-
PDF
- Split View
-
Views
-
Cite
Cite
Kwang-Seuk Jeong, Keon-Young Jeong, Young-ShiCk Hong, Dong-Kyun Kim, Hye-Ji Oh, Kwang-Hyeon Chang, Application of nuclear magnetic resonance for analyzing metabolic characteristics of winter diatom blooms, Journal of Plankton Research, Volume 42, Issue 1, January 2020, Pages 31–39, https://doi.org/10.1093/plankt/fbz069
Close - Share Icon Share
Abstract
We compared two metabolome profiles of a small centric diatom species, Stephanodiscus hantzschii Grun., grown under conditions with enriched nutrients but different temperatures. This species proliferates in eutrophic rivers during winter. We investigated the population dynamics and internal metabolite changes of Stephanodiscus by performing a simple culture experiment at different temperatures (5 and 15°C). We applied the 1H nuclear magnetic resonance (NMR) technique to fully grown cells to obtain the metabolite profiles of S. hantzschii. Growth rates were significantly different at different temperature conditions (0.99 ± 0.11 day−1 at 15°C and 0.21 ± 0.12 day−1 at 5°C, n = 10). Characterized metabolites included saturated and unsaturated fatty acids, AXP (including AMP, ADP and ATP), and UDP-glucose and UDP-galactose, all of which are important for energy metabolism. These metabolites were abundant within S. hantzschii cells grown at 15°C but were not prolific in those grown at 5°C. Furthermore, other 1H NMR spectrum uncovered very little amounts of metabolites. Based on these observations of cell growth rate, although required nutrients were supplied, colder temperatures suppressed population growth through the deactivation of various internal metabolisms. Thus, winter proliferation of this species is opportunistic, implying that survival success led to dominance in freshwater ecosystems with neither resource competition nor grazing pressure.
INTRODUCTION
Microalgal proliferation in freshwater systems is recognized as the primary cause of water quality degradation and damage to aquatic ecosystems. Various environmental circumstances, such as temperature changes and nutrient accumulation, are associated with this phenomenon. To date, numerous studies have reported a causal relationship between environmental factors and bloom formation patterns from various species, including cyanobacteria such as Microcystis (Liu et al., 2011) and Oscillatoria (Chu et al., 2007) and the green algae Chlorella (Barati et al., 2018). In contrast, due to numerous concerns related to the application of microalgal population dynamics, such as production of biofuels and environmental remediation, optimal conditions for their growth, including temperature, have become an important research topic (Smith and Mcbride, 2015; Hammed et al., 2016).
Diatoms have been used as important bioindicators in oceanic and riverine ecosystems as their diversity reflects the environmental conditions. However, in recent years, their roles are expanding to biodiesel production and wastewater treatments (Li et al., 2009; Datta et al., 2019). In particular, the winter-growing diatom Stephanodiscus hantzschii has attracted much interest in freshwater systems compared with the summer-growing species. A series of ecological modeling and batch culture experiments have stressed the influence of diatom proliferation on water quality exacerbation in rivers such as increase of turbidity (Jeong et al., 2007; Kim et al., 2007b; Jung et al., 2009; Jung et al., 2011; Lenard and Ejankowski, 2017; Kim et al., 2018), as well as its advantages, disadvantages and potential for environmental applications (Li et al., 2009; Joh et al., 2011; Yang et al., 2012; Lang et al., 2017).
The proliferation of microalgae occurs when the surrounding environment is favorable for the growth of these species. Several diatoms and green algal species replicate between 15 and 25°C, and their relative abundance typically increases in spring (Abdelaziz et al., 2013; Abdelaziz et al., 2014). Some studies have previously dealt with S. hantzschii in lentic systems within the Northern Hemisphere and found that this species usually appears in early spring (15–20°C) before domination by green algae. Lynn et al. (2000) successfully evaluated the growth pattern of Stephanodiscus minutulus from multifactorial experiments, indicating that it requires high phosphorus content under silica-limited conditions, resulting in a different phospholipid accumulation pattern at 20°C. However, in some river systems in East Asian, this diatom species proliferates during winter (i.e. typically between December and February), and its relative abundance represents more than 90% (>100 000 cells mL−1) of the total phytoplankton (Jeong et al., 2007). However, although several studies have been conducted on optimal conditions for the growth of S. hantzschii, physiological mechanisms that facilitate their growth at low temperatures remain poorly understood.
Metabolome analysis of microalgae via 1H nuclear magnetic resonance (NMR) has been popularly implemented in recent years, and these studies have primarily focused on the overall metabolite profile analysis (Chauton et al., 2003; Azizan et al., 2018) or the comparison of lipid contents (Nuzzo et al., 2013; Sarpal et al., 2016). In the present study, we conducted a simple growth experiment to investigate the metabolic status of S. hantzschii using proton NMR spectroscopy to understand the accumulated metabolite profiles in S. hantzschii cells cultured under two different temperature conditions (i.e. 15 and 5°C). Based on statistical analyses applied to the NMR signals, we evaluated the adaptability of S. hantzschii to a cold temperature environment and phytoplankton–zooplankton interactions (Choi et al., 2014; Choi et al., 2016).
MATERIALS AND METHOD
Strain obtainment and growth assay
To investigate the response of the diatom S. hantzschii to low temperatures, we experimented on S. hantzschii based on a series of batch cultures. The diatom strain S. hantzschii Grunow was obtained from the Canadian Phycological Culture Centre (strain number CPCC 267), and subcultures were maintained in diatom medium (DM; Beakes et al., 1988) in a growth chamber (SDI-432C, Chemtopia, South Korea) at 15°C, with cold-white LED illumination (light intensity, 180 μM m−2 s−1) with a 12 h light/12 h dark cycle.
We set two temperature conditions, 15°C as the control and 5°C as the tested treatment, and the remaining growth conditions were identical to the aforementioned subculture maintenance. The former temperature setting was selected according to the report from Jung et al. (2009) stating that this species grew well under temperature conditions of 15–17°C. A comparatively cold temperature (i.e. 5°C) was chosen based on a long-term ecological research at the lower Nakdong River, South Korea, where the recurrent winter proliferation of S. hantzschii has been reported at 4–7°C (Kim et al., 2007a; Kim et al., 2007b). Prior to the experiment, one subculture of the strain (grown at 15°C) was allowed to acclimatize to cold temperature conditions for three subculture generations. First, we inoculated approximately 5 × 106 cells of S. hantzschii which were grown at 15°C, into a flask filled with a newly prepared DM, and the flask was incubated in the growth chamber (the temperature was set to 5°C). We allowed the subculture to grow in the cold temperature, and the two inoculations were implemented using cells adapted to 5°C temperature in the same way.
Each experimental group encompassed 10 biological replicates. We used 300-mL Erlenmeyer flasks filled with 150-mL DM stocks for the replicates. When both 15 and 5°C acclimated cultures were ready to undergo the growth experiments, we measured the cell density of the cultures and inoculated cells into newly prepared fresh DM (initial density, 10 000 cells mL−1), respectively, resulting in 10 replicate flasks for 15°C growth and another 10 flasks for 5°C growth. The flasks were then placed in two different growth chambers, set at either 15 or 5°C.
The cultures were allowed to proliferate for 14 days. During this growth experiment, we subsampled 2-mL culture medium containing diatom cells from each flask at daily intervals, which were then stored in polypropylene tubes, and 0.2-mL Lugol’s solution was added to every tube to preserve the cells for further density measurements. We used Sedgewick-Rafter counting chamber for cell enumeration. We injected 1 mL of well suspended samples into the chamber and allowed the cells to settle down (~2 h). Cell numbers were measured under a Nikon inverted microscope (Diaphot, Nikon, Japan). We calculated the maximum growth rate by using the equation shown below (Equation 1):
|${\mu}_{\mathrm{max}}=\ln \left(\left({x}_2/{x}_1\right)/\left({t}_2-{t}_1\right)\right),$|
where x2 and x1 are the densities of S. hantzschii (cells mL−1) at culture times t2 and t1 days, respectively.
On the 14th day, we enumerated the number of S. hantzschii cells of each culture flask immediately, in order to determine the threshold of cell amount for NMR analysis. This was also to avoid cell condensation effect (i.e. larger cell amount would result in stronger NMR signal production). Based on the culture sample that exhibited the smallest cell amount, we set the threshold as 12 × 106 cells per flask. We used most of the volume of the 5°C flasks to meet the threshold, while more or less than 18–20 mL of 15°C flasks were used. We gently but sufficiently shook the flasks to mix the cells in every flask and then carefully sampled and transferred the cells into new polypropylene tubes for centrifugation at 4000 rpm for 5 min. The supernatant was carefully eliminated and discarded, and cell pellets were transferred to sterile 1.5-mL centrifugation tubes. The harvested S. hantzschii cells were washed thrice with sterile Dulbecco’s phosphate buffered saline. The tubes were then immediately flash-frozen with liquid nitrogen and stored at −80°C (Forma −86C Ultra Low Temperature Freezer, Thermo Fisher Scientific, Columbus, OH, USA) until the next required sample process.
Metabolome analysis using cultured S. hantzschii
The pelleted frozen cells were thawed and resuspended in a mixture of 700 μL of 70% methanol-d4 and 30% deuterium oxide (D2O) with 1-mM sodium 3-trimethylsilyl [2,2,3,3-2H4]-1-propionate (TSP). Metabolites were extracted by vortexing the mixture at 2.10 m s−1, 0.30 cycle time, with three cycles and 0.05 pause dwell using a Mini Bead Ruptor 24 (Omni International, Kennesaw, GA, USA).
Subsequently, 550 μL of the supernatant were transferred to an NMR tube. The chemical shift reference compound TSP used in the NMR experiment (see previous section) and D2O provided a field lock for the NMR spectrometer. The 1D 1H NMR spectra of cell extracts were acquired using a Bruker 700-MHz spectrometer equipped with a cryogenic triple-resonance probe and an automatic sample changer (Bruker Biospin, Rheinstetten, Germany) operating at a frequency of 700.40 MHz 1H and a temperature of 300 K. The “zgpresat” pulse sequence was used to suppress the water signal and acquire the 1D 1H NMR spectrum. For each sample, 128 transients were collected into 32 K data points using a spectrum at 8403.36 Hz with a relaxation delay of 2.0 s. A line-broadening function of 0.3 Hz was applied to all spectra before Fourier transformation. Both phase and baseline of the resultant NMR spectra were manually corrected, and the chemical shift was calibrated to |$\delta$| 0.00 to the signal from the TSP with the phosphate buffer. Signal assignment of representative sample was facilitated by 2D total correlation spectroscopy (TOCSY) and heteronuclear single-quantum correlation (HSQC). Statistical total correlation spectroscopy (STOCSY) method (Cloarec et al., 2005) was also used to identify the metabolite.
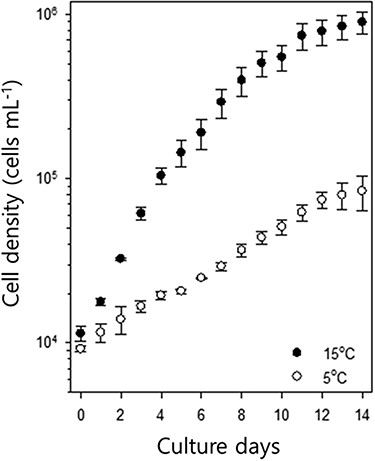
Growth curves of S. hantzschii in two different temperature conditions (control, 15°C; experimental, 5°C). Error bars indicate standard errors (n = 10 per experimental group).
The 1D 1H NMR spectra were imported into MATLAB (R2010b, Mathworks, Inc., 2010) without binning or spectral segments, using an in-house software afterward. Then, the probabilistic quotient normalization of spectra using the mean spectrum was conducted to estimate the most probabilistic quotient after total integral normalization for avoiding the dilution effects of the samples and the effects of metabolites in massive amounts on changes in the overall concentration of samples (Dieterle et al., 2006), after the removal of the region corresponding to residual water (|$\delta$| 4.57–4.90) and methanol (|$\delta$| 3.36–3.42). The resultant data were analyzed using multivariate statistical models, such as principal component analysis (PCA), partial least-squares discriminant analysis (PLS-DA) and orthogonal projections to the latent structures discriminant analysis (OPLS-DA; Hotelling, 1992; Johan and Svante, 2002; Bylesjö et al., 2006). In addition, the OPLS loading coefficient or OPLS loading plots in the OPLS-DA models between the two classes were back-scaled to improve interpretability and identify discriminant metabolites, as described by Cloarec et al. (2005). To prevent the over-fitting of spectra data into the OPLS-DA models, a 7-fold cross-validation method was used, and the cross-validation parameter Q2, which is defined as the proportion of variance in the data predictable by the model and indicates the predictability, was calculated.
RESULTS
Growth of S. hantzschii
The growth of S. hantzschii differed between the two temperature regimes (Fig. 1), and cell density readily increased at 15°C. In contrast, although low-temperature-acclimated cells were used, the population of S. hantzschii only gradually increased at 5°C, and its growth rate was substantially lower at 5°C than at 15°C (control group). The average maximum growth rate (μmax) of the control group was 0.99 ± 0.11 day−1, whereas that of the low-temperature group was 0.21 ± 0.12 day−1 (n = 10). In case of the control group, a sharp increase in the cell density was observed on day 2 of culture, and the population reached a stable plateau after day 10. The low-temperature group did not show this proliferation pattern, and they reached plateau on day 12.
Metabolome analysis
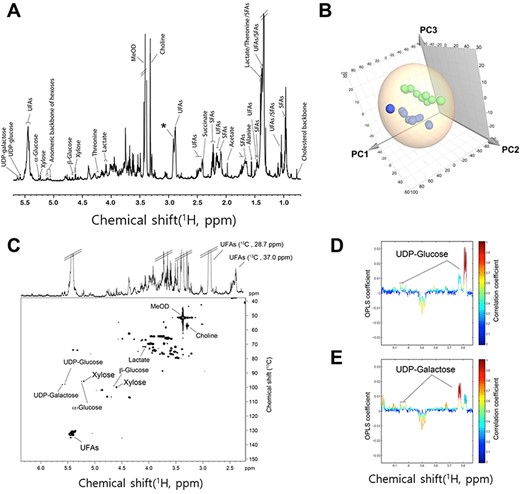
The representative 1H NMR spectra of the S. hantzschii extract are shown in Fig. 2. The assigned metabolites comprised both saturated fatty acids (SFAs) and unsaturated fatty acids, alanine, acetate, succinate, choline, lactate, threonine, xylose, glucose, UDP-glucose and UDP-galactose, with the dominant metabolites being SFAs and unsaturated fatty acids (Fig. 2a). The application of PCA to the 1H NMR spectrum data of S. hantzschii exhibited clear metabolic differentiations in the species subcultures cultivated at 5°C from those cultivated at 15°C (Fig. 2b), which was accounted by PC1 (0.595), PC2 (0.209) and PC3 (0.071).
Metabolite profile of S. hantzschii subcultures from two different temperature groups, and experimental group differentiation. (A) Representative 700 MHz 1H NMR spectrum of the species extracts. (B) 3D PCA score plot derived from 1H NMR spectra (green, 5°C; blue, 15°C). (C) HSQC NMR spectrum with identification of the metabolite selected. Partial regions of STOCSY of (D) UDP-glucose and (E) UDP-galactose.
We generated an OPLS-DA model from the 1H NMR spectra of S. hantzschii cells to identify intracellular metabolites responsible for the differentiation. This OPLS model was generated with one predictive component and one orthogonal component and validated by a 200 times permutation test in the PLS-DA model with the same number of components (data not shown). The model showed good fitness and high predictability, as indicated by R2X = 0.66 and Q2 = 0.93, respectively.
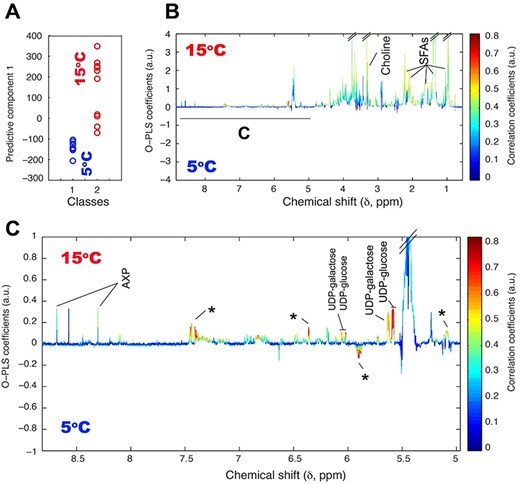
The upper segment in the OPLS coefficient plot (Fig. 3) denotes high metabolite levels within S. hantzschii grown at 15°C compared with those grown at 5°C, whereas the lower segment reveals decreased metabolite levels in S. hantzschii grown at 15°C. As shown in Fig. 3, S. hantzschii cells cultivated at 15°C had higher levels of SFAs, choline, UDP-glucose, UDP-galactose and AXP (a collective indication of AMP, ADP and ATP) than those grown at 5°C (Fig. 3b and c).
(A) OPLS score and (B) coefficient plots derived from 1H NMR spectra of S. hantzschii extract, revealing a clear metabolic differentiation between S. hantzschii grown under different temperatures of 5 and 15°C, and the identification of S. hantzschii metabolites distinctly dependents on different growing conditions. Panel (C) is the expansion selected at (C) indicated in (B). Asterisks indicate unknown metabolites. AXP denotes AMP, ADP and ATP.
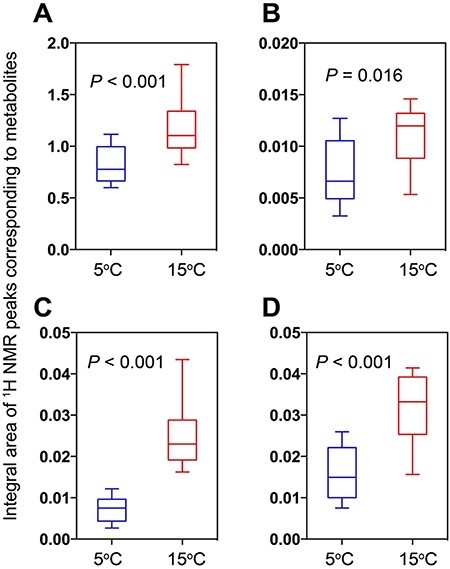
Metabolite levels between the cells grown at 5°C and those grown at 15°C are compared in Fig. 4. In all cases, S. hantzschii produced more metabolites at 15°C than at 5°C.
Relative amounts of metabolites showing a distinct difference between groups grown at differing temperatures, which were calculated from integral areas of 1H NMR peaks of individual metabolites. (A) SFAs. (B) AXP. (C) UDP-glucose. (D) UDP-galactose.
DISCUSSION
Metabolite patterns
In this study, we considered that NMR analysis allows us to understand the biochemical response pattern of microalgal species to different environmental conditions. Although some chemicals are still not assigned clearly, the well-assigned metabolites provided useful information to characterize the S. hantzschii response strategy to low temperature stress. Based on the results, we believe that S. hantzschii just maintained their population size in 5°C in constrast to 15°C treatment where the species actively grew.
Overall, the low production level of SFA, AXP, UDP-glucose and UDP-galactose in 5°C treatment would be due to the inefficiency of photosynthesis in S. hantzschii cells. Photosynthesis is a system that automatically transports electron to generate energy storage products in the presence of light. Generally, microalgae exhibit the highest photosynthetic rates under optimum growth temperature; the lower the growth temperature is, the lower the photosynthetic rates are found (Claquin et al., 2008). It is known that low temperature stress increases the membrane viscosity of cellular organelles in chloroplasts (e.g. thylakoid) and lowers the activity of carbon, nitrogen or sulfate reduction enzymes, which leads to the inhibition of the electron transport process (Huner et al., 1998; Ensminger et al., 2006). Under autotrophic conditions, microalgae yields ATP (~40%) from oxidative phosphorylation process in the mitochondria, and the majority of ATP is consumed in Calvin cycle (Yang et al., 2000). Unfortunately we did not measure photosynthetic rates in this study, the significant decrease of AXP in 5°C treatment would be due to the low photosynthetic activity resulting in low growth rates of S. hantzschii.
SFA, UDP-glucose and UDP-galactose contents showed a similar pattern to AXP. Relatively higher contents of SFA were observed in 15°C treatment. In their extensive review, Paliwal et al. (2017) insisted that relatively higher temperature allowed microalgae to accumulate non-polar lipids such as triacyl glycerol under sufficient light conditions. We believe that a relatively high photosynthetic rate would make S. hantzschii obtain carbon source to generate a large quantity of SFA, but the species would suffer from lack of carbon accumulation at lower temperature, resulting in low SFA contents. UDP-glucose and UDP-galactose are intermittent metabolites that are used to form Chrysolaminaran (although not shown in this study), a storage carbohydrate that is found in Bascillariophyta (Zhu et al., 2015; Zill et al., 2017). It is not apparent whether the increase of UDP-glucose and UDP-galactose 15°C treatment is from the degradation or generation of Chrysolaminaran, the relatively highly activated metabolic process in this higher temperature treatment would allow the increase of these metabolites.
S. hantzschii increase would not be active result
Understanding the interactions between microalgal populations and environmental factors is important not only for the management of water quality but also for the control of algal biomass in cultures of biomass energy and biofloc ponds (Du et al., 2018). Various environmental factors, including temperature and nutrient conditions, affect the growth patterns of microalgae, and increased proliferation of microalgae occurs under favorable environmental conditions (Pohnert, 2005; Kim et al., 2006; Kim et al., 2008; Paerl et al., 2011). In the Northern Hemisphere, an increase in the water temperature in eutrophic waters occurs during spring, usually between 15 and 20°C, and triggers the fast growth of diatoms, followed by the proliferation of green algae (Mechling and Kilham, 1982; Lynn et al., 2000; Reynolds, 2006) or cyanobacteria (Paerl et al., 2001; Paerl and Paul, 2012). Thus, winter density increase of diatoms is an extraordinary phenomenon that attracts both ecological and physiological interests.
To date, it is known that diatoms are relatively insensitive to temperature than are other microalgae (Anderson, 2000); thus, it is expected that some diatom species have the potential to dominate a winter microalgal assemblage (Løvstad, 1984; Ellen and Soltau, 1990; Kim et al., 2008; Jung et al., 2009). Based on our results, however, the increase of S. hantzschii density in freshwater systems in winter would not be an active proliferation but a result of slow adaptation and maintenance of population to cold water temperature. Long-term weekly monitoring and modeling studies showed that, unlike other excessively grown microalgae or cyanobacteria, S. hantzschii usually required more than 2 or 3 weeks to form large population size in winter (December to next January; Jeong et al., 2007; Kim et al., 2007b). Cold adaptation of microalgae has been under steadily increasing interest (Smith et al., 1994; Thomas and Dieckmann, 2002; Svenning et al., 2019), which might be due to the extensive overexpression of cold adaptation genes, including heat- and cold-shock proteins (e.g. Thomas and Klaus, 2004). Further transcriptomic analysis for S. hantzschii is requested to make clear this hypothesis.
CONCLUSION
In this study, we observed that the small centric diatom S. hantzschii could survive in cold temperature conditions (5°C), but the growth pattern was different from the control group (15°C). The comparison of the metabolite profile obtained from NMR analysis revealed that cold temperature stress affected the metabolism activation negatively. Some biochemical metabolite contents, including AXP and SFA, were significantly lower in the cold treatment samples compared with the control group. Although the species grew slowly in the 5°C condition, based on the metabolite profile pattern, the growth would be concluded as survival. Further transcriptomic analytic studies should be conducted to identify how the species adapted to the temperature stress.
FUNDING
This study was financially supported by the research project "Prediction and elucidation of diatom proliferation in freshwater systems using molecular ecological models based on transcriptome/metabolome and artificial intelligence" from the National Research Foundation of South Korea (Project No. 2019019984).