-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey T. Leek, svaseq: removing batch effects and other unwanted noise from sequencing data, Nucleic Acids Research, Volume 42, Issue 21, 1 December 2014, Page e161, https://doi.org/10.1093/nar/gku864
Close - Share Icon Share
Abstract
It is now known that unwanted noise and unmodeled artifacts such as batch effects can dramatically reduce the accuracy of statistical inference in genomic experiments. These sources of noise must be modeled and removed to accurately measure biological variability and to obtain correct statistical inference when performing high-throughput genomic analysis. We introduced surrogate variable analysis (sva) for estimating these artifacts by (i) identifying the part of the genomic data only affected by artifacts and (ii) estimating the artifacts with principal components or singular vectors of the subset of the data matrix. The resulting estimates of artifacts can be used in subsequent analyses as adjustment factors to correct analyses. Here I describe a version of the sva approach specifically created for count data or FPKMs from sequencing experiments based on appropriate data transformation. I also describe the addition of supervised sva (ssva) for using control probes to identify the part of the genomic data only affected by artifacts. I present a comparison between these versions of sva and other methods for batch effect estimation on simulated data, real count-based data and FPKM-based data. These updates are available through the sva Bioconductor package and I have made fully reproducible analysis using these methods available from: https://github.com/jtleek/svaseq.
INTRODUCTION
Batch effects and other technological artifacts introduce spurious correlation, create bias and add variability to the results of genomic experiments (1–3). The basic problem is that batch effects introduce a new source of signal into the data that can be confused with the signal an analyst is looking for. This signal is consistent across transcripts, exons or genes and so may lead to gross errors in the calculation of statistical significance, estimates of effect sizes or other statistical measures (4,5). These types of noise also prevent analysts from appropriately modeling biological variation and group-specific changes in gene expression (6). Unfortunately we rarely know all of the potential artifacts in most high-throughput experiments (4,7). In some cases, it is possible to rely on the date the samples were processed as a surrogate for unmeasured artifacts (8) and correct for them to get statistically accurate results. However, each new technology may suffer from different artifacts and it may take time for the community to discover which variables must be measured and included in an analysis (9).
General sva estimation framework. In this general framework, Step 1 allows for transformations specific to different data types, Step 2 allows for either estimating or defining the probabilities of being affected by unknown artifacts but not known variables and Step 4 allows for a variety of matrix decompositions and factor analysis approaches.
Approach for simulating RNA-seq data with Polyester package (34).
In this paper I discuss two new extensions of the sva approach for sequencing data. The first extension deals with Step 1 of the sva approach (Figure 2). The idea is to use external information to estimate the genes that are likely to be affected by unknown artifacts. The original sva algorithm attempted to identify genes affected by artifacts directly from the data itself, but sometimes there is external information about which genes are unlikely to be differential expressed. This external information could be control probes (7) or estimates of batch-related probes from previous studies (11). Supervised sva uses this information in Step 1 of the sva approach, reducing computational time and reliance on estimation procedures for identifying the right genes to use for artifact estimation. The second idea, svaseq, is based on performing an appropriate transformation of the count or Fragments Per Kilobase Of Exon Per Million Fragments Mapped (FPKM) data during Steps 1 and 2 of the sva approach (12). Here I focus on the moderated log transform, which has been widely adopted both for the analysis of sequence count data and FPKM estimates. I then perform a thorough and reproducible comparison between the standard methods for removing batch effects from sequencing data. I demonstrate that svaseq and supervised sva perform comparably to existing approaches for removing batch effects from sequencing data.
MATERIALS AND METHODS
General form of the surrogate variable analysis mathematical model
In Step 5, the covariates may be included in a standard linear regression modeling analysis on an appropriately transformed scale or the covariates can be directly used in software that models counts with generalized linear models (GLMs) including edgeR (13) and DESeq (14). They can be directly included in these models as they are estimated on the same scale as standard link functions for GLMs.
Relationship of surrogate variable analysis to other approaches
The general form of the sva algorithm relies on the idea that there is a subset of genes, probes or transcripts that are affected by unknown batch effects or other artifacts but are not affected by the biological relationship of interest. This is the main way that a sva approach is distinguished from a standard principal component regression. Standard principal component-based regression methods, such as EIGENSTRAT (15), are sufficient when the number of probes, genes or features expected to show a signal is small. Then the principal components will be consistent estimates of a linear transformation of the artifacts and not the phenotype/outcome of interest (16).A number of extensions and variations on the sva algorithm have been introduced. For Step 1 in the sva algorithm, identifying probes only associated with unmeasured artifacts, it has been proposed to use control probes (7,12). For Step 2 of the sva algorithm, estimating latent factors only associated with unmeasured artifacts, it has been proposed to use factor analysis (17), independent components analysis (18) and principal components analysis (19). Another extension of the surrogate variable approach in Step 2 has been to model known sources of technical or biological covariation between the measurements for probes, for example in eQTL studies (20,21).
Supervised sva (ssva)
Supervised sva (ssva) sets λi = 1 for all negative controls and λi = 0 for all other genes in Step 2 of the sva algorithm. The assumption is that control probes will capture all of the variation due to unknown artifacts and none of the variation due to the phenotype. Control probes may miss biological artifacts. For example, we showed that trans-eQTL that are associated with multiple gene expression levels may act like an artifact when measuring the association between gene expression and phenotype (4). These artifacts may be missed by the ssva approach. However ssva is particularly useful for unfortunate experimental designs where the phenotype variable and unknown artifacts are highly correlated (8), making empirical estimates unstable (7).
Moderated log link sva (svaseq)
The second extension involves the choice of function f() in (1). In our original work, we used the identity function for data measured on an approximately symmetric and continuous scale. For sequencing data, which are often represented as counts, a more suitable model may involve the use of a moderated log function (22,23). For example in Step 1 of the algorithm we may first transform the gene expression measurements by applying the function log(gij + c) for a small positive constant. In the analyses that follow I will set c = 1. The choice of the moderating constant is an important one and is beyond the scope of this manuscript. Intuitively a choice of c = 0 corresponds to no moderation and as c increases you decrease the variation in the data. After performing Steps 1–5 of the sva estimation algorithm, the estimated covariates are included in downstream models as adjustment variables. For the analyses that follow, I will use the limma package (24) with the voom method (25) for differential expression analysis. The voom method is an approach for estimating the mean–variance relationship when performing differential expression analysis on sequencing experiments.
Combining svaseq and ssva
Supervised svaseq proceeds by applying the transformation log(gij + c) to the gene expression count data in Step 1 and setting λi = 1 for all negative controls and λi = 0 for all other genes in Step 2 of the sva algorithm.
Zebrafish data
I use data from Zebrafish sensory neurons with three control samples and three gallein treated samples as the comparison groups (26). These data are available as part of the zebrafishRNASeq Bioconductor package. I loaded the data and filtered as described in the removing unwanted variation in sequencing data (RUVSeq) package. Then I estimated batch effects using supervised and unsupervised sva for sequencing, principal components analysis, RUV with control probes, RUV with empirical controls and residual RUV. I compared the model estimates and I compared differential expression analysis results when each of the different batch effect estimates was included in the model in place of the study variable.
ReCount data
ReCount is a database of pre-processed RNA-sequencing data, processed to be comparable across samples (27). In this analysis, I downloaded pre-counted RNA-sequencing datasets measuring gene expression in two separate Hapmap populations (28,29). For my analysis, I downloaded the count data from ReCount and downloaded the pedigree information from the Hapmap website. I then performed differential expression analyses looking for differences in expression between males and females and estimated unknown latent structure. I calculated estimates of batch effects using unsupervised sva for sequencing, principal components analysis, RUV with empirical control probes, and RUV on residuals. I compared the estimates to the variable indicating whether the data came from the Pickrell or Montgomery study. I compared two scenarios, one where the sex and study variables were balanced and one where they were imbalanced. I also compared the differential expression analysis results when each of the different batch effect estimates was included in the model in place of the study variable.
GEUVADIS data
I downloaded the processed GEUVADIS (30,31) Ballgown object (32) from:https://github.com/alyssafrazee/ballgown_codeI then subset the data to only the non-duplicated samples (31) and performed a differential expression analysis comparing populations. I calculated estimates of batch effects using unsupervised sva for sequencing, principal components analysis, RUV with empirical control probes and RUV on residuals. I compared the estimates to the variable indicating which lab the sequencing was performed in. I also compared the differential expression analysis results when each of the different batch effect estimates was included in the model in place of the laboratory variable.
Simulating data
I simulated data from a negative binomial model for count based RNA-sequencing data (Figure 3) (33). For complete details see the simulated data R markdown document and accompanying HTML file.I estimated the model parameters from the Zebrafish data described above. I simulated two scenarios, one where the group and batch variable were not correlated and one where they were correlated. Here we consider both batch effects that are correlated with the outcome and batch effects that are orthogonal. This is a critical distinction as unsupervised methods that estimate batch effects directly from the data will often perform worse when batch and outcome are correlated unless this relationship is explicitly modeled. Data were simulated with the Polyester R package (34). Then I estimated batch effects using supervised and unsupervised sva for sequencing, principal components analysis, RUV with control probes, RUV with empirical controls and residual RUV. I compared the model estimates to the true simulated batch variable and I also compared differential expression analysis results when each of the different batch effect estimates was included in the model in place of the study variable.
Code and availability
ssva and svaseq are currently implemented in the sva Bioconductor package version 3.11.2 or greater (http://www.bioconductor.org/packages/devel/bioc/html/sva.html). All data and code used to perform this analysis are available as R markdown files (35) available from: https://github.com/jtleek/svaseq. You can view the individual analyses as webpages at:
Zebrafish analysis: http://jtleek.com/svaseq/zebrafish.html
ReCount analysis: http://jtleek.com/svaseq/recount.html
GEUVADIS analysis: http://jtleek.com/svaseq/geuvadis.html
Simulated data analysis: http://jtleek.com/svaseq/simulateData.html
RESULTS
Simulated data
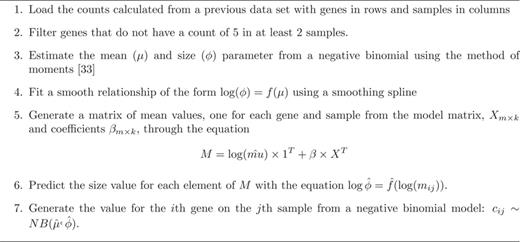
I estimated simulation parameters from the Zebrafish data as described in the methods. I then performed several checks to confirm that (i) the data generated by the simulated model recapitulated the qualitative behavior of the data used to estimate the model parameters (Figure 4), (ii) that data generated without signal did not show statistically significant results, (iii) data could be simulated with differential expression signal and (iv) that data with batch effects displayed the expected conservative bias of P-values (36) (See supplementary analysis files (http://jtleek.com/svaseq/zebrafish.html).
Distribution of means and variances for simulated and real Zebrafish data. To confirm that my simulation procedure produced reasonable simulated counts, I plotted the gene-specific means and variances for (left panel) the simulated data set and (right panel) the observed Zebrafish data set. The two distributions are qualitatively similar. Additional checks on the simulation procedure are provided in the simulated data analysis at http://jtleek.com/svaseq/simulateData.html.
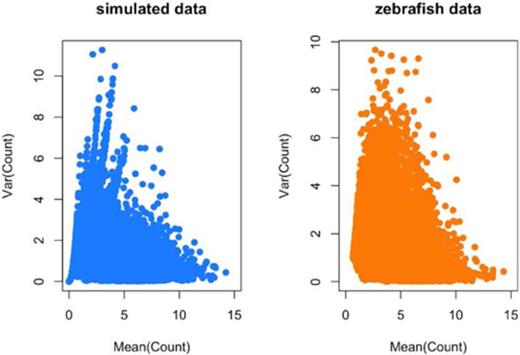
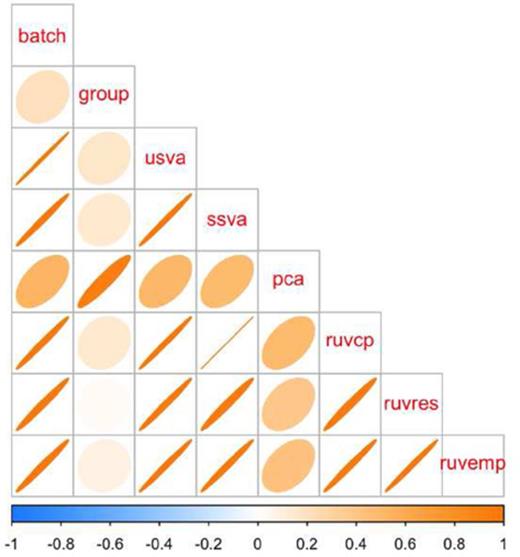
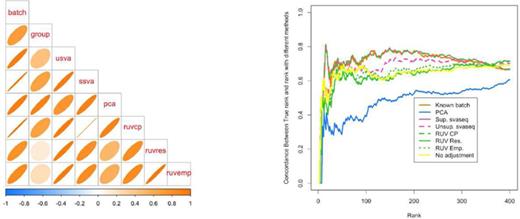
In the Zebrafish study there was a single simulated batch effect. I simulated two scenarios, in the first scenario the batch effect and the group effect had low correlation. As expected, all methods that aim to estimate batch effects while taking into account multiple sources of signal (svaseq and RUV methods) produce estimates that are highly correlated with the simulated batch effect. The estimate of batch based on principal components is biased, because the principal component is estimating a linear combination of the group and batch variable (Figure 5). In this scenario all the P-value distributions are approximately correct, with the exception of the analysis using principal component based estimates of batch. This is because the principal components do not estimate accurate versions of the batch effect and bias the statistical significance calculation (see: http://jtleek.com/svaseq/simulateData.html for plots).
Correlation between simulated batch and group variables and various batch estimates. Light circles indicate low correlation and dark, tight ellipses indicate high correlation. In this case, all estimates that respect multiple sources of signal (sva and RUV based) methods are highly correlated with the simulated batch effect. Principal components estimates a linear combination of the group and batch variable and has lower concordance with the true simulated batch and the other estimates. Additional details at http://jtleek.com/svaseq/simulateData.html.
Differential expression results for simulated data. A concordance at the top plot (CAT plot) shows the fraction of DE results that are concordant between the analysis with the true batch and the analyses using different batch estimates. Supervised (pink solid) and unsupervised (pink dotted) sva for sequencing, RUV with control probes (green dashed), RUV with empirical controls (green dotted) and residual RUV (green solid) all outperform not adjusting for batch effects (yellow) while principal components analysis (blue) performs worse than no adjustment. Additional details at http://jtleek.com/svaseq/simulateData.html.
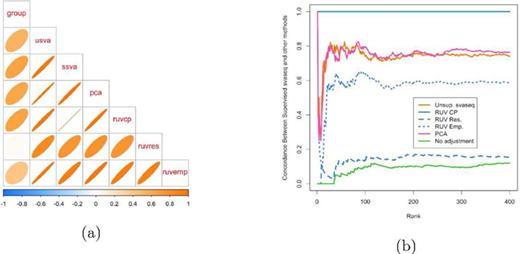
We performed an identical analysis where the batch was now correlated with the group variable. Qualitatively similar results hold in this second simulated scenario with one exception. The empirical RUV methods attempt to define control probes by identifying genes that do not show differential expression with respect to batch. But when batch and group are correlated, this may also through away genes that show signal with respect to the group variable. Similar the residual RUV approach estimates the batch variable after taking the residuals from the model fit of the counts on the group variable. However, when batch and group are correlated, this again removes batch signal and leads to slightly lower performance of the RUV approaches (4). Unsupervised svaseq does not use the control probes but avoids some of these difficulties by iteratively identifying probes associated with group but not associated with batch (5) (Figure 7). The P-value histograms here show a strong difference between supervised and unsupervised approaches. The unsupervised approaches attempt to estimate the artifacts, but they are correlated with the group. Since the estimates are off, the statistical significance calculations are not correct (see: http://jtleek.com/svaseq/simulateData.html for plots). But the supervised methods correct the statistical significance calculations accurately.
Comparison of batch effect results when group and batch are correlated. (a) A plot of the correlation between the different batch estimates and the batch variable analogous to Figure 5. (b) A concordance at the top plot measuring concordance between the analysis using the true batch variable and the various estimates analogous to Figure 6. Here the unsupervised RUV approaches using empirical control probes and residuals perform worse than no adjustment, because the methods can not distinguish signal from the known group variable and the unknown batch variable. Additional details at http://jtleek.com/svaseq/simulateData.html.
Comparison of batch effect results on Zebrafish data. (a) A plot of the correlation between the different batch estimates analogous to Figure 5, but with no gold standard. (b) A concordance at the top plot measuring concordance between the analysis using the supervised SVA estimates and the various other batch estimates analogous to Figure 6. The control probes RUV approach (blue solid in (b)) and supervised sva approach produce identical results. The unsupervised sva (orange solid) and principal components (pink solid) approaches are most similar to the supervised estimates in this scenario. Additional details at http://jtleek.com/svaseq/zebrafish.html.
Zebrafish data
Next I performed an analysis on the Zebrafish data as described in the methods section. Here, the batch variable is not known, but we do have negative control probes which can be used to estimate the batch effects. When comparing the batch estimates, I noted that the supervised sva estimates and the RUV control probes estimates were perfectly correlated (R2 = 1) and that they produced identical differential expression results (Figure 8a). The unsupervised sva and principal components approaches are most similar to the supervised estimates from SVA or RUV for the Zebrafish data.
ReCount data
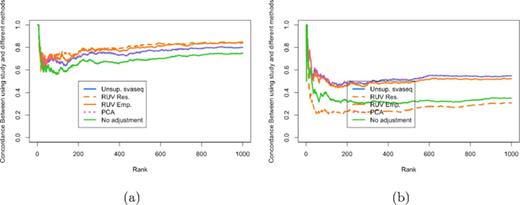
In the original data, the batch effect and the group variable are nearly perfectly orthogonal. In this situation the empirical and residual RUV approaches produce estimates of the batch variable more highly correlated than the unsupervised svaseq approach (see http://jtleek.com/svaseq/recount.html) and produce correspondingly more similar differential expression results to using the true study variable as an adjustment in the differential expression analysis (Figure 9a). However, I next re-sampled the data to mimic a scenario where the group and batch variable showed modest correlation (r2 = 0.33). In this scenario the unsupervised sva and principal components analysis approaches outperform the empirical control RUV approach. The residual RUV approach performs worse than no adjustment for study, because signal due to the batch variable was removed when the residuals from the model relating sex to phenotype was calculated (Figure 9b).
Comparison of differential expression results for ReCount experiment. (a) A concordance at the top plot measuring concordance between the analysis using the true study and the various other batch estimates analogous to Figure 6. (b) A concordance at the top plot measuring concordance between the analysis using the true study and the various other batch estimates analogous to Figure 6 when data were resampled to make the sex and study variables moderately correlated (r2 = 0.33.) When sex and study are uncorrelated, RUV performs slightly better and when sex and study are correlated, svaseq performs slightly better. Additional details at http://jtleek.com/svaseq/recount.html.
GEUVADIS data
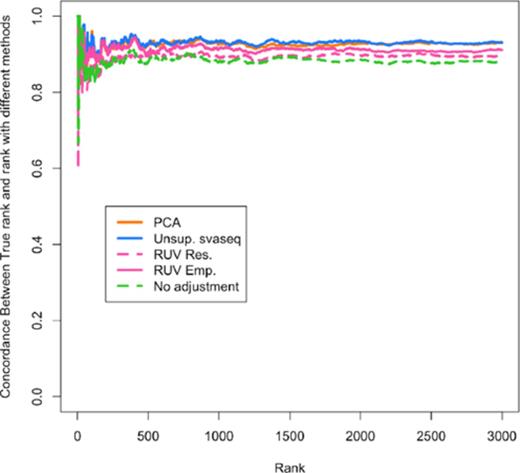
Differential expression results for GEUVADIS data. A concordance at the top plot (CAT plot) shows the fraction of DE results that are concordant between the analysis with the true laboratory and the analyses using different batch estimates. Unsupervised sva for sequencing (blue) and principal components analysis (orange) outperform the RUV based methods (pink) and no batch adjustment (green). Additional details at http://jtleek.com/svaseq/geuvadis.html.
DISCUSSION
Here I have described the general sva framework and I have introduced two extensions of the sva approach. The first takes advantage of known control probes to simplify the sva algorithm and the second addresses the distribution of count and FPKM data typically observed in sequencing experiments. The question of whether to use FPKM or count based approaches for the analysis of RNA-sequencing data is beyond the scope of this paper. However, I have demonstrated in this paper that regardless of the choice for measurement summary, svaseq can be applied to remove batch effects.I have shown that sva-based approaches perform comparably to other batch effect estimation procedures for sequencing when the group and unknown batch variables are uncorrelated and outperform other approaches when the batch and group variable are correlated. These extensions are currently available from the devel branch of the sva software http://bioconductor.org/packages/devel/bioc/html/sva.html and all analyses are fully reproducible and available as R markdown documents from https://github.com/jtleek/svaseq.
FUNDING
National Institutes of Health (NIH) [R01 GM10570502, GM083084 to J.L.]. Funding for open access charge: NIH [R01 GM10570502].
Conflict of interest statement. None declared.






Comments