-
PDF
- Split View
-
Views
-
Cite
Cite
Alexandra M. Montoya, Alejandro Sánchez González, José P. Palma-Nicolás, Alberto Gómez-Treviño, José G. González, Gloria M. González, Genotyping, extracellular compounds, and antifungal susceptibility testing of Trichosporon asahii isolated from Mexican patients, Medical Mycology, Volume 53, Issue 5, June 2015, Pages 505–511, https://doi.org/10.1093/mmy/myv009
Close - Share Icon Share
Abstract
Trichosporon asahii is considered an opportunistic pathogen responsible for severe infections, mainly in immunocompromised patients. The aims of this study were to investigate the prevalent genotypes among 39 clinical isolates of this microorganism by sequencing the IGS1 region and to determine the in vitro production of DNAse, hemolysin, aspartyl proteinase, phospholipase and esterase, as well as the susceptibilities of the isolates to amphotericin B, anidulafungin, micafungin, caspofungin, voriconazole, posaconazole, fluconazole and 5-flucytosine. Our findings showed that genotype I was the most prevalent comprising 69.23% of the isolates. We confirmed the production of esterase for all our isolates, and report the production of DNAse and aspartyl proteinase in 84.62% and 23% of the isolates, respectively. Only one isolate of T. asahii produced hemolysin. None of the isolates showed phospholipase activity. Fifty-three percent of the T. asahii strains exhibited amphotericin B MICs ≥ 2 μg/ml. The three echinocandins evaluated yielded high MICs (≥2 μg/ml) in all isolates. Thirty-five percent of the isolates had high MICs for 5-flucytosine (≥32 μg/ml), and 97% of the isolates were susceptible to the evaluated triazoles.
Introduction
Trichosporon spp. is a yeastlike fungus that can colonize mainly the gastrointestinal and respiratory tracts and the skin of humans. T. cutaneum, T. inkin, and T. ovoides are commonly associated to white piedra, an asymptomatic and superficial hair infection [1]. However, in the last decades T. asahii has become clinically important as the etiological agent of deep-seated infections or trichosporonosis. This pathogen is considered an important cause of disseminated yeast infections by non-Candida species, specifically in the setting of patients with malignant hematological diseases [2,3]. In addition, the administration of parenteral hyper-alimentation solutions, as well as the use of intravascular devices and prosthetic materials represent an important risk factor that could lead to infections in susceptible hosts [4–6].
It is well known that the conventional identification methods for species of Trichosporon regularly yield contradictory results [7,8]. The accurate identification of the members of this genus requires analysis of the rDNA intergenic spacer 1 (IGS1) region, which also allows for the genotyping of T. asahii isolates [9]. In the clinical scenario, precise recognition of these microorganisms can be significant to improve antifungal treatment, since it has been shown that T. asahii seems to be more resistant in vitro to amphotericin B than triazole compounds [10,11].
Regarding the role of lytic compounds in the pathogenicity of microorganisms, it is known that some enzymes allow protein degradation and destabilization of host cell membranes increasing virulence [12]. These compounds have been reported mostly in Cryptococcus neoformans and Candida species [13–15]; however, these determinants of pathogenicity have remained substantially unrecognized for T. asahii. In 2006, Dag and Cerikcioglu detected esterase activity in all clinical isolates of T. asahii evaluated [16]. Recently, Sun et al. worked with 23 isolates of T. asahii and reported that 100% of the strains produced hemolysin [17]. In both studies, neither proteinases nor phospholipases were detected under the methodologies utilized for them.
In the present study, we investigated the prevalent genotypes among 39 clinical isolates of T. asahii by IGS1 sequencing and determined the production of extracellular lytic compounds (DNAse, hemolysin, aspartyl proteinase, phospholipase, and esterase). We also present the in vitro activities of eight commonly used antifungal agents against the isolates.
Material and methods
Strain identification
We examined 39 isolates previously identified as T. asahii by the API 20C AUX system (bioMérieux, France) and positive for urease activity on Christensen's agar. Each isolate was obtained from different patients between 2003 and 2014 at the Microbiology Reference Center of the School of Medicine, Universidad Autónoma de Nuevo León, in Monterrey, Mexico. Table 1 lists the 39 strains and their origin. All isolates were sub-cultured on Sabouraud glucose agar (SGA) and Mycosel agar at 30°C, 37°C, and 40°C. Macroscopic and microscopic examinations were done after 10 days at 30°C.
Genotype and clinical sources of Trichosporon asahii isolates analyzed.
| Genotype . | Strain . | Source . | GenBank accession no. . |
|---|---|---|---|
| I | E-1384 | Urine | KM269297 |
| F-1291 | Urine | KM269299 | |
| F-2178 | Urine | KM269300 | |
| G-898 | Urine | KM269301 | |
| G-1995 | Urine | KM269302 | |
| 07–2042 | Hair | KM269309 | |
| L-1579 | Urine | KM269310 | |
| 08–1660 | Urine | KM269312 | |
| 09–206 | Urine | KM269313 | |
| 09–324 | Urine | KM269314 | |
| 09–481 | Urine | KM269315 | |
| 09–730 | Urine | KM269316 | |
| 09–928 | Urine | KM269317 | |
| 09–1238 | Urine | KM269318 | |
| 09–1487 | Urine | KM269319 | |
| 09–1572 | Urine | KM269320 | |
| 10–552 | Urine | KM269321 | |
| 10–814 | Urine | KM269322 | |
| 10–874 | Urine | KM269323 | |
| 10–1002 | Urine | KM269324 | |
| 10–1065 | Urine | KM269325 | |
| H-498 | Blood | KM269326 | |
| 11–804 | Urine | KM269328 | |
| 12–287 | Urine | KM269329 | |
| 12–877 | Urine | KM269330 | |
| 12–1238 | Urine | KM269331 | |
| 13–0297 | Nail | KM269332 | |
| III | FM-545 | Hair | KM269303 |
| FM-200 | Hair | KM269304 | |
| 07–335 | Blood | KM269306 | |
| IV | E-1592 | Nail | KM269298 |
| 08–535 | Urine | KM269311 | |
| 14–0705 | Skin | KM269333 | |
| VII | D-142 | Nail | KM269295 |
| D-2621 | Wound secretion | KM269296 | |
| 07–230 | Skin | KM269305 | |
| 07–769 | Nail | KM269307 | |
| 07–1694 | Nail | KM269308 | |
| 11–217 | Urine | KM269327 |
| Genotype . | Strain . | Source . | GenBank accession no. . |
|---|---|---|---|
| I | E-1384 | Urine | KM269297 |
| F-1291 | Urine | KM269299 | |
| F-2178 | Urine | KM269300 | |
| G-898 | Urine | KM269301 | |
| G-1995 | Urine | KM269302 | |
| 07–2042 | Hair | KM269309 | |
| L-1579 | Urine | KM269310 | |
| 08–1660 | Urine | KM269312 | |
| 09–206 | Urine | KM269313 | |
| 09–324 | Urine | KM269314 | |
| 09–481 | Urine | KM269315 | |
| 09–730 | Urine | KM269316 | |
| 09–928 | Urine | KM269317 | |
| 09–1238 | Urine | KM269318 | |
| 09–1487 | Urine | KM269319 | |
| 09–1572 | Urine | KM269320 | |
| 10–552 | Urine | KM269321 | |
| 10–814 | Urine | KM269322 | |
| 10–874 | Urine | KM269323 | |
| 10–1002 | Urine | KM269324 | |
| 10–1065 | Urine | KM269325 | |
| H-498 | Blood | KM269326 | |
| 11–804 | Urine | KM269328 | |
| 12–287 | Urine | KM269329 | |
| 12–877 | Urine | KM269330 | |
| 12–1238 | Urine | KM269331 | |
| 13–0297 | Nail | KM269332 | |
| III | FM-545 | Hair | KM269303 |
| FM-200 | Hair | KM269304 | |
| 07–335 | Blood | KM269306 | |
| IV | E-1592 | Nail | KM269298 |
| 08–535 | Urine | KM269311 | |
| 14–0705 | Skin | KM269333 | |
| VII | D-142 | Nail | KM269295 |
| D-2621 | Wound secretion | KM269296 | |
| 07–230 | Skin | KM269305 | |
| 07–769 | Nail | KM269307 | |
| 07–1694 | Nail | KM269308 | |
| 11–217 | Urine | KM269327 |
Genotype and clinical sources of Trichosporon asahii isolates analyzed.
| Genotype . | Strain . | Source . | GenBank accession no. . |
|---|---|---|---|
| I | E-1384 | Urine | KM269297 |
| F-1291 | Urine | KM269299 | |
| F-2178 | Urine | KM269300 | |
| G-898 | Urine | KM269301 | |
| G-1995 | Urine | KM269302 | |
| 07–2042 | Hair | KM269309 | |
| L-1579 | Urine | KM269310 | |
| 08–1660 | Urine | KM269312 | |
| 09–206 | Urine | KM269313 | |
| 09–324 | Urine | KM269314 | |
| 09–481 | Urine | KM269315 | |
| 09–730 | Urine | KM269316 | |
| 09–928 | Urine | KM269317 | |
| 09–1238 | Urine | KM269318 | |
| 09–1487 | Urine | KM269319 | |
| 09–1572 | Urine | KM269320 | |
| 10–552 | Urine | KM269321 | |
| 10–814 | Urine | KM269322 | |
| 10–874 | Urine | KM269323 | |
| 10–1002 | Urine | KM269324 | |
| 10–1065 | Urine | KM269325 | |
| H-498 | Blood | KM269326 | |
| 11–804 | Urine | KM269328 | |
| 12–287 | Urine | KM269329 | |
| 12–877 | Urine | KM269330 | |
| 12–1238 | Urine | KM269331 | |
| 13–0297 | Nail | KM269332 | |
| III | FM-545 | Hair | KM269303 |
| FM-200 | Hair | KM269304 | |
| 07–335 | Blood | KM269306 | |
| IV | E-1592 | Nail | KM269298 |
| 08–535 | Urine | KM269311 | |
| 14–0705 | Skin | KM269333 | |
| VII | D-142 | Nail | KM269295 |
| D-2621 | Wound secretion | KM269296 | |
| 07–230 | Skin | KM269305 | |
| 07–769 | Nail | KM269307 | |
| 07–1694 | Nail | KM269308 | |
| 11–217 | Urine | KM269327 |
| Genotype . | Strain . | Source . | GenBank accession no. . |
|---|---|---|---|
| I | E-1384 | Urine | KM269297 |
| F-1291 | Urine | KM269299 | |
| F-2178 | Urine | KM269300 | |
| G-898 | Urine | KM269301 | |
| G-1995 | Urine | KM269302 | |
| 07–2042 | Hair | KM269309 | |
| L-1579 | Urine | KM269310 | |
| 08–1660 | Urine | KM269312 | |
| 09–206 | Urine | KM269313 | |
| 09–324 | Urine | KM269314 | |
| 09–481 | Urine | KM269315 | |
| 09–730 | Urine | KM269316 | |
| 09–928 | Urine | KM269317 | |
| 09–1238 | Urine | KM269318 | |
| 09–1487 | Urine | KM269319 | |
| 09–1572 | Urine | KM269320 | |
| 10–552 | Urine | KM269321 | |
| 10–814 | Urine | KM269322 | |
| 10–874 | Urine | KM269323 | |
| 10–1002 | Urine | KM269324 | |
| 10–1065 | Urine | KM269325 | |
| H-498 | Blood | KM269326 | |
| 11–804 | Urine | KM269328 | |
| 12–287 | Urine | KM269329 | |
| 12–877 | Urine | KM269330 | |
| 12–1238 | Urine | KM269331 | |
| 13–0297 | Nail | KM269332 | |
| III | FM-545 | Hair | KM269303 |
| FM-200 | Hair | KM269304 | |
| 07–335 | Blood | KM269306 | |
| IV | E-1592 | Nail | KM269298 |
| 08–535 | Urine | KM269311 | |
| 14–0705 | Skin | KM269333 | |
| VII | D-142 | Nail | KM269295 |
| D-2621 | Wound secretion | KM269296 | |
| 07–230 | Skin | KM269305 | |
| 07–769 | Nail | KM269307 | |
| 07–1694 | Nail | KM269308 | |
| 11–217 | Urine | KM269327 |
For molecular identification, strains were cultured in SGA for 48 h at 30°C, after which their genomic DNA was extracted using the phenol-chloroform method [18], precipitated with 70% ethanol and resuspended in sterile deionized water. DNA was used for the amplification of the ribosomal region IGS1, using the forward primer 26SF (5′-ATC CTT TGC AGA CGA CTT GA-3′) and reverse primer 5SR (5′-AGC TTG ACT TCG CAG ATC GG -3′). Amplification reactions were performed in final volumes of 50 μl using Taq polymerase Master Mix (Promega, Madison, WI), 10 ng/μl of sample DNA, and final concentrations of 0.5 μm for each primer. The reaction mixtures were amplified in a C1000 Touch thermal cycler (Bio-Rad), using the following program: 94°C for 3 min, followed by 35 cycles consisting of 94°C for 30 s, 57°C for 30 s, and 72°C for 1 min, with a final extension period at 72°C for 10 min. The polymerase chain reaction (PCR) products were sequenced by the Sanger method on a Genetic Analyzer 3130 sequencer (Applied Biosystems). Species identification and genotyping of T. asahii were performed by comparison against previously reported strains (GenBank accession numbers AB066386, AB066397, AB066399, AB066401, AB072606, AB180192, AB180193, AB439002, AB439003, EU441158, EU441160, and JF412789). Sequence alignment was performed using the GeneStudio Professional software version 2.2.0.0 (www.genestudio.com).
Lytic compounds activity assays
All the isolates were cultured in SGA for 24 h at 30°C. For the assessment of DNAse activity, strains were directly striate-inoculated on DNAse test agar with methyl green medium (Becton Dickinson, Le Pont de Claix, France), incubated for 7 d at 37°C and interpreted according to the instructions of the manufacturer. Staphylococcus aureus ATCC 25923 was used as a positive control. For the analysis of hemolysin, phospholipase, aspartyl proteinase and esterase, fungal cells were re-suspended in sterile deionized water at a concentration of 1×107 cfu/ml, and 5 μl of this suspension were spot-inoculated on the appropriate medium. Hemolytic activity testing was performed on Sabouraud blood medium (65 g/l SGA, 3% w/v glucose and 7% v/v blood) [19]. Phospholipase activity was assayed on egg yolk medium (65 g/l SGA, 3% w/v glucose, 1 m NaCl, 5 mm CaCl2 and 8% egg yolk emulsion) [20]. Aspartyl proteinase activity was carried out on bovine serum albumin (BSA) medium (1.17% w/v yeast carbon base, 0.01% w/v yeast extract, 0.2% w/v BSA and 1.12% agar) [21]. Esterase activity was evaluated on Tween 80 medium (1% w/v Bacto peptone, 0.5%w/v NaCl, 0.01% w/v CaCl2, 1.5% w/v agar and 0.5% v/v Tween 80) [22]. Plates were incubated at 37°C for 2, 5, 7, and 10 days, respectively. Candida albicans ATCC 90028 was used as a positive control. Sterile deionized water was used as a negative control for all enzymatic assays.
Hemolytic activity was determined positive by the presence of a translucent halo around the inoculation site, visible with transmitted light. Phospholipase and proteinase activities were determined positive by the formation of a precipitation halo around the fungal growth. Positivity of esterase activity was established by the presence of opaque crystals around the colony, visible with transmitted light. Enzymatic activity was expressed as a Pz value, which measures the ratio of the colony diameter to the diameter of the activity halo. Activity was thus categorized as “very strong” (Pz ≤ 0.69), “strong” (Pz = 0.70–0.79), “mild” (Pz = 0.80–0.89), “weak” (Pz = 0.90–0.99), and “negative” (Pz = 1.0) [23].
Antifungal susceptibility testing
Susceptibility testing was performed according to the M27-A3 broth microdilution method published by the Clinical and Laboratory Standards Institute (CLSI) [24]. RPMI 1640 with L-glutamine (Hardy Diagnostics, Santa Maria, CA) buffered at pH 7.0 with 0.165 m morpholinepropanesulfonic acid was used as a medium in flat bottom 96-well plates. Medium was inoculated for a final concentration of 0.5 × 103 to 2.5 × 103 cfu/ml in each well. Plates were incubated at 37°C for 24 or 48 h.
Amphotericin B (Apothecon, Princeton, NJ), anidulafungin (Ben Venve, Northfield Road Bedford, OH), micafungin (Astellas Pharma, Inc., Tokyo), caspofungin (Merck, Rahway, NJ), voriconazole (Pfizer, Inc., Mexico), posaconazole (Merck, Rahway, NJ), fluconazole (Pfizer, Inc., Amboise, France) and 5-flucytosine (Sigma Chemical, St. Louis, MO) were prepared in stock solutions and diluted using RPMI 1640 medium with L-glutamine. The final antifungal concentrations used were 0.03 to 16 μg/ml for amphotericin B, voriconazole and posaconazole; 0.015 to 8 μg/ml for the echinocandins; and 0.125 to 64 μg/ml for fluconazole and 5-flucytosine. Candida krusei ATCC 6258 and Candida parapsilosis 22019 were used as quality control strains.
The minimum inhibitory concentrations (MIC) were determined according to the CLSI guidelines. Readings were done after 24 h for amphotericin B and after 48 h for all other antifungals. The MIC of amphotericin B was taken from the well with the lowest concentration with 100% inhibition of visual growth compared to growth in the control well. The MICs of the other antifungals were taken from the wells with approximately ≥50% growth inhibition compared to the growth in the control well.
Results
Strain identification and genotype distribution
All 39 isolates grew on SGA at 30°C reaching an average of 20 mm after 10 days, producing a cream colored yeastlike colony that developed furrows and irregular folds with age. Microscopic examination revealed septate and hyaline hyphae with oval or rectangular arthroconidia and round to oval blastoconidia. Every isolate grew at 37°C but failed to grow at 40°C or in the presence of cycloheximide on Mycosel agar.
PCR amplification of the IGS1 region produced fragments of ∼637 bp (data not shown). All isolates were identified as T. asahii by sequence homology with previously reported IGS1 sequences of type strains. The genotype and GenBank accession number for the 39 isolates are listed in Table 1. The genotype distribution was 27 (69.23%) for type I; 6 (15.39%) for type VII; and 3 (7.69%) for each of the genotypes III and IV. T. asahii isolated from urine samples comprised most of the analyzed strains (26; 66.67%); 24 out of 26 belonging to genotype I, followed by genotypes IV and VII with one isolate each. Two isolates of T. asahii were recovered from blood samples (5.13%); one belonging to genotype I and the other to genotype III. Two isolates from skin corresponded one each to types IV and VII. T. asahii was also isolated from cases of white piedra (3; 7.69%), with a distribution of one isolate of type I and two isolates belonging to type III. Also, five isolates from nails (12.82%) exhibited the following distribution: three of type VII, followed by types I and IV with one isolate each. In addition, one strain corresponding to genotype VII was isolated from a wound secretion.
Production of extracellular lytic compounds
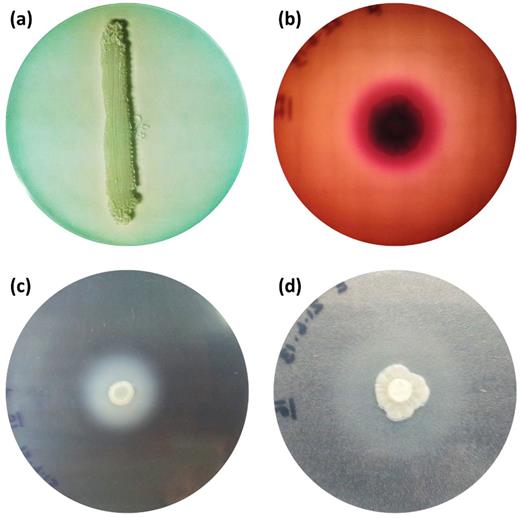
Results for enzymatic activity are summarized in Table 2 and depicted in Figure 1. None of the isolates was positive for phospholipase activity. DNAse activity was evident in isolates of all genotypes: 85% belonging to genotype I, 100% of the isolates comprising genotypes III and IV, and 67% of genotype VII. Only one isolate of T. asahii genotype VII showed very strong hemolysin activity. Regarding aspartyl proteinase activity, 23% of T. asahii isolates, all from genotype I, showed strong or very strong activity. All our isolates were positive for esterase activity. Ninety-seven percent of T. asahii isolates tested had very strong esterase activity, and 68% of these isolates were composed of genotype I.
In vitro evaluation of the DNAse (a), hemolysin (b), aspartyl proteinase (c), and esterase (d) activity of the isolates tested in this study.
Extracellular compounds activities of Trichosporon asahii isolates per genotype.
| Enzymatic activity . | Pz valuea . | Number of T. asahii isolates . | |||
|---|---|---|---|---|---|
| . | . | Genotype I (n = 27) . | Genotype III (n = 3) . | Genotype IV (n = 3) . | Genotype VII (n = 6) . |
| DNAse | |||||
| Positive | – | 23 | 3 | 3 | 4 |
| Negative | – | 4 | 0 | 0 | 2 |
| Hemolysin | |||||
| Very strong | ≤0.69 | 0 | 0 | 0 | 1 |
| Strong | 0.70–0.79 | 0 | 0 | 0 | 0 |
| Negative | 1.0 | 27 | 3 | 3 | 5 |
| Aspartyl proteinase | |||||
| Very strong | ≤0.69 | 6 | 0 | 0 | 0 |
| Strong | 0.70–0.79 | 3 | 0 | 0 | 0 |
| Negative | 1.0 | 18 | 3 | 3 | 6 |
| Esterase | |||||
| Very strong | ≤0.69 | 26 | 3 | 3 | 6 |
| Strong | 0.70–0.79 | 1 | 0 | 0 | 0 |
| Negative | 1.0 | 0 | 0 | 0 | 0 |
| Enzymatic activity . | Pz valuea . | Number of T. asahii isolates . | |||
|---|---|---|---|---|---|
| . | . | Genotype I (n = 27) . | Genotype III (n = 3) . | Genotype IV (n = 3) . | Genotype VII (n = 6) . |
| DNAse | |||||
| Positive | – | 23 | 3 | 3 | 4 |
| Negative | – | 4 | 0 | 0 | 2 |
| Hemolysin | |||||
| Very strong | ≤0.69 | 0 | 0 | 0 | 1 |
| Strong | 0.70–0.79 | 0 | 0 | 0 | 0 |
| Negative | 1.0 | 27 | 3 | 3 | 5 |
| Aspartyl proteinase | |||||
| Very strong | ≤0.69 | 6 | 0 | 0 | 0 |
| Strong | 0.70–0.79 | 3 | 0 | 0 | 0 |
| Negative | 1.0 | 18 | 3 | 3 | 6 |
| Esterase | |||||
| Very strong | ≤0.69 | 26 | 3 | 3 | 6 |
| Strong | 0.70–0.79 | 1 | 0 | 0 | 0 |
| Negative | 1.0 | 0 | 0 | 0 | 0 |
aPz value: the ratio of the colony diameter to the diameter of the activity halo.
Extracellular compounds activities of Trichosporon asahii isolates per genotype.
| Enzymatic activity . | Pz valuea . | Number of T. asahii isolates . | |||
|---|---|---|---|---|---|
| . | . | Genotype I (n = 27) . | Genotype III (n = 3) . | Genotype IV (n = 3) . | Genotype VII (n = 6) . |
| DNAse | |||||
| Positive | – | 23 | 3 | 3 | 4 |
| Negative | – | 4 | 0 | 0 | 2 |
| Hemolysin | |||||
| Very strong | ≤0.69 | 0 | 0 | 0 | 1 |
| Strong | 0.70–0.79 | 0 | 0 | 0 | 0 |
| Negative | 1.0 | 27 | 3 | 3 | 5 |
| Aspartyl proteinase | |||||
| Very strong | ≤0.69 | 6 | 0 | 0 | 0 |
| Strong | 0.70–0.79 | 3 | 0 | 0 | 0 |
| Negative | 1.0 | 18 | 3 | 3 | 6 |
| Esterase | |||||
| Very strong | ≤0.69 | 26 | 3 | 3 | 6 |
| Strong | 0.70–0.79 | 1 | 0 | 0 | 0 |
| Negative | 1.0 | 0 | 0 | 0 | 0 |
| Enzymatic activity . | Pz valuea . | Number of T. asahii isolates . | |||
|---|---|---|---|---|---|
| . | . | Genotype I (n = 27) . | Genotype III (n = 3) . | Genotype IV (n = 3) . | Genotype VII (n = 6) . |
| DNAse | |||||
| Positive | – | 23 | 3 | 3 | 4 |
| Negative | – | 4 | 0 | 0 | 2 |
| Hemolysin | |||||
| Very strong | ≤0.69 | 0 | 0 | 0 | 1 |
| Strong | 0.70–0.79 | 0 | 0 | 0 | 0 |
| Negative | 1.0 | 27 | 3 | 3 | 5 |
| Aspartyl proteinase | |||||
| Very strong | ≤0.69 | 6 | 0 | 0 | 0 |
| Strong | 0.70–0.79 | 3 | 0 | 0 | 0 |
| Negative | 1.0 | 18 | 3 | 3 | 6 |
| Esterase | |||||
| Very strong | ≤0.69 | 26 | 3 | 3 | 6 |
| Strong | 0.70–0.79 | 1 | 0 | 0 | 0 |
| Negative | 1.0 | 0 | 0 | 0 | 0 |
aPz value: the ratio of the colony diameter to the diameter of the activity halo.
In vitro antifungal susceptibility of Trichosporon asahii isolates.
| Antifungal . | MIC (μg/ml) . | |||
|---|---|---|---|---|
| . | Range . | GM . | MIC50 . | MIC90 . |
| Amphotericin Ba | 0.5–16 | 1.84 | 2 | 4 |
| Anidulafunginb | 4–>8 | ND | >8 | >8 |
| Micafunginb | >8 | ND | >8 | >8 |
| Caspofunginb | 8–>8 | ND | 8 | >8 |
| Voriconazolea | 0.03–1 | 0.04 | 0.03 | 0.03 |
| Posaconazolea | 0.03–0.5 | 0.08 | 0.06 | 0.25 |
| Fluconazolea | 0.125–16 | 0.78 | 0.5 | 1 |
| 5-flucytosinea | 4–>64 | ND | 16 | 32 |
| Antifungal . | MIC (μg/ml) . | |||
|---|---|---|---|---|
| . | Range . | GM . | MIC50 . | MIC90 . |
| Amphotericin Ba | 0.5–16 | 1.84 | 2 | 4 |
| Anidulafunginb | 4–>8 | ND | >8 | >8 |
| Micafunginb | >8 | ND | >8 | >8 |
| Caspofunginb | 8–>8 | ND | 8 | >8 |
| Voriconazolea | 0.03–1 | 0.04 | 0.03 | 0.03 |
| Posaconazolea | 0.03–0.5 | 0.08 | 0.06 | 0.25 |
| Fluconazolea | 0.125–16 | 0.78 | 0.5 | 1 |
| 5-flucytosinea | 4–>64 | ND | 16 | 32 |
Notes: MIC: minimum inhibitory concentration; GM: geometric mean; MIC50: MIC at which 50% of the isolates tested were inhibited; MIC90: MIC at which 90% of the isolates tested were inhibited; ND: not done.
aMIC at 24 h; bMIC at 48 h.
In vitro antifungal susceptibility of Trichosporon asahii isolates.
| Antifungal . | MIC (μg/ml) . | |||
|---|---|---|---|---|
| . | Range . | GM . | MIC50 . | MIC90 . |
| Amphotericin Ba | 0.5–16 | 1.84 | 2 | 4 |
| Anidulafunginb | 4–>8 | ND | >8 | >8 |
| Micafunginb | >8 | ND | >8 | >8 |
| Caspofunginb | 8–>8 | ND | 8 | >8 |
| Voriconazolea | 0.03–1 | 0.04 | 0.03 | 0.03 |
| Posaconazolea | 0.03–0.5 | 0.08 | 0.06 | 0.25 |
| Fluconazolea | 0.125–16 | 0.78 | 0.5 | 1 |
| 5-flucytosinea | 4–>64 | ND | 16 | 32 |
| Antifungal . | MIC (μg/ml) . | |||
|---|---|---|---|---|
| . | Range . | GM . | MIC50 . | MIC90 . |
| Amphotericin Ba | 0.5–16 | 1.84 | 2 | 4 |
| Anidulafunginb | 4–>8 | ND | >8 | >8 |
| Micafunginb | >8 | ND | >8 | >8 |
| Caspofunginb | 8–>8 | ND | 8 | >8 |
| Voriconazolea | 0.03–1 | 0.04 | 0.03 | 0.03 |
| Posaconazolea | 0.03–0.5 | 0.08 | 0.06 | 0.25 |
| Fluconazolea | 0.125–16 | 0.78 | 0.5 | 1 |
| 5-flucytosinea | 4–>64 | ND | 16 | 32 |
Notes: MIC: minimum inhibitory concentration; GM: geometric mean; MIC50: MIC at which 50% of the isolates tested were inhibited; MIC90: MIC at which 90% of the isolates tested were inhibited; ND: not done.
aMIC at 24 h; bMIC at 48 h.
Antifungal susceptibility
The in vitro antifungal susceptibilities of the 39 T. asahii isolates are summarized in Table 3. Of note, 53% of the isolates tested had MICs ≥ 2 μg/ml for amphotericin B. All isolates had MICs ≥ 4 μg/ml for the three echinocandins tested. Concerning voriconazole, posaconazole and fluconazole, the isolates were susceptible to these compounds, obtaining MIC90 of 0.03, 0.25, and 1 μg/ml, respectively. With regard to 5-flucytosine, we observed a variable response among the isolates tested; 35% of all strains displayed a MIC ≥ 32 μg/ml, corresponding to 7 isolates of genotype I, 3 isolates of genotype VII, and 2 isolates each of genotypes III and IV.
Discussion
At present, 12 genotypes of T. asahii have been reported. Genotypes I, III, IV, and VII were found among our isolates. Genotype I was the predominant type and was obtained in 27 isolates. This result is similar to those found in Brazil [25,26], Spain [27], Turkey [28], Taiwan [29], and Thailand [30]. Genotype III was reported as the most predominant in Argentina and the United States, followed by genotypes I and V, respectively [9,31]. Also in Japan, genotype III corresponded to the majority of the environmental strains obtained from the houses of patients with Summer-type hypersensitivity pneumonitis caused by T. asahii [32]. Genotype IV has been the most reported in China, followed by genotype III [8,17]. Furthermore, the most recently described genotypes seem to be geographically restricted: genotypes VIII and IX have been reported in Turkey [28], and genotypes X, XI and XII are present in China [33]. Nevertheless, there is only one report for each of these genotypes, therefore no data on their distribution is yet available.
Regarding virulence factors, little is known of the production of lytic compounds and their role in the pathogenicity of T. asahii. DNAse activity was recently reported in eight out of 18 T. asahii clinical isolates [34]. Similarly, 84.6% of the isolates in our study were producers of this compound.
Phospholipases are involved in the disruption of host cell membranes [35]. In our study, none of the isolates were positive for phospholipase activity. This agrees with previous reports on the phospholipase capability of T. asahii clinical isolates [16,17], although one study has recently reported positive activity by six out of 18 clinical strains [34]. Furthermore, phospholipase activity has been reported in Trichosporon strains isolated from bovine raw milk [36] and from the droppings and cloacae of wild birds [37].
Hemolysins are pore-forming toxins capable of lysing red blood cells, releasing iron needed as a growth factor by several fungi [38]. Variable hemolytic activity has been shown in T. asahii isolates of genotypes I, III, and IV [17]. This differs greatly with our findings in which no hemolytic activity was found in any of our isolates, except for one isolate of genotype VII. At the moment, we cannot explain the discrepancy between these results.
Regarding proteinases, their role as a virulence factor has been best described in C. albicans, where it has been shown that they participate in the degradation of human proteins, adherence to different host tissues and cell types, and the regulation of phenotypic switching. BSA has been routinely used for the evaluation of aspartyl proteinase production by Candida spp. [39], and has been used for the assessment of activity by T. asahii, T. cutaneum and T. japonicum [16,17,40]. Only one of such study has reported positive aspartyl-proteinase activity in 12 out of 18 clinical isolates [34]. Our findings showed that 23% of the isolates had very strong or strong lytic activity against BSA. All but one of our proteinase-positive strains were isolated from urine samples.
Esterases hydrolyze ester bonds for the release of fatty acids and, as opposed to other lipases, are capable of acting on soluble substrates [41]. All our strains were positive for esterase activity when Tween 80 was used as a medium. The extracellular production of esterases has been previously studied on T. japonicum, T. montevideense, and T. asahii using the same substrate. With the exception of one T. montevideense isolated from llama meat, all strains tested have been reported positive for esterase activity [16,42,43]. Most significantly, all studies published to date have reported positive activity in all the clinical isolates tested, which suggests a role for esterases in the pathogenicity of Trichosporon.
Another important aspect of T. asahii in the clinical scenario is its lack of response to some commonly used antifungals. It is accepted that the genus Trichosporon is not inhibited by amphotericin B, and our results showed that 53% of all strains had MICs ≥2 μg/ml for this antifungal compound. Similar results have been previously reported by other authors [26,28,30]. To date, only one study has reported 100% inhibition of 18 T. asahii isolates by this drug [27].
Regarding echinocandins, lack of activity against Trichosporon spp. has been previously reported [44]. As expected, we found MICs ≥ 4 μg/ml in all our isolates in accordance to that found in T. asahii [25,45]. Guo et al. and Chagas-Neto et al. have reported MICs of 2 μg/ml for caspofungin, which is the C. albicans breakpoint for this drug [8,26]. Triazole compounds were the most effective antifungals in vitro against Trichosporon. Previous reports state low MICs for voriconazole and fluconazole [8,26–28,30], which agree with our results for these antifungals. Two additional studies have evaluated the efficacy of posaconazole against clinical isolates of T. asahii, with variable results: a broad MIC range of 0.12–16 μg/ml reported by Araujo Ribeiro et al., [25] and a narrow range of 0.015–1 μg/ml reported by Taverna et al. [31] Our results showed excellent antifungal activity against isolates of T. asahii, obtaining a range of 0.03–0.5 μg/ml. Finally, response to 5-flucytosine has been reported as varied [25–27,30] in accordance with our results.
This is the first study on genotyping carried out in our country. The genotype distribution of the Mexican isolates was similar to those previously reported in some countries. Regarding the extracellular hydrolytic compounds, we confirmed the production of DNAse, hemolysins, esterase and aspartyl-proteinase by T. asahii. Finally, our study emphasizes the importance of in vitro susceptibility testing of clinical isolates, because response to the different commonly used antifungals may vary with each isolate.
We thank Dr. Sergio Lozano of the “Dr. José E. González” University Hospital (Monterrey, Mexico) for his review of the manuscript prior to submission.
Funding
This work was supported by the Programa de Apoyo a la Investigación Científica y Tecnológica (PAICyT) [CS-656-11].
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and the writing of the paper.
References
- amphotericin b
- antifungal agents
- flucytosine
- aspartic acid endopeptidases
- deoxyribonuclease i
- deoxyribonucleases
- esterase
- fluconazole
- genotype
- hemolysin
- immunocompromised host
- phospholipase
- triazoles
- trichosporon
- infections
- pathogenic organism
- caspofungin
- voriconazole
- posaconazole
- echinocandins
- antifungal susceptibility test
- anidulafungin
- micafungin
- microorganisms
- pathogenicity
- genotype determination
- malnutrition-inflammation-cachexia syndrome