14. A. Single-stem with opposite rows. Larger palmate anisochela upper teeth and alae about 50% of total length of spicule (Fig. 42I) Asbestopluma (A.) pennatula
B. Sponge is a single stem, but with four to six filament rows evenly placed around the stem. Larger palmate anisochela upper teeth and alae clearly less than 50% of total length of spicule (Fig. 42G) Asbestopluma (A.) bihamatifera
15. A. Palmate anisochelae are clearly palmate, with a roughly oval outline when viewed from the front (Fig. 42K, L). Morphology is single stem with filaments, partly coalesced or leaf-shaped, or small stipitate with filaments 16
B. Palmate anisochelae are palmate to arcuate, with a more angular outline (Fig. 42M). Morphology is stipitate with a bud- or cup-like body without obvious long filaments 22
16. A. Small stipitate sponge ~1 cm in total length with a roughly spherical body with filaments mostly directed upwards Lycopodina hydra
B. Sponge is erect, with a short stem and an elongated cylindrical or partly leaf-shaped body with filaments or coalesced ridges 17
17. A. Sponge is stipitate with a roughly cylindrical elongated body with filaments in all directions. Forceps spicules, when found, are not straight, but have both legs curving in a convex shape toward the ends (Fig. 42N) 18
B. Sponge is stipitate and cylindrical, roughly pinnate or with an apical plate that may give the sponge a leaf-shaped morphology. Single filaments may be present, but are mostly coalesced into ridges or swellings on the surface of the sponge. Forceps spicules, when found, have straight legs (Fig. 42O). Anisochelae are usually 21–28 µm long 20
18. A. Styles can reach a length of 3 mm and are routinely longer than 1500 µm. Basal stem is short, and sponge lacks apical grooved swelling Lycopodina tendali
B. Styles are less than 1500 µm 19
19. A. Palmate anisochelae are around 16–24 µm Lycopodina novangliae
B. Palmate anisochelae are around 11–16 µm Lycopodina lycopodium
20. A. Sponge is stipitate and elongate, cylindrical and somewhat club-shaped with side branches directed upwards causing a grooved surface Lycopodina robusta
B. Sponge is erect, but pennate or flattened, with an apical structure that can range in size from a couple of mm at the top to large parts of the stem giving creating a partial leaf-shaped habit 21
21. A. Sponge has a cylindrical lower stem with a clear, abrupt transition into an apical plate-like upper part Lycopodina ruijsi
B. Sponge is either pennate to club-like with partly coalesced filaments or cylindrical gradually tapering off into a top plate-like part creating a flattened drop or leaf shape Lycopodina cupressiformis.
22. A. Sponge stipitate with a cup-shaped body, megascleres include special tapering styles to subtylostyles 23
B. Sponge is stipitate with a cup-shaped or bud-shaped body. Tapering subtylostyles are lacking 24
23. A. Tapering subtylostyles are polytylote and may reach 1400 µm, total sponge length ~10 mm, body 2 mm in diameter Lycopodina comata
B. Tapering subtylostyles only very slightly tylote, usually in the range of 400–800 µm, total sponge length up to 3–4 cm Lycopodina infundibulum
24. A. Body is either cup-shaped or bud-shaped. Forceps spicules lacking. Megascleres include styles, subtylostyles and smaller tylostyles. Anisochelae 11–15 µm. Total length 3–22 mm Lycopodina minuta
B. Body is bud-shaped. Forceps spicules present. Anisochelae are 27 µm Lycopodina versatilis
Selected microscope images of cladorhizid microscleres for use with the key provided. (A) anchorate isochela (Chondrocladia (C.) grandis), (B) anchorate anisochela (Cladorhiza abyssicola), (C–E) sigmas (Cl. abyssicola, Cl. corticocancellata and Cl. tenuisigma), (F) sigmancistra (Cl. tenuisigma), (G–J) palmate anisochelae (Asbestopluma (A.) bihamatifera, A. (A.) furcata, A. (A.) pennatula and A. (A.) ruetzleri), (K, L) palmate anisochelae (Lycopodina lycopodium and L. cupressiformis), (M) palmate/arcuate anisochela (L. infundibulum), (N, O) forceps spicules (L. lycopodium and L. cupressiformis).
PHYLOGENETIC RESULTS
Suitable specimens were sequenced in order to complement existing sequences from Hestetun et al. (2016b), further adding to the molecular data currently available for cladorhizid sponges. The analysis includes all cladorhizid sequences from Hestetun et al. (2016b) with some clades collapsed in order to focus on the position of the newly sequenced species Chondrocladia (C.) grandis, Cladorhiza kenchingtonae, C. oxeata and Lycopodina minuta. In addition to generally showing the phylogenetic relationships of these species, we also wanted to use this molecular data to help address to questions in particular: Whether C. (C.) gigantea represents a synonym of C. (C.) grandis, and whether L. minuta represents a species distinct from L. infundibulum.
The general results are similar to those of the phylogeny, using mostly the same data set, presented in Hestetun et al. (2016b), including the polyphyly of subgenus Chondrocladia along a split between ‘Crinorhiza’ and ‘concrescens’ type Chondrocladia species (Fig. 43). A clade containing all C. (C.) grandis and C. (C.) gigantea sequences was recovered with high support (BS = 98; PP = 1), close to C. (C.) virgata (BS = 98; PP = 1), and sequences from specimens identified as C. (C.) grandis/gigantea from the Scotian Shelf (Sable Gully) and Baffin Bay were indistinguishable from earlier sequences of C. (C.) gigantea from Svalbard (all sequences identical except two silent third codon changes for the Scotian Shelf specimen). This strongly supports the hypothesis that C. (C.) gigantea is a synonym of C. (C.) grandis. The two specimens from Jan Mayen identified as Lycopodina minuta were recovered as a sister group to the existing sequences of L. infundibulum from Hestetun et al. (2016b) (BS = 100; PP = 1), with a distance supporting the hypothesis that they are closely related, but separate species. This finding is in accordance with the similar, but distinct, spicule and other morphological characters for these two species. The new species Lycopodina tendali was, as in Hestetun et al. (2016b), recovered in a sister position to the clade containing L. infundibulum and L. minuta. Sequences could not be obtained from the remaining new Lycopodina species, L. novangliae and the new Asbestopluma species Asbestopluma (A.) ruetzleri.
As expected based on morphological characters, Cladorhiza oxeata was recovered within the group of Cladorhiza species representing the North Atlantic arbuscular forms, which are closely related to each other. In concordance with its different morphology and the presence of accessory acanthoxeas, C. kenchingtonae, while part of Cladorhiza, was recovered outside this particular clade in a sister position (BS = 99; PP = 1).
DISCUSSION
The GIN seas
The Greenland-Icelandic-Norwegian (GIN) Seas is a partly isolated deep-sea basin with the Arctic Mid-Ocean Ridge (AMOR) in the middle, bordered by ample shelf areas and the shallower Barents and Kara Seas. To the south, it is enclosed by the Greenland-Iceland-Faroe (GIF) Ridge and is connected to the polar basin through the Fram Strait, west of Svalbard (Fig. 44). The area represents the main part of the species records known for the NE Atlantic, and the species composition of adjacent areas is similar, or a subset of, the cladorhizid fauna of the GIN Seas discussed here. For the wider distribution of NE Atlantic species, see Figs 1, 2, 9, 14, 15, 29 and 30.
A map showing collection records of known cladorhizids from the GIN Seas and adjacent areas.
This area is comparatively well sampled, and thus, there are many species records from this area (see references in section 1). Seventeen species have been registered here, with most records on the surrounding shelf and slope, especially the heavily sampled GIF Ridge (Fig. 44). Of these, five species have been reported only from the GIN Seas, nine species have a GIN Seas and wider Arctic distribution including Baffin Bay and the Davis Strait, two species are shared between the GIN Seas and the North American Atlantic Shelf and four species have been reported from both the GIN Seas and the southern North Atlantic (Table 10).
Summary of the distribution of Atlantic boreo-Arctic Cladorhizidae
| Southern N Atlantic | NW Atlantic | NE Atlantic Banks | GIN Seas | Barents, Kara Seas | Baffin Bay, Labrador Sea | |
| Asbestopluma | ||||||
| A. (A.) bihamatifera | X | X | X | |||
| A. (A.) furcata | X | X | X | |||
| A. (A.) pennatula | X | X | X | X | ||
| A. (A.) ruetzleri | X | X | ||||
| Chondrocladia | ||||||
| C. (C.) grandis | X | X | X | X | ||
| Cladorhiza | ||||||
| C. abyssicola | X | X | X | X | X | |
| C. arctica | X | |||||
| C. corticocancellata | X | |||||
| C. gelida | X | X | X | X | ||
| C. iniquidentata | X | |||||
| C. kenchingtonae | X | |||||
| C. oxeata | X | X | ||||
| C. tenuisigma | X | |||||
| Lycopodina | ||||||
| L. comata | X | |||||
| L. cupressiformis | X | X | X | X | ||
| L. hydra | X | |||||
| L. infundibulum | X | X | X | |||
| L. lycopodium | X | X | X | X | ||
| L. novangliae | X | |||||
| L. minuta | X | X | X | |||
| L. robusta | X | |||||
| L. ruijsi | X | |||||
| L. tendali | X | |||||
| L. versatilis | X |
| Southern N Atlantic | NW Atlantic | NE Atlantic Banks | GIN Seas | Barents, Kara Seas | Baffin Bay, Labrador Sea | |
| Asbestopluma | ||||||
| A. (A.) bihamatifera | X | X | X | |||
| A. (A.) furcata | X | X | X | |||
| A. (A.) pennatula | X | X | X | X | ||
| A. (A.) ruetzleri | X | X | ||||
| Chondrocladia | ||||||
| C. (C.) grandis | X | X | X | X | ||
| Cladorhiza | ||||||
| C. abyssicola | X | X | X | X | X | |
| C. arctica | X | |||||
| C. corticocancellata | X | |||||
| C. gelida | X | X | X | X | ||
| C. iniquidentata | X | |||||
| C. kenchingtonae | X | |||||
| C. oxeata | X | X | ||||
| C. tenuisigma | X | |||||
| Lycopodina | ||||||
| L. comata | X | |||||
| L. cupressiformis | X | X | X | X | ||
| L. hydra | X | |||||
| L. infundibulum | X | X | X | |||
| L. lycopodium | X | X | X | X | ||
| L. novangliae | X | |||||
| L. minuta | X | X | X | |||
| L. robusta | X | |||||
| L. ruijsi | X | |||||
| L. tendali | X | |||||
| L. versatilis | X |
Note: Southern N Atlantic refers to the area south of the NAC, NW Atlantic to the area between New England and the Flemish Cap and NE Atlantic Banks to the banks to the west of the British Isles.
Summary of the distribution of Atlantic boreo-Arctic Cladorhizidae
| Southern N Atlantic | NW Atlantic | NE Atlantic Banks | GIN Seas | Barents, Kara Seas | Baffin Bay, Labrador Sea | |
| Asbestopluma | ||||||
| A. (A.) bihamatifera | X | X | X | |||
| A. (A.) furcata | X | X | X | |||
| A. (A.) pennatula | X | X | X | X | ||
| A. (A.) ruetzleri | X | X | ||||
| Chondrocladia | ||||||
| C. (C.) grandis | X | X | X | X | ||
| Cladorhiza | ||||||
| C. abyssicola | X | X | X | X | X | |
| C. arctica | X | |||||
| C. corticocancellata | X | |||||
| C. gelida | X | X | X | X | ||
| C. iniquidentata | X | |||||
| C. kenchingtonae | X | |||||
| C. oxeata | X | X | ||||
| C. tenuisigma | X | |||||
| Lycopodina | ||||||
| L. comata | X | |||||
| L. cupressiformis | X | X | X | X | ||
| L. hydra | X | |||||
| L. infundibulum | X | X | X | |||
| L. lycopodium | X | X | X | X | ||
| L. novangliae | X | |||||
| L. minuta | X | X | X | |||
| L. robusta | X | |||||
| L. ruijsi | X | |||||
| L. tendali | X | |||||
| L. versatilis | X |
| Southern N Atlantic | NW Atlantic | NE Atlantic Banks | GIN Seas | Barents, Kara Seas | Baffin Bay, Labrador Sea | |
| Asbestopluma | ||||||
| A. (A.) bihamatifera | X | X | X | |||
| A. (A.) furcata | X | X | X | |||
| A. (A.) pennatula | X | X | X | X | ||
| A. (A.) ruetzleri | X | X | ||||
| Chondrocladia | ||||||
| C. (C.) grandis | X | X | X | X | ||
| Cladorhiza | ||||||
| C. abyssicola | X | X | X | X | X | |
| C. arctica | X | |||||
| C. corticocancellata | X | |||||
| C. gelida | X | X | X | X | ||
| C. iniquidentata | X | |||||
| C. kenchingtonae | X | |||||
| C. oxeata | X | X | ||||
| C. tenuisigma | X | |||||
| Lycopodina | ||||||
| L. comata | X | |||||
| L. cupressiformis | X | X | X | X | ||
| L. hydra | X | |||||
| L. infundibulum | X | X | X | |||
| L. lycopodium | X | X | X | X | ||
| L. novangliae | X | |||||
| L. minuta | X | X | X | |||
| L. robusta | X | |||||
| L. ruijsi | X | |||||
| L. tendali | X | |||||
| L. versatilis | X |
Note: Southern N Atlantic refers to the area south of the NAC, NW Atlantic to the area between New England and the Flemish Cap and NE Atlantic Banks to the banks to the west of the British Isles.
Species that have also been reported from the southern part of the North Atlantic are Cladorhiza abyssicola, C. gelida, Asbestopluma (A.) pennatula and Lycopodina infundibulum. Cladorhiza abyssicola seems to be a special case: It has the widest distribution of all cladorhizids presented here, ranging from Cape Verde, the Mediterranean to the GIN Seas, North American Shelf and Baffin Bay. Most records of this species are comparatively shallow (<1000 m) with more southern records a mix of shallow and larger depths. Cladorhiza gelida seems to have a eurybathic, although generally deeper distribution, and could be found in the North Atlantic basin, giving it a wide distribution range. Asbestopluma (A.) pennatula and L. infundibulum both have a few scattered records at the Mid-Atlantic Ridge and (for L. infundibulum) in the Gulf of Cadiz and at the Barbados Accretionary Prism. These records are in the deeper part of their depth range, compared to their typical boreo-Arctic depth (Table 11).
A list of species from Atlantic continental shelf areas and islands south of the North Atlantic Current and north of the equator, including the Mediterranean and Caribbean Seas
| Species | Areas | Source | Depth |
| N Atlantic south of NAC | |||
| Asbestopluma (A.) gracilior | Florida Straits | Schmidt, 1870 | 580–640 m |
| Asbestopluma (A.) pennatula* | Lucky Strike (MAR) | Desbruyères et al., 2001 | 1700 m |
| Asbestopluma (H.) stylivarians | Tenerife | Topsent, 1929 | 1340–1530 m |
| Chondrocladia (C.) amphactis | Barbados | Schmidt, 1880 | 527 m |
| Chondrocladia (C.) concrescens | Caribbean Sea | Schmidt, 1880 | 825–1342 m |
| Chondrocladia (C.) verticillata | Caribbean Sea | Topsent, 1920; Hestetun et al., 2016a | 1000–1472 m |
| Chondrocladia (C.) robertballardi | Gorringe and Galicia Banks | Cristobo et al., 2015 | 1400–1738 m |
| Chondrocladia (C.) virgata** | Gibraltar, Madeira, Canary Islands | Thomson, 1873; Arnesen, 1920 | 872–2603 m |
| Cladorhiza abyssicola* | Azores, Madeira, Tenerife, Cape Verde, Gulf of Cadiz | Topsent, 1909; van Soest, 1993; Boury-Esnault et al., 1994 | 1182–1968 m |
| Cladorhiza grimaldii | Madeira; Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 1700–1968 m |
| Cladorhiza methanophila | Barbados | unpub. R/V ‘Atlantis’ 21-02 material | 1450–4930 |
| Euchelipluma congeri | Loggerhead Key (Caribbean) | de Laubenfels, 1936 | 1047 m |
| Euchelipluma pristina | Cape Verde, Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 91–1700 m |
| Lycopodina hypogea | English Channel, Bay of Biscay, Galician Coast, Gulf of Cadiz | Chevaldonné et al., 2015 | 5–50; 494 m |
| Lycopodina infundibulum* | Lucky Strike (MAR), Barbados Accretionary Prism, Gulf of Cadiz | Boury-Esnault et al., 1994; Desbruyères et al., 2001; Hestetun et al., 2015 | 1590–1947 m |
| Mediterranean | |||
| Cladorhiza abyssicola* | Alboran Sea, West Mediterranean and the Adriatic Sea | Babić, 1922; Vacelet, 1969; Boury-Esnault et al., 1994 | 200–715 m |
| Lycopodina hypogea | Submarine caves and deep-sea distribution from Alboran Sea to Adriatic | Vacelet & Boury-Esnault, 1996; Bakran-Petricioli et al., 2007; Aguilar et al., 2011; Chevaldonné et al., 2015 | 18–26; 100–623 m |
| Species | Areas | Source | Depth |
| N Atlantic south of NAC | |||
| Asbestopluma (A.) gracilior | Florida Straits | Schmidt, 1870 | 580–640 m |
| Asbestopluma (A.) pennatula* | Lucky Strike (MAR) | Desbruyères et al., 2001 | 1700 m |
| Asbestopluma (H.) stylivarians | Tenerife | Topsent, 1929 | 1340–1530 m |
| Chondrocladia (C.) amphactis | Barbados | Schmidt, 1880 | 527 m |
| Chondrocladia (C.) concrescens | Caribbean Sea | Schmidt, 1880 | 825–1342 m |
| Chondrocladia (C.) verticillata | Caribbean Sea | Topsent, 1920; Hestetun et al., 2016a | 1000–1472 m |
| Chondrocladia (C.) robertballardi | Gorringe and Galicia Banks | Cristobo et al., 2015 | 1400–1738 m |
| Chondrocladia (C.) virgata** | Gibraltar, Madeira, Canary Islands | Thomson, 1873; Arnesen, 1920 | 872–2603 m |
| Cladorhiza abyssicola* | Azores, Madeira, Tenerife, Cape Verde, Gulf of Cadiz | Topsent, 1909; van Soest, 1993; Boury-Esnault et al., 1994 | 1182–1968 m |
| Cladorhiza grimaldii | Madeira; Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 1700–1968 m |
| Cladorhiza methanophila | Barbados | unpub. R/V ‘Atlantis’ 21-02 material | 1450–4930 |
| Euchelipluma congeri | Loggerhead Key (Caribbean) | de Laubenfels, 1936 | 1047 m |
| Euchelipluma pristina | Cape Verde, Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 91–1700 m |
| Lycopodina hypogea | English Channel, Bay of Biscay, Galician Coast, Gulf of Cadiz | Chevaldonné et al., 2015 | 5–50; 494 m |
| Lycopodina infundibulum* | Lucky Strike (MAR), Barbados Accretionary Prism, Gulf of Cadiz | Boury-Esnault et al., 1994; Desbruyères et al., 2001; Hestetun et al., 2015 | 1590–1947 m |
| Mediterranean | |||
| Cladorhiza abyssicola* | Alboran Sea, West Mediterranean and the Adriatic Sea | Babić, 1922; Vacelet, 1969; Boury-Esnault et al., 1994 | 200–715 m |
| Lycopodina hypogea | Submarine caves and deep-sea distribution from Alboran Sea to Adriatic | Vacelet & Boury-Esnault, 1996; Bakran-Petricioli et al., 2007; Aguilar et al., 2011; Chevaldonné et al., 2015 | 18–26; 100–623 m |
Note: Species that are also found in the boreal Atlantic and Arctic are marked with an asterisk.
*Have also been reported from the area covered by this article.
**Included as species description in this article even if no known presence in the boreal Atlantic.
A list of species from Atlantic continental shelf areas and islands south of the North Atlantic Current and north of the equator, including the Mediterranean and Caribbean Seas
| Species | Areas | Source | Depth |
| N Atlantic south of NAC | |||
| Asbestopluma (A.) gracilior | Florida Straits | Schmidt, 1870 | 580–640 m |
| Asbestopluma (A.) pennatula* | Lucky Strike (MAR) | Desbruyères et al., 2001 | 1700 m |
| Asbestopluma (H.) stylivarians | Tenerife | Topsent, 1929 | 1340–1530 m |
| Chondrocladia (C.) amphactis | Barbados | Schmidt, 1880 | 527 m |
| Chondrocladia (C.) concrescens | Caribbean Sea | Schmidt, 1880 | 825–1342 m |
| Chondrocladia (C.) verticillata | Caribbean Sea | Topsent, 1920; Hestetun et al., 2016a | 1000–1472 m |
| Chondrocladia (C.) robertballardi | Gorringe and Galicia Banks | Cristobo et al., 2015 | 1400–1738 m |
| Chondrocladia (C.) virgata** | Gibraltar, Madeira, Canary Islands | Thomson, 1873; Arnesen, 1920 | 872–2603 m |
| Cladorhiza abyssicola* | Azores, Madeira, Tenerife, Cape Verde, Gulf of Cadiz | Topsent, 1909; van Soest, 1993; Boury-Esnault et al., 1994 | 1182–1968 m |
| Cladorhiza grimaldii | Madeira; Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 1700–1968 m |
| Cladorhiza methanophila | Barbados | unpub. R/V ‘Atlantis’ 21-02 material | 1450–4930 |
| Euchelipluma congeri | Loggerhead Key (Caribbean) | de Laubenfels, 1936 | 1047 m |
| Euchelipluma pristina | Cape Verde, Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 91–1700 m |
| Lycopodina hypogea | English Channel, Bay of Biscay, Galician Coast, Gulf of Cadiz | Chevaldonné et al., 2015 | 5–50; 494 m |
| Lycopodina infundibulum* | Lucky Strike (MAR), Barbados Accretionary Prism, Gulf of Cadiz | Boury-Esnault et al., 1994; Desbruyères et al., 2001; Hestetun et al., 2015 | 1590–1947 m |
| Mediterranean | |||
| Cladorhiza abyssicola* | Alboran Sea, West Mediterranean and the Adriatic Sea | Babić, 1922; Vacelet, 1969; Boury-Esnault et al., 1994 | 200–715 m |
| Lycopodina hypogea | Submarine caves and deep-sea distribution from Alboran Sea to Adriatic | Vacelet & Boury-Esnault, 1996; Bakran-Petricioli et al., 2007; Aguilar et al., 2011; Chevaldonné et al., 2015 | 18–26; 100–623 m |
| Species | Areas | Source | Depth |
| N Atlantic south of NAC | |||
| Asbestopluma (A.) gracilior | Florida Straits | Schmidt, 1870 | 580–640 m |
| Asbestopluma (A.) pennatula* | Lucky Strike (MAR) | Desbruyères et al., 2001 | 1700 m |
| Asbestopluma (H.) stylivarians | Tenerife | Topsent, 1929 | 1340–1530 m |
| Chondrocladia (C.) amphactis | Barbados | Schmidt, 1880 | 527 m |
| Chondrocladia (C.) concrescens | Caribbean Sea | Schmidt, 1880 | 825–1342 m |
| Chondrocladia (C.) verticillata | Caribbean Sea | Topsent, 1920; Hestetun et al., 2016a | 1000–1472 m |
| Chondrocladia (C.) robertballardi | Gorringe and Galicia Banks | Cristobo et al., 2015 | 1400–1738 m |
| Chondrocladia (C.) virgata** | Gibraltar, Madeira, Canary Islands | Thomson, 1873; Arnesen, 1920 | 872–2603 m |
| Cladorhiza abyssicola* | Azores, Madeira, Tenerife, Cape Verde, Gulf of Cadiz | Topsent, 1909; van Soest, 1993; Boury-Esnault et al., 1994 | 1182–1968 m |
| Cladorhiza grimaldii | Madeira; Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 1700–1968 m |
| Cladorhiza methanophila | Barbados | unpub. R/V ‘Atlantis’ 21-02 material | 1450–4930 |
| Euchelipluma congeri | Loggerhead Key (Caribbean) | de Laubenfels, 1936 | 1047 m |
| Euchelipluma pristina | Cape Verde, Lucky Strike (MAR) | Topsent, 1909; Desbruyères et al., 2001 | 91–1700 m |
| Lycopodina hypogea | English Channel, Bay of Biscay, Galician Coast, Gulf of Cadiz | Chevaldonné et al., 2015 | 5–50; 494 m |
| Lycopodina infundibulum* | Lucky Strike (MAR), Barbados Accretionary Prism, Gulf of Cadiz | Boury-Esnault et al., 1994; Desbruyères et al., 2001; Hestetun et al., 2015 | 1590–1947 m |
| Mediterranean | |||
| Cladorhiza abyssicola* | Alboran Sea, West Mediterranean and the Adriatic Sea | Babić, 1922; Vacelet, 1969; Boury-Esnault et al., 1994 | 200–715 m |
| Lycopodina hypogea | Submarine caves and deep-sea distribution from Alboran Sea to Adriatic | Vacelet & Boury-Esnault, 1996; Bakran-Petricioli et al., 2007; Aguilar et al., 2011; Chevaldonné et al., 2015 | 18–26; 100–623 m |
Note: Species that are also found in the boreal Atlantic and Arctic are marked with an asterisk.
*Have also been reported from the area covered by this article.
**Included as species description in this article even if no known presence in the boreal Atlantic.
The North American Atlantic Coast and Arctic
Compared to the NE Atlantic, the cladorhizid fauna of the Eastern North America is less studied, and the only previously published information is a couple of records by Lambe (1896, 1900b) in the Gulf of Saint Lawrence and records by Verrill mainly of specimens from the Scotian Shelf (Verrill, 1879, 1885). In this article, we present these and new records from an area of the shelf from off North Carolina up to and including the Flemish Cap (Fig. 45). While the southern extent of this fauna is not known due to lack of records from the Atlantic off the SE United States, records from the Florida Keys and Caribbean show that this area has a different species composition (Hestetun, Pomponi & Rapp, 2016; Schmidt, 1870, 1880).
A map showing collection records of all cladorhizids from the shelf of North-east USA and South-East Canada.
Based on the records examined here, the cladorhizid fauna of this region seems to be made up of either (1) amphi-Atlantic species also found in the NE Atlantic or (2) indigenous species that are closely related to NE Atlantic fauna. In the first category are Asbestopluma (A.) furcata, Chondrocladia (C.) grandis, Cladorhiza gelida and possibly C. abyssicola (a couple of records are unreliable, but the species is present in adjacent Arctic areas), while Asbestopluma (A.) ruetzleri sp. nov., Lycopodium novangliae sp. nov. and possibly L. versatilis are in the second category. Cl. kenchingtonae, collected from the Southern Slope of Flemish Cap, represents a special case, as the depth reported for this species is lower bathyal (2935 m) rather than on the shelf itself.
In adjacent Arctic areas, R/V ‘Paamiut’ trawl surveys have collected large numbers of Ch. (C.) grandis and Cl. oxeata in Baffin Bay and at the Davis Strait. Earlier records from Lambe (1900a) and Brøndsted (1933) show that Cl. abyssicola, L. cupressiformis, L. lycopodium (or possibly L. tendali) and L. minuta are also present in this area, and numerous cladorhizids strongly resembling Cl. abyssicola were recorded from video transects during the Lar2012-002 cruise. Collection activity (Hud2013-029) from the Hatton Basin Voluntary Closure Area (Northern Labrador Sea) and nearby Davis Strait recovered a mix of Asbestopluma (A.) bihamatifera, A. (A.) pennatula and A. (A.) ruetzleri (Fig. 2), which suggests at least some overlap in the distribution of these species, although any further extent is unknown. The current limited Asbestopluma distribution in this area may also in part reflect selectivity in sampling gear. For example, Asbestopluma specimens were not recovered during the R/V ‘Paamiut’ trawl surveys, but were recovered in van Veen grab samples from the same areas. Presumably they are either so small they pass through the trawl net or are passed over by the trawl.
The Russian Arctic and its Relationship with the North Pacific
Almost all of the species treated here with a Russian Arctic distribution also have records farther south, especially in the GIN Seas (Table 10) (Koltun, 1970b). Precise locality information is lacking for most of the species records from the Soviet expeditions in the Laptev, East Siberian and Chukchi Seas. However, the general distribution records indicate that the cladorhizid species in these areas are species also found in, for example the GIN Seas, including records of Asbestopluma (A.) pennatula, Lycopodina infundibulum, L. Lycopodium and L. cupressiformis (Gorbunov, 1946; Koltun, 1959, 1970b; Rezvoj, 1928). Most of these Arctic records are from comparatively shallow depths. One species is known from the slopes of the Polar Basin only, Cladorhiza arctica Koltun, 1959 at 2040–2365 m depth (Gorbunov, 1946; Koltun, 1959), although Koltun also considers C. gelida to be a part of the lower bathyal to abyssal Arctic fauna (Koltun, 1970b).
Unsurprisingly, a shift in the species distribution occurs at the Bering Strait, with a different set of species reported from the Bering Sea and farther south, such as at the Aleutian Islands, Kuriles and the Okhotsk Sea (e.g. Koltun, 1955, 1958, 1970a) (see Downey & Janussen, 2015 for a review).
Southern North Atlantic and Mediterranean Cladorhizids
While not implying a causal relationship, the North Atlantic Current coincides with a shift in the cladorhizid species composition and thus provides a good cut-off point for the Arctic and boreal North-Atlantic fauna. The Southern North Atlantic is used here to define the Atlantic south of the NAC, but north of the equator (see Section 1). Several indigenous species have been reported this area, but the separation is not complete, and a couple of species present in the boreal Atlantic have been reported at specific localities south of the NAC, usually at lower depths. From the Mediterranean, only the species Lycopodina hypogea (also found on the SW Europe Atlantic coast) and Cladorhiza abyssicola (wide Atlantic distribution) have been recorded. A list of known cladorhizid species from south of the NAC and the Mediterranean is given in Table 11.
Depth Distribution
The vast majority of the records of the species treated here are at less than 2000 m depth, but with scattered deeper records (Fig. 46). Exceptions to this are the species Cladorhiza kenchingtonae and C. arctica, and the special case of C. gelida, which has an unusually large eurybathic depth range. The deep Atlantic Ocean (>2500 m depth) features a mostly different, but less well-known species composition to that of the rest of the North Atlantic. Some species, such as C. methanophila, are clearly close relatives of shallower species, while others, such as the reported Abyssocladia species, or the mostly stipitate Chondrocladia species, are clearly different (Table 12).
Depth distribution of Atlantic boreo-Arctic cladorhizid species described in this article.
A list of species from the lower bathyal and abyssal North Atlantic
| Species | Areas | Source | Depth (m) |
| Abyssocladia polycephalus | Muir Seamount | Hestetun et al. (2016a) | 2829 |
| Abyssocladia tecta | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Asbestopluma (A.) caribica | Venezuela Basin | Hestetun et al. (2016a) | 4007 |
| Asbestopluma (A.) quadriserialis | West of the Azores | Tendal (1973) | 4600 |
| Chondrocladia (C.) albatrossi | Off NE Brazil | Tendal (1973) | 4450 |
| Chondrocladia (C.) burtoni | Cape Verde Plain | Tendal (1973) | 4870 |
| Chondrocladia (C.) guiteli | Off Galicia | Topsent (1904) | 4900 |
| Chondrocladia (C.) vaceleti | Off Iberian Peninsula | Hestetun et al. (2015) | 4260 |
| Cladorhiza aff. gelida* | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Cladorhiza kenchingtonae** | Flemish Cap | This article | 2935 |
| Cladorhiza methanophila | Barbados Accretionary Prism, Mid-Atlantic Ridge fracture zone at 15°20ʹN | Vacelet & Boury-Esnault (2002); Hestetun (2015); unpub. R/V ‘Atlantis’ 21-02 material | 2600–4935 |
| Cladorhiza thomsoni | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 4000 |
| Lycopodina parvula | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
| Lycopodina rastrichela | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
| Species | Areas | Source | Depth (m) |
| Abyssocladia polycephalus | Muir Seamount | Hestetun et al. (2016a) | 2829 |
| Abyssocladia tecta | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Asbestopluma (A.) caribica | Venezuela Basin | Hestetun et al. (2016a) | 4007 |
| Asbestopluma (A.) quadriserialis | West of the Azores | Tendal (1973) | 4600 |
| Chondrocladia (C.) albatrossi | Off NE Brazil | Tendal (1973) | 4450 |
| Chondrocladia (C.) burtoni | Cape Verde Plain | Tendal (1973) | 4870 |
| Chondrocladia (C.) guiteli | Off Galicia | Topsent (1904) | 4900 |
| Chondrocladia (C.) vaceleti | Off Iberian Peninsula | Hestetun et al. (2015) | 4260 |
| Cladorhiza aff. gelida* | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Cladorhiza kenchingtonae** | Flemish Cap | This article | 2935 |
| Cladorhiza methanophila | Barbados Accretionary Prism, Mid-Atlantic Ridge fracture zone at 15°20ʹN | Vacelet & Boury-Esnault (2002); Hestetun (2015); unpub. R/V ‘Atlantis’ 21-02 material | 2600–4935 |
| Cladorhiza thomsoni | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 4000 |
| Lycopodina parvula | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
| Lycopodina rastrichela | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
*Have also been reported from the area covered by this article.
**Included as species description in this article.
A list of species from the lower bathyal and abyssal North Atlantic
| Species | Areas | Source | Depth (m) |
| Abyssocladia polycephalus | Muir Seamount | Hestetun et al. (2016a) | 2829 |
| Abyssocladia tecta | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Asbestopluma (A.) caribica | Venezuela Basin | Hestetun et al. (2016a) | 4007 |
| Asbestopluma (A.) quadriserialis | West of the Azores | Tendal (1973) | 4600 |
| Chondrocladia (C.) albatrossi | Off NE Brazil | Tendal (1973) | 4450 |
| Chondrocladia (C.) burtoni | Cape Verde Plain | Tendal (1973) | 4870 |
| Chondrocladia (C.) guiteli | Off Galicia | Topsent (1904) | 4900 |
| Chondrocladia (C.) vaceleti | Off Iberian Peninsula | Hestetun et al. (2015) | 4260 |
| Cladorhiza aff. gelida* | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Cladorhiza kenchingtonae** | Flemish Cap | This article | 2935 |
| Cladorhiza methanophila | Barbados Accretionary Prism, Mid-Atlantic Ridge fracture zone at 15°20ʹN | Vacelet & Boury-Esnault (2002); Hestetun (2015); unpub. R/V ‘Atlantis’ 21-02 material | 2600–4935 |
| Cladorhiza thomsoni | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 4000 |
| Lycopodina parvula | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
| Lycopodina rastrichela | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
| Species | Areas | Source | Depth (m) |
| Abyssocladia polycephalus | Muir Seamount | Hestetun et al. (2016a) | 2829 |
| Abyssocladia tecta | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Asbestopluma (A.) caribica | Venezuela Basin | Hestetun et al. (2016a) | 4007 |
| Asbestopluma (A.) quadriserialis | West of the Azores | Tendal (1973) | 4600 |
| Chondrocladia (C.) albatrossi | Off NE Brazil | Tendal (1973) | 4450 |
| Chondrocladia (C.) burtoni | Cape Verde Plain | Tendal (1973) | 4870 |
| Chondrocladia (C.) guiteli | Off Galicia | Topsent (1904) | 4900 |
| Chondrocladia (C.) vaceleti | Off Iberian Peninsula | Hestetun et al. (2015) | 4260 |
| Cladorhiza aff. gelida* | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 3550 |
| Cladorhiza kenchingtonae** | Flemish Cap | This article | 2935 |
| Cladorhiza methanophila | Barbados Accretionary Prism, Mid-Atlantic Ridge fracture zone at 15°20ʹN | Vacelet & Boury-Esnault (2002); Hestetun (2015); unpub. R/V ‘Atlantis’ 21-02 material | 2600–4935 |
| Cladorhiza thomsoni | Mid-Atlantic Ridge fracture zone at 15°20ʹN | Hestetun et al. (2015) | 4000 |
| Lycopodina parvula | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
| Lycopodina rastrichela | Porcupine abyssal plain | Hestetun et al. (2015) | 4845 |
*Have also been reported from the area covered by this article.
**Included as species description in this article.
CONCLUDING REMARKS
We have reviewed the distribution of carnivorous sponges from the Boreal North Atlantic and Arctic using historical records and new collections and altogether have provided descriptions of 25 cladorhizid species – including 4 species that are new to science.
The results show that the boreal Atlantic and adjoining Arctic is home to a rich and distinct cladorhizid fauna roughly demarcated by the NAC to the south and extending to the Bering Strait. Despite being among the most heavily sampled marine regions in the world, uncertainties remain regarding the cladorhizid fauna of the NE Atlantic and adjoining Arctic, especially with regard to genus Lycopodina, where further molecular data are needed to demarcate species boundaries. The NW Atlantic contains a mix of amphi-Atlantic species also found in the NE Atlantic and Arctic and closely related indigenous species, although the extent of the distribution of several species from this area is still poorly known. Generally, the comparatively large (for deep-sea species) number of records in the boreal Atlantic and Arctic allows for the qualitative approximation of the distribution of cladorhizid species in these areas, which is a step up from the state of cladorhizids in other areas, where the extent and distribution of described species is virtually unknown.
The best tree from the maximum likelihood analysis of partial 28S, COI and ALG11 molecular data shown with rapid bootstrap values (1–100) from the ML analysis and posterior probabilities (0–1) from the Bayesian analysis indicated for nodes with support over 50/0.5. Sequences from this article indicated in bold. The tree is rooted using the non-carnivorous species Guitarra antarctica (Guitarridae). All other sequences are carnivorous sponges (Cladorhizidae). For accession numbers, see Table 9 (bold), Hestetun et al., 2016b (non-bold) and Hestetun et al. (2016a) (non-bold, indicated with one asterisk). L. tendali sp. nov., indicated with two asterisks, was given as L. lycopodium clade 2 in Hestetun et al. (2016b).
A list of specimens sequenced for this article with accession numbers indicated. For collection information, see the list of examined material for the species indicated
| Species | Sample | 28S | COI | ALG11 |
| Asbestopluma | ||||
| A. (A.) bihamatifera (Carter, 1876) | LAR2012-003 75-11 | KX266191 | KX266207 | KX266224 |
| Chondrocladia | ||||
| C. (C.) grandis (Verrill, 1879) 1 | PAA2012007025 | KX266196 | KX266210 | KX266226 |
| C. (C.) grandis (Verrill, 1879) 2 | PAA2012007031 | KX266211 | ||
| C. (C.) grandis (Verrill, 1879) 3 | PAA2012007042 | KX266197 | KX266212 | |
| C. (C.) grandis (Verrill, 1879) 4 | HUD20070251058 | KX266198 | KX266213 | KX266227 |
| C. (C.) grandis (Verrill, 1879) 5 | PAA2010009159 | KX266214 | ||
| C. (C.) grandis (Verrill, 1879) 6 | PAA2010009042 | KX266199 | KX266215 | |
| C. (C.) grandis (Verrill, 1879) 7 | PAA2010009044 | KX266200 | KX266216 | |
| C. (C.) grandis (Verrill, 1879) 8 | PAA2012007096 | KX266201 | KX266217 | |
| C. (C.) grandis (Verrill, 1879) 9 | PAA2013008021 | KX266202 | KX266218 | KX266228 |
| C. (C.) grandis (Verrill, 1879) 10 | PAA2012007148 | KX266203 | KX266219 | |
| Cladorhiza | ||||
| C. kenchingtonae sp. nov. | HUD20100291336 | KX266208 | ||
| C. oxeata Lundbeck, 1905 | PAA2010009147 | KX266193 | KX266221 | |
| C. oxeata Lundbeck, 1905 | PAA2012007030 | KX266192 | KX266209 | KX266225 |
| C. oxeata Lundbeck, 1905 | PAA2012007104 | KX266194 | ||
| C. oxeata Lundbeck, 1905 | PAA2013008057 | KX266195 | ||
| Lycopodina | ||||
| L. minuta (Lambe, 1900) | GeoBio 12ROV04 | KX266205 | KX266222 | KX266230 |
| L. minuta (Lambe, 1900) | GeoBio 12ROV14 | KX266206 | KX266223 | KX266231 |
| L. tendali sp. nov. | BIODEEP07ROV09 | KX266204 | KX266220 | KX266229 |
| Species | Sample | 28S | COI | ALG11 |
| Asbestopluma | ||||
| A. (A.) bihamatifera (Carter, 1876) | LAR2012-003 75-11 | KX266191 | KX266207 | KX266224 |
| Chondrocladia | ||||
| C. (C.) grandis (Verrill, 1879) 1 | PAA2012007025 | KX266196 | KX266210 | KX266226 |
| C. (C.) grandis (Verrill, 1879) 2 | PAA2012007031 | KX266211 | ||
| C. (C.) grandis (Verrill, 1879) 3 | PAA2012007042 | KX266197 | KX266212 | |
| C. (C.) grandis (Verrill, 1879) 4 | HUD20070251058 | KX266198 | KX266213 | KX266227 |
| C. (C.) grandis (Verrill, 1879) 5 | PAA2010009159 | KX266214 | ||
| C. (C.) grandis (Verrill, 1879) 6 | PAA2010009042 | KX266199 | KX266215 | |
| C. (C.) grandis (Verrill, 1879) 7 | PAA2010009044 | KX266200 | KX266216 | |
| C. (C.) grandis (Verrill, 1879) 8 | PAA2012007096 | KX266201 | KX266217 | |
| C. (C.) grandis (Verrill, 1879) 9 | PAA2013008021 | KX266202 | KX266218 | KX266228 |
| C. (C.) grandis (Verrill, 1879) 10 | PAA2012007148 | KX266203 | KX266219 | |
| Cladorhiza | ||||
| C. kenchingtonae sp. nov. | HUD20100291336 | KX266208 | ||
| C. oxeata Lundbeck, 1905 | PAA2010009147 | KX266193 | KX266221 | |
| C. oxeata Lundbeck, 1905 | PAA2012007030 | KX266192 | KX266209 | KX266225 |
| C. oxeata Lundbeck, 1905 | PAA2012007104 | KX266194 | ||
| C. oxeata Lundbeck, 1905 | PAA2013008057 | KX266195 | ||
| Lycopodina | ||||
| L. minuta (Lambe, 1900) | GeoBio 12ROV04 | KX266205 | KX266222 | KX266230 |
| L. minuta (Lambe, 1900) | GeoBio 12ROV14 | KX266206 | KX266223 | KX266231 |
| L. tendali sp. nov. | BIODEEP07ROV09 | KX266204 | KX266220 | KX266229 |
A list of specimens sequenced for this article with accession numbers indicated. For collection information, see the list of examined material for the species indicated
| Species | Sample | 28S | COI | ALG11 |
| Asbestopluma | ||||
| A. (A.) bihamatifera (Carter, 1876) | LAR2012-003 75-11 | KX266191 | KX266207 | KX266224 |
| Chondrocladia | ||||
| C. (C.) grandis (Verrill, 1879) 1 | PAA2012007025 | KX266196 | KX266210 | KX266226 |
| C. (C.) grandis (Verrill, 1879) 2 | PAA2012007031 | KX266211 | ||
| C. (C.) grandis (Verrill, 1879) 3 | PAA2012007042 | KX266197 | KX266212 | |
| C. (C.) grandis (Verrill, 1879) 4 | HUD20070251058 | KX266198 | KX266213 | KX266227 |
| C. (C.) grandis (Verrill, 1879) 5 | PAA2010009159 | KX266214 | ||
| C. (C.) grandis (Verrill, 1879) 6 | PAA2010009042 | KX266199 | KX266215 | |
| C. (C.) grandis (Verrill, 1879) 7 | PAA2010009044 | KX266200 | KX266216 | |
| C. (C.) grandis (Verrill, 1879) 8 | PAA2012007096 | KX266201 | KX266217 | |
| C. (C.) grandis (Verrill, 1879) 9 | PAA2013008021 | KX266202 | KX266218 | KX266228 |
| C. (C.) grandis (Verrill, 1879) 10 | PAA2012007148 | KX266203 | KX266219 | |
| Cladorhiza | ||||
| C. kenchingtonae sp. nov. | HUD20100291336 | KX266208 | ||
| C. oxeata Lundbeck, 1905 | PAA2010009147 | KX266193 | KX266221 | |
| C. oxeata Lundbeck, 1905 | PAA2012007030 | KX266192 | KX266209 | KX266225 |
| C. oxeata Lundbeck, 1905 | PAA2012007104 | KX266194 | ||
| C. oxeata Lundbeck, 1905 | PAA2013008057 | KX266195 | ||
| Lycopodina | ||||
| L. minuta (Lambe, 1900) | GeoBio 12ROV04 | KX266205 | KX266222 | KX266230 |
| L. minuta (Lambe, 1900) | GeoBio 12ROV14 | KX266206 | KX266223 | KX266231 |
| L. tendali sp. nov. | BIODEEP07ROV09 | KX266204 | KX266220 | KX266229 |
| Species | Sample | 28S | COI | ALG11 |
| Asbestopluma | ||||
| A. (A.) bihamatifera (Carter, 1876) | LAR2012-003 75-11 | KX266191 | KX266207 | KX266224 |
| Chondrocladia | ||||
| C. (C.) grandis (Verrill, 1879) 1 | PAA2012007025 | KX266196 | KX266210 | KX266226 |
| C. (C.) grandis (Verrill, 1879) 2 | PAA2012007031 | KX266211 | ||
| C. (C.) grandis (Verrill, 1879) 3 | PAA2012007042 | KX266197 | KX266212 | |
| C. (C.) grandis (Verrill, 1879) 4 | HUD20070251058 | KX266198 | KX266213 | KX266227 |
| C. (C.) grandis (Verrill, 1879) 5 | PAA2010009159 | KX266214 | ||
| C. (C.) grandis (Verrill, 1879) 6 | PAA2010009042 | KX266199 | KX266215 | |
| C. (C.) grandis (Verrill, 1879) 7 | PAA2010009044 | KX266200 | KX266216 | |
| C. (C.) grandis (Verrill, 1879) 8 | PAA2012007096 | KX266201 | KX266217 | |
| C. (C.) grandis (Verrill, 1879) 9 | PAA2013008021 | KX266202 | KX266218 | KX266228 |
| C. (C.) grandis (Verrill, 1879) 10 | PAA2012007148 | KX266203 | KX266219 | |
| Cladorhiza | ||||
| C. kenchingtonae sp. nov. | HUD20100291336 | KX266208 | ||
| C. oxeata Lundbeck, 1905 | PAA2010009147 | KX266193 | KX266221 | |
| C. oxeata Lundbeck, 1905 | PAA2012007030 | KX266192 | KX266209 | KX266225 |
| C. oxeata Lundbeck, 1905 | PAA2012007104 | KX266194 | ||
| C. oxeata Lundbeck, 1905 | PAA2013008057 | KX266195 | ||
| Lycopodina | ||||
| L. minuta (Lambe, 1900) | GeoBio 12ROV04 | KX266205 | KX266222 | KX266230 |
| L. minuta (Lambe, 1900) | GeoBio 12ROV14 | KX266206 | KX266223 | KX266231 |
| L. tendali sp. nov. | BIODEEP07ROV09 | KX266204 | KX266220 | KX266229 |
FUNDING
This study was supported by the Norwegian Biodiversity Information Centre (grant to HTR, project number 70184219), the Norwegian Academy of Science and Letters (grant to HTR) and the Research Council of Norway (through contract number 179560). This work has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 679849 (the SponGES project). The project was supported by an NSERC Visiting Fellowship to Gabrielle Tompkins MacDonald and Intergovernmental Strategy (IGS) funding.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Appendix 1. List of examined specimens.
ACKNOWLEDGEMENTS
Numerous people have helped us for different parts of this study: We are greatly indebted to Ole S. Tendal for his help in providing access to the large number of cladorhizids found in the zoological collections at the Natural History Museum of Denmark. We would like to thank Megan Best for collecting SEM images of DFO specimens, and Ellen Kenchington and Tim Siferd for providing access to DFO specimens and data. We would like to thank Elena Gerasimova for help and training in making thin sections for species descriptions, and Lindsay Beazley and Lynne Anstey for providing records from benthic imagery and assisting with barcoding of specimens, respectively. We would like to thank Emma Sherlock at the Museum of Natural History (London), Eric Lazo-Wasem at the Yale Peabody Museum, Klaus Rützler and William Moser from the Smithsonian Institution Museum of Natural History, Torkild Bakken from the NTNU University Museum, Anne Helene Tandberg and Jon Anders Kongsrud at the University Museum of Bergen and the MAREANO program, and Rob van Soest and Elly Beglinger at the Naturalis Biodiversity Center for assistance in borrowing samples from their respective collections. We would also like to thank Javier Cristobo, Pilar Ríos, Jean Vacelet, Nicole Boury-Esnault and Michelle Kelly for sending additional samples or subsamples for study or comparison, and Saskia Brix (Senckenberg Naturmuseum Frankfurt) for inviting us to take part in the IceAge project. We would like to thank Nataliya Budaeva and Alexander Plotkin for help in obtaining and translating Russian sources. Finally, we would like to thank Javier Murillo for proofreading and providing input on the manuscript.
REFERENCES
Author notes
[Version of record, published online on 7 July 2017; http://zoobank.org urn:lsid:zoobank.org:pub: 4B2DBF9B-D84D-47C2-AEB3-CE97E89398DA]



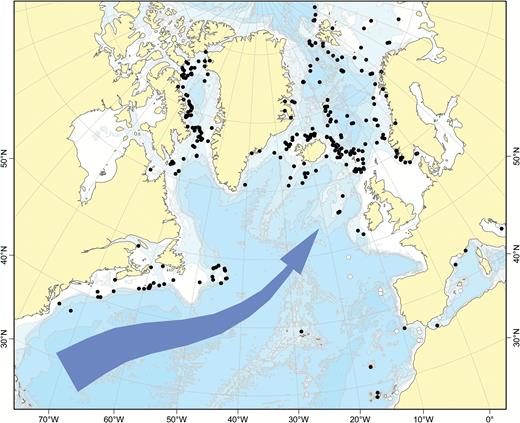
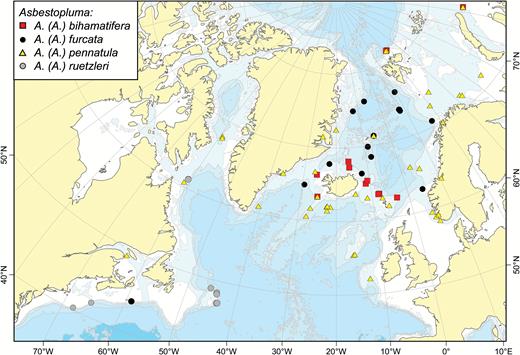
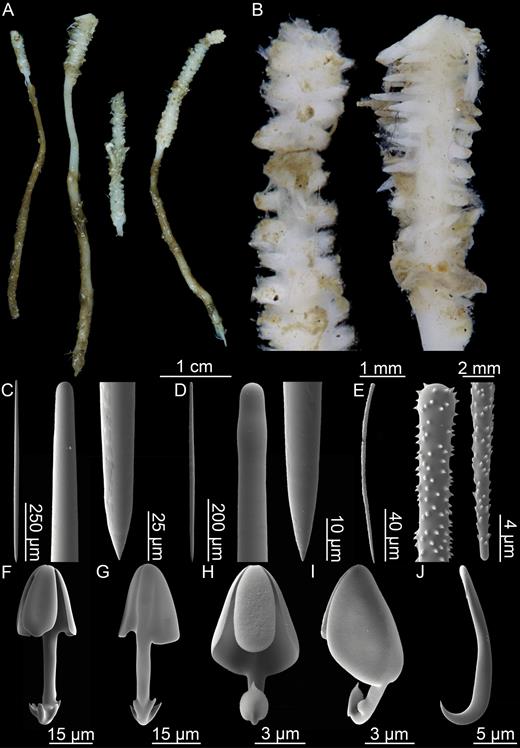

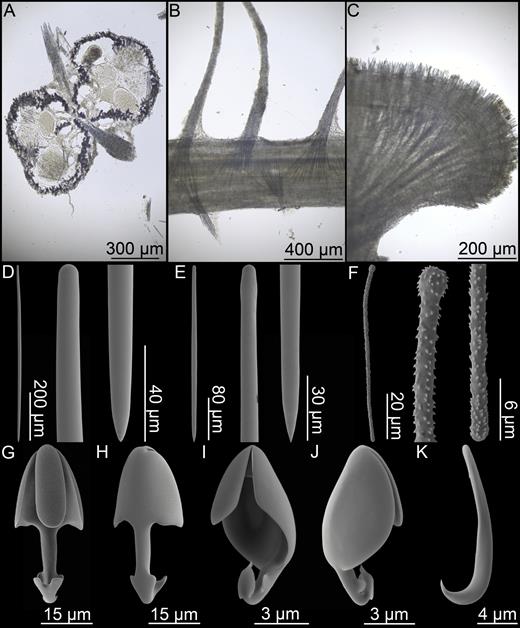

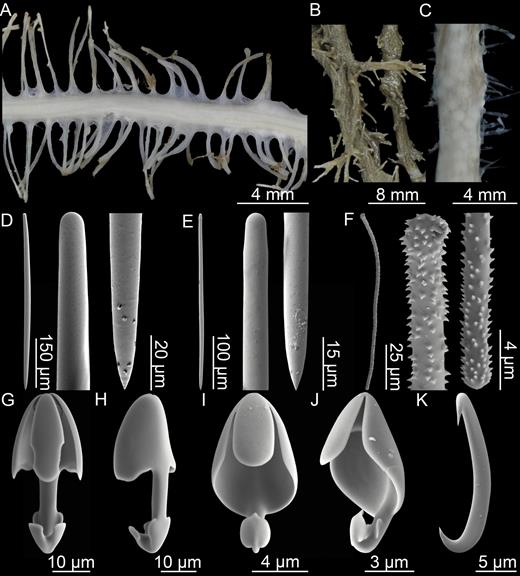
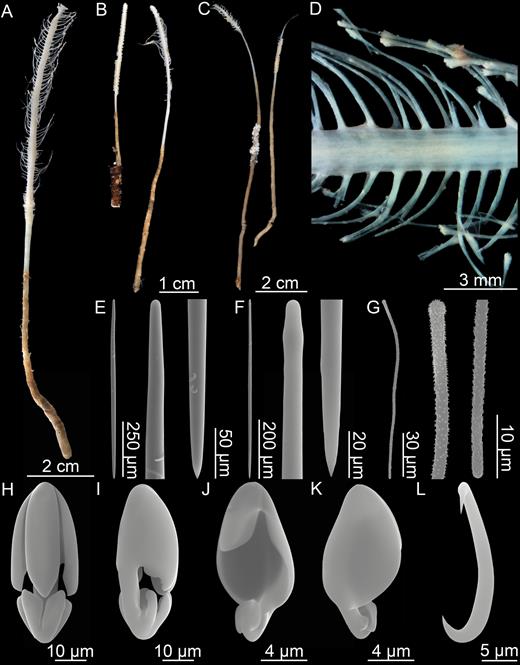
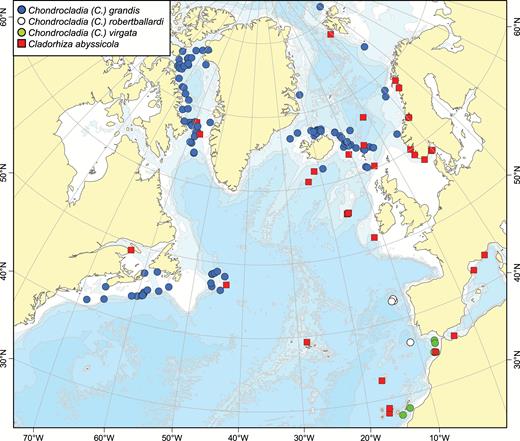

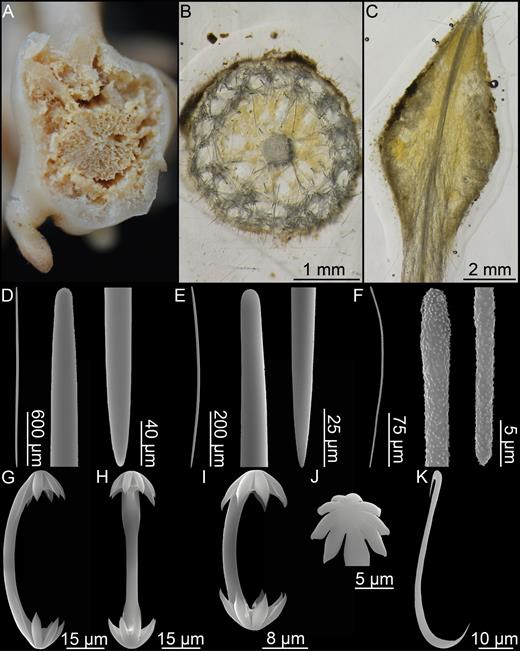
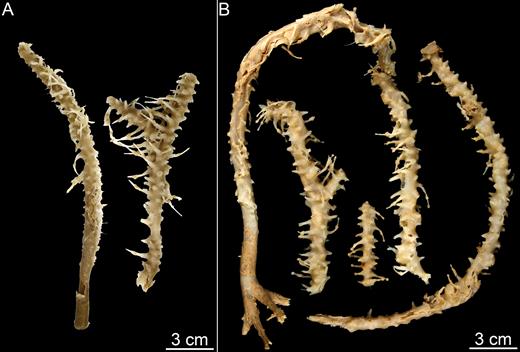
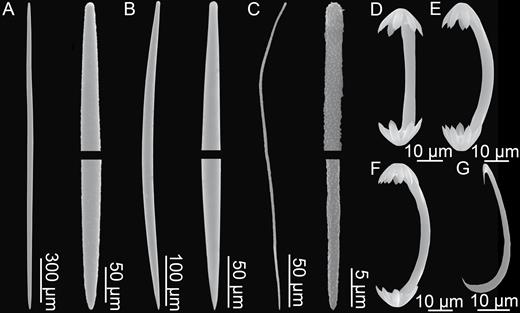
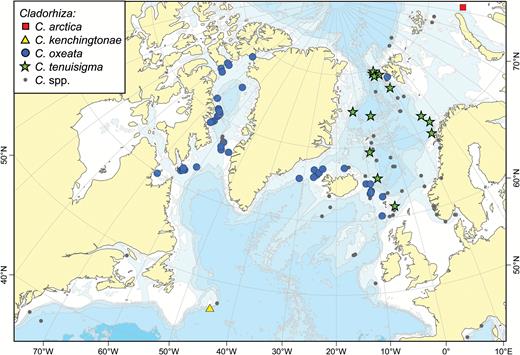
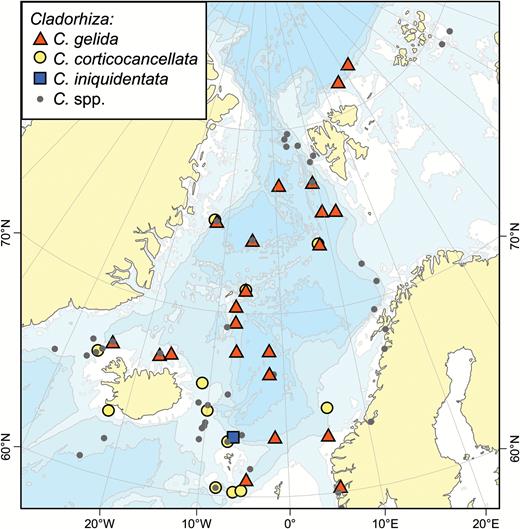

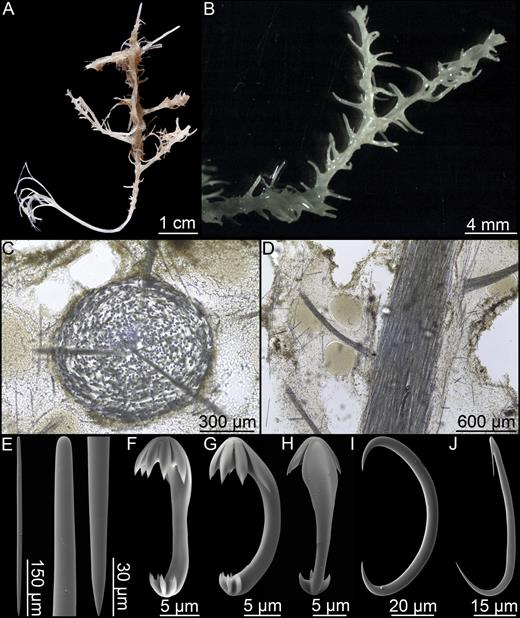
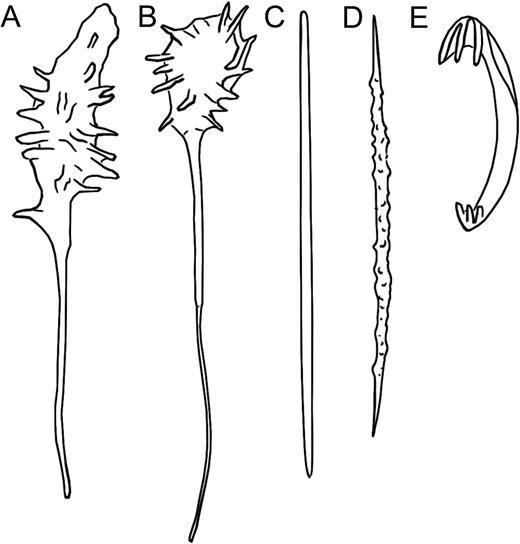

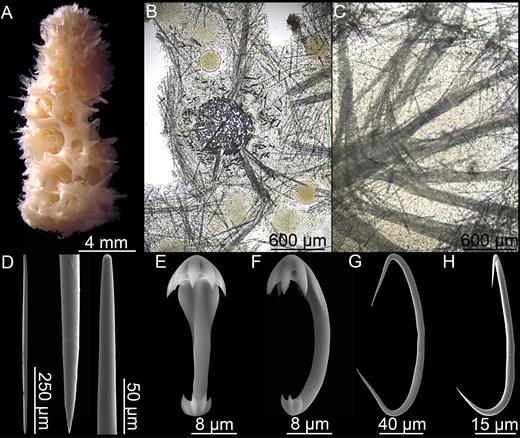
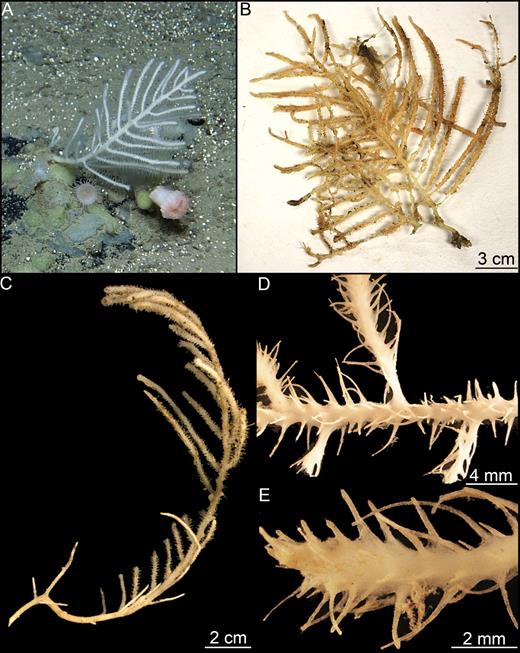
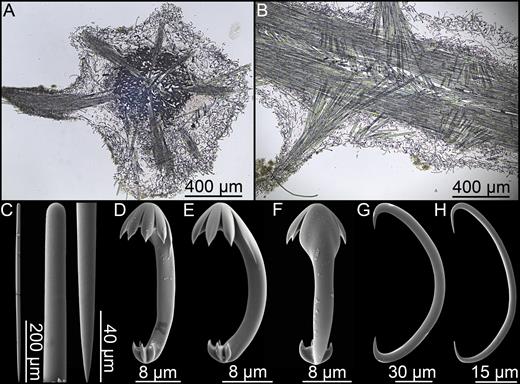
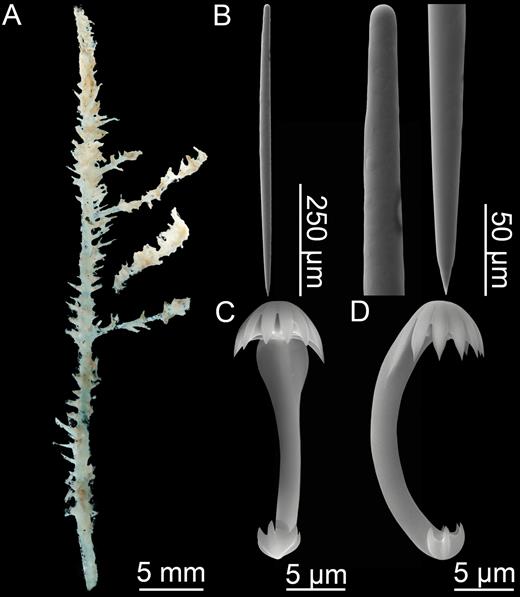
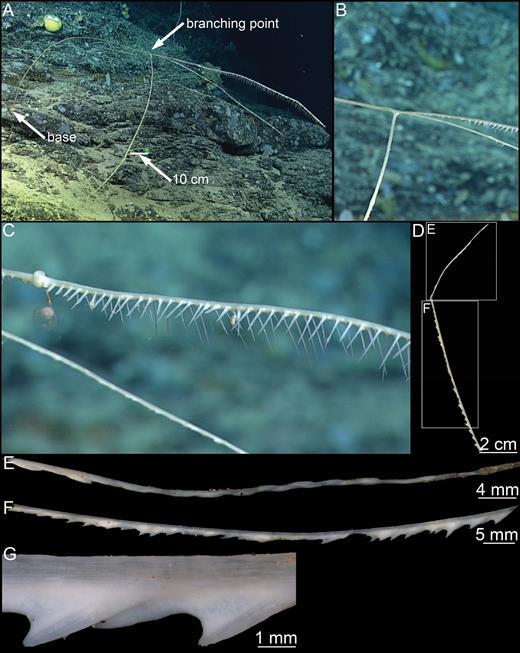
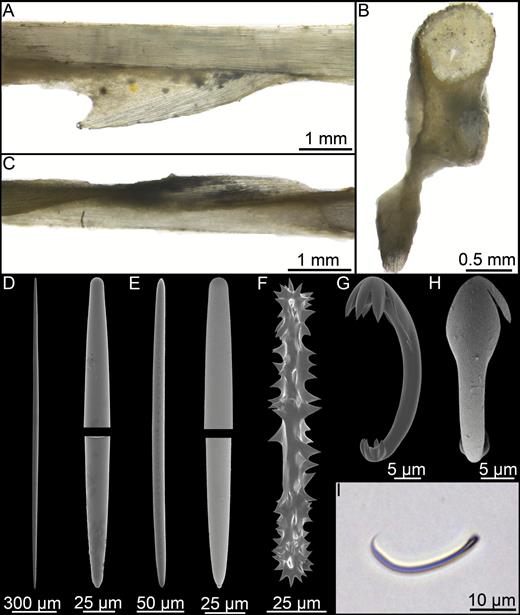
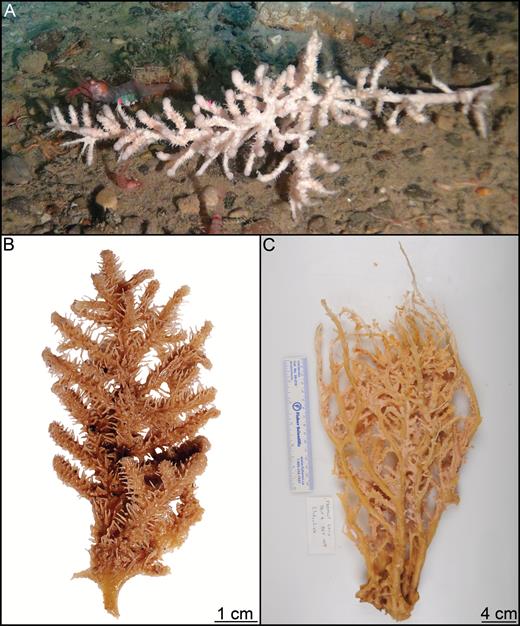
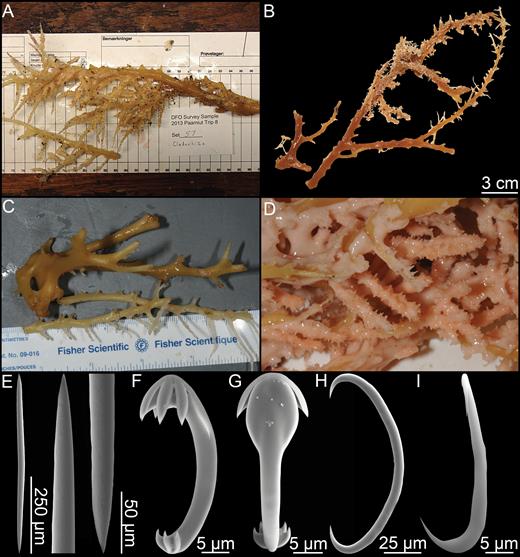

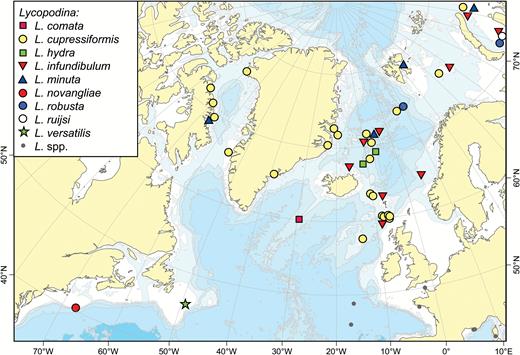
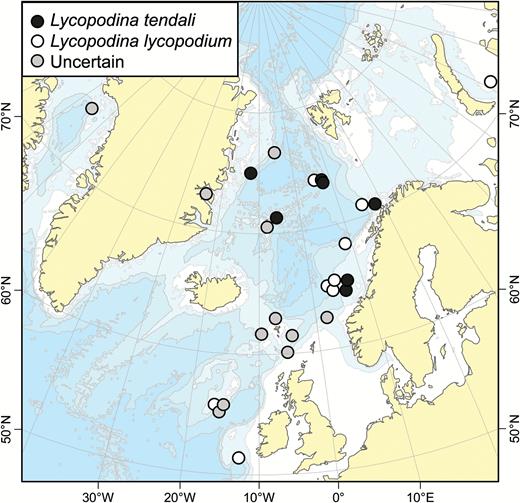
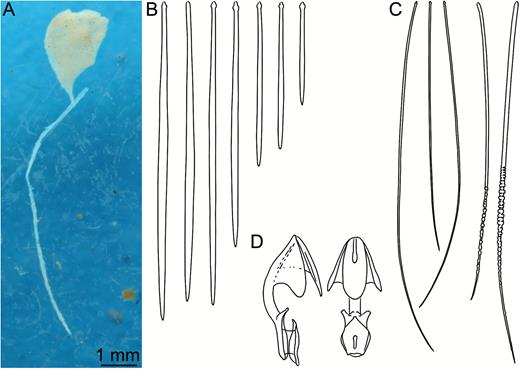
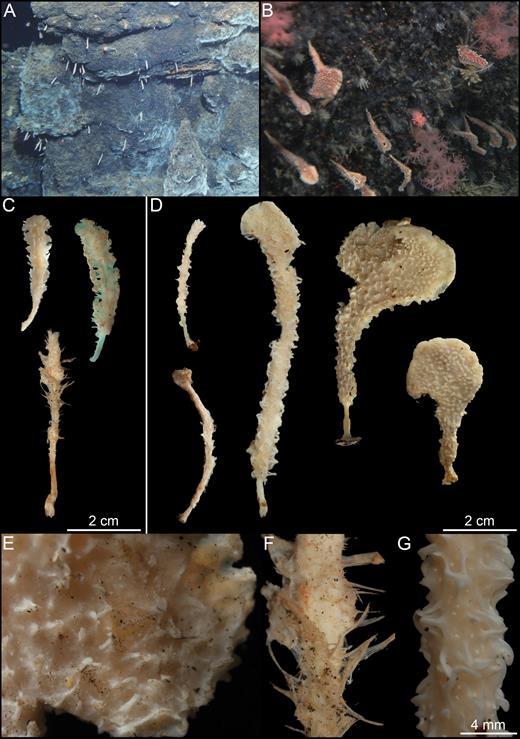
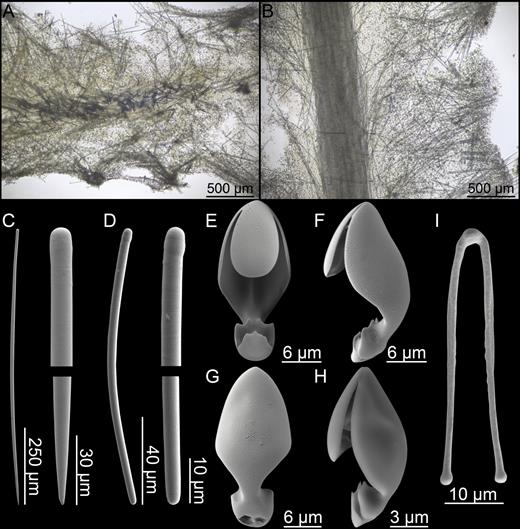
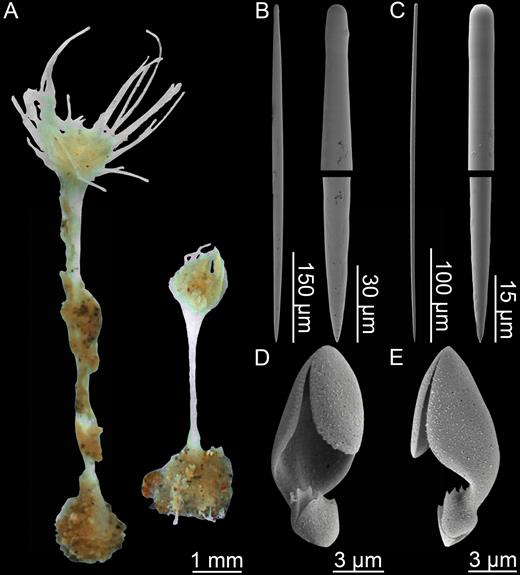
![Lycopodina infundibulum. (A) Specimen ZMBN103460, (B) detail of ZMBN103460, (C) style, (D) subtylostyle, (E) tortuous, tapering subtylostyle, (F, G) arcuate to palmate anisochela front and back views, (H) forceps spicule [redrawn from Levinsen (1887) Pl. XXXI, Fig. 19].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/zoolinnean/181/1/10.1093_zoolinnean_zlw022/1/m_zlw02235.jpeg?Expires=1716324893&Signature=3btBeEJWzvvJBpOmAdQ~uMTyvw9Le81CvMsO43FSvlw3FJa7jco089ri-roSQB9Plkvn13Tklb8u03x0jIKGNlFl0UC63kIQX-Ne3sNEr0rEDqdvVV9YKKX4XfnsxtCzZ1~NdVX3Jpbx3Zcw2IJMlj5ILtsmZedSzbOlalZmw5tzadovC2q3BZ-OB7JSzaw42aEvZCxouK-rGfHin89GE3WPdPvkR0Bvs7FXT9otqqGzOkI6cMoZjgzqVuib55uTg0iPW8ItEza8sWDc9-RwfNs0izOAZ4JQniisTfPzvkDW~uXVBeGFRrsnHwIxXMPLAdGkr1glrbN2c5LWoUQOiQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)