-
PDF
- Split View
-
Views
-
Cite
Cite
Monic M. M. Tomassen, Diane M. Barrett, Henry C. P. M. van der Valk, Ernst J. Woltering, Isolation and characterization of a tomato non-specific lipid transfer protein involved in polygalacturonase-mediated pectin degradation, Journal of Experimental Botany, Volume 58, Issue 5, March 2007, Pages 1151–1160, https://doi.org/10.1093/jxb/erl288
Close - Share Icon Share
Abstract
An important aspect of the ripening process of tomato fruit is softening. Softening is accompanied by hydrolysis of the pectin in the cell wall by pectinases, causing loss of cell adhesion in the middle lamella. One of the most significant pectin-degrading enzymes is polygalacturonase (PG). Previous reports have shown that PG in tomato may exist in different forms (PG1, PG2a, PG2b, and PGx) commonly referred to as PG isoenzymes. The gene product PG2 is differentially glycosylated and is thought to associate with other proteins to form PG1 and PGx. This association is thought to modulate its pectin-degrading activity in planta. An 8 kDa protein that is part of the tomato PG1 multiprotein complex has been isolated, purified, and functionally characterized. This protein, designated ‘activator’ (ACT), belongs to the class of non-specific lipid transfer proteins (nsLTPs). ACT is capable of ‘converting’ the gene product PG2 into a more active and heat-stable form, which increases PG-mediated pectin degradation in vitro and stimulates PG-mediated tissue breakdown in planta. This finding suggests a new, not previously identified, function for nsLTPs in the modification of hydrolytic enzyme activity. It is proposed that ACT plays a role in the modulation of PG activity during tomato fruit softening.
Introduction
Ripening of tomato fruit involves a series of complex metabolic processes. Modifications in cell wall polymers during ripening are intricate and considered to involve the co-ordinated and interdependent action of a range of cell wall-modifying enzymes and proteins such as polygalacturonase (PG), β-subunit, pectin methylesterase (PME), β-galactosidase, α-L-arabinofuranosidase, endo-(1,4) β-D-glucanase, expansin, and xyloglucan endotransglycosylase (Brummell and Harpster, 2001). One of these processes is the depolymerization of pectic polymers in the middle lamella and cell wall, which is brought about by the action of the ripening-associated enzyme PG (Crookes and Grierson, 1983; Huber, 1983). The appearance of PG correlates with the rate of pectin degradation and the rate of fruit softening, which has led to the suggestion that PG enzyme activity is primarily responsible for softening during ripening. To investigate the role of PG in fruit softening, PG-antisense tomato plants with greatly reduced PG mRNA and PG protein levels have been produced (Gray et al., 1992). Despite the low PG activity in transgenic fruit (<1% of the wild-type level), softening was not prevented (Smith et al., 1988, 1990). This result suggests that softening is not solely a PG-dependent process (DellaPenna et al., 1987; Speirs and Brady, 1991). The specific contribution of PG in tomato fruit ripening still remains an open question.
In vitro, PG can be separated into four isoenzymes termed PG1, PGx, PG2a, and PG2b (Pressey and Avants, 1973; Knegt et al., 1988). All of these isoenzymes originate from one single PG gene (Fischer and Bennett, 1991; Pogson et al., 1991). PG2a and PG2b, which are 45 kDa and 46 kDa proteins, respectively, differ only in their degree of glycosylation (DellaPenna et al., 1989). Because of the physical and biochemical similarity of PG2a and PG2b, these two PG isoenzymes will be treated as a single isoenzyme, namely PG2. PG2 is a heat-labile protein that is inactivated readily at 65 °C (Tucker et al., 1981). PG2 mRNA accumulates to high levels during fruit ripening (Brady, 1992). PG2 is present in the cytoplasm but is not directly in contact with the middle lamella of the cell walls (van Loon and Bruinsma, 1992). It was hypothesized that PG2 migrates through the plasma membrane aided by a wall-bound protein called the ‘convertor’ (CV) (Tucker et al., 1981; Pressey, 1984; Knegt et al., 1988, 1991). The molecular mass of CV has been estimated to be between 81 kDa and 102 kDa (Pressey, 1984; Knegt et al., 1991). It is generally assumed that the CV itself has no known enzyme activity (Pressey, 1984) and that the CV has two binding sites that can bind PG2 (Knegt et al., 1991). It was hypothesized that the association between CV and PG2 creates either the isoenzyme PG1 (Tucker et al., 1981) or PGx (Knegt et al., 1988), depending on the number of CV-binding sites occupied.
PG1 is a heat-stable protein complex with a molecular mass of ∼100 kDa and a pH optimum of 3.6 (Pressey and Avants, 1973). PG1 is believed to be an artefactual complex, formed during extraction. It was hypothesized that, in PG1, both binding sites of the CV are associated with PG2 (PG2–CV–PG2) (Pressey, 1986, 1988; Bruinsma et al., 1989). Other authors have suggested that PG1 may consist of one molecule of PG2 that is tightly associated with a well-characterized cell wall glycoprotein, called the β-subunit (Zheng et al., 1992; Moore and Bennett, 1994). The β-subunit is an acidic, heat-stable, heavily glycosylated protein with an apparent molecular mass of 38 kDa (Pogson et al., 1991; Brady, 1992). The levels of the β-subunit protein increase ∼4-fold during ripening (Pogson and Brady, 1993). Several authors have suggested that the β-subunit performs the function of the hypothetical CV (Osteryoung et al., 1990; DellaPenna et al., 1996). However, fruits from antisense β-subunit plants demonstrate an increased release of pectic polysaccharides, which would indicate that the β-subunit inhibits rather than activates PG (Chun and Huber, 1997). Although the exact nature is currently not known, PG1 is considered to be a multiprotein complex consisting of PG2 and other proteins (CV and/or the β-subunit) (Pogson et al., 1991).
PGx is thought to be formed when one binding site of the CV is engaged (PG2–CV). PGx has similar heat stability to PG1 and similar pH optimum to PG2, and the molecular weight of PGx is ∼71 kDa (Knegt et al., 1991). PGx is thought to be the predominant protein complex that occurs in vivo and is reported to be the controlling factor in tomato fruit softening (Knegt et al., 1991; van Loon and Bruinsma, 1992).
The above considerations about the involvement of a PG-modulating protein in PG-mediated pectin degradation, however, are merely hypothetical as the CV has never been isolated as a single protein, and molecular characterization of CV has not been described. Judging from the molecular masses of the different PG isoenzymes, it is hard to estimate the exact molecular weight of the hypothetical CV. Differences in the extraction and purification procedures and unpredictable elution profiles of multiprotein complexes may have caused the observed discrepancies.
Here the isolation and characterization of a protein, present in the tomato PG1 multiprotein complex, with some of the properties of the long-sought CV, are described. The protein, which belongs to the class of non-specific lipid transfer proteins (nsLTPs), is capable of ‘converting’ PG2 into a more active, heat-stable form, which increases PG-mediated pectin degradation in vitro and stimulates PG-mediated tissue breakdown in vivo. It is proposed that this protein plays an important regulatory role in PG-mediated pectin depolymerization and fruit softening.
Materials and methods
Plant material
Tomato plants (Lycopersicon esculentum cv. Counter) and tomato fruit harvested at different ripening stages were grown in a greenhouse under standard cultivation conditions at the Plant Research International (PRI), Wageningen, The Netherlands.
Isolation and separation of polygalacturonase isoenzymes from ripe tomato fruit
The PG isoenzymes were isolated from fully ripe tomato fruit pericarp tissue according to the method of Knegt et al. (1988). PG2 was extracted by 0.5 M NaCl and PG1 by 1.25 M NaCl. The extracts were desalted and concentrated using an Amicon filtration system (YM10 filters) and purified as described by Knegt et al. (1988) using a Sephadex G100 column eluted with 100 mM NaAc and 200 mM NaCl buffer at pH 4.5. Standards were blue dextran 2000 (molecular weight 2000 kDa), bovine serum albumin (67 kDa), trypsin (24 kDa), and cytochrome c (12.5 kDa). The molecular weight of the various peaks was calculated from their relative retention volumes. To check the purity of the proteins, SDS-PAGE (12% acrylamide) was performed using a Bio-Rad (Richmond, CA, USA) Mini Protein apparatus. To determine the molecular weight of the different proteins, a low molecular weight marker (Pharmacia; 94, 67, 43, 30, 20, and 14 kDa) was used. Gels were stained for protein with Coomassie Brilliant Blue R (Laemmli, 1970). These PG isoenzymes were used for further investigations.
Isolation of proteins present in the multiprotein PG1 complex
Fractions of the PG1 peak of ripe tomato fruit showing the highest PG activity were pooled and heated for a period of 5 min from 65 °C to 100 °C to separate the multiprotein complex into its individual proteins. After reaching 100 °C, the solution was immediately cooled on ice, concentrated using an Amicon filtration system (YM10 filters), and applied to a Sephacryl S300 column with a length of 1.0 m and an internal diameter of 16 mm. The linear flow rate was 8.4 ml h−1 and samples of 2–2.5 ml were applied and developed with 100 mM NaAc and 200 mM NaCl buffer at pH 4.5. The molecular weight of the various peaks was calculated from their relative retention volumes using standards as described above.
Isolation of an 8 kDa protein from green tomato fruit
Mature green tomato pericarp was cut into 1.0 cm pieces and mixed with an equal volume (w/v) of cold water in a small 0.02 mm thick plastic bag (15×20 cm). The material was heated at 65 °C in a shaking water bath for 5 min to inactivate PG2. After cooling on iced water for 20 min, the mixture was homogenized in a cooled Edmund Bühler HO4 for 1 min (35 000 rpm). The pH of the suspension was adjusted to 3.0 with 1.0 M HCl. The suspension was stirred for 15 min at room temperature and centrifuged at 47 500 g at 4 °C for 15 min. The pellet was homogenized (cooled Edmund Bühler HO4) in a 1.25 M NaCl solution, using twice the volume of the previous amount of water. The suspension was adjusted to pH 5.5 with 1.0 M NaOH, stirred for 15 min at room temperature, and centrifuged at 47 500 g for 15 min at 4 °C. The supernatant was filtered through a 0.45 μM filter and stored at −20 °C. The supernatant was desalted, concentrated, and gel filtration was performed as described for the isolation of proteins from the multiprotein PG1 complex (see previous section). To check the purity of the protein, analysis of filtration fractions was performed on a PhastSystem using a PhastGel homogeneous high density gel. Gels were stained with a PhastGel Protein Silver Staining Kit (Amersham Biosciences). To determine the molecular weight of the different proteins, a myoglobin marker (Pharmacia; 16.9, 14.4, 8.1, 6.2, and 2.5 kDa) was used.
Sequencing of the 8 kDa protein
The N-terminal sequencing, internal peptide sequencing, and amino acid analysis were carried out by Eurosequence BV, Groningen, The Netherlands. The 8 kDa protein isolated from green tomato fruit pericarp was sequenced before and after pyridylethylation and subsequent degradation using the Edman procedure (Edman, 1956). The sequences of the N-terminus and an internal peptide were determined on an automated sequenator (Model 477A, Applied Biosystems) according to the method of Hewick et al. (1981) coupled with a high-pressure liquid chromatography (HPLC; Model 120A ABI). Amino acid analysis was performed with an HP 1090 Aminoqant (Schuster, 1988) by means of a two-step derivation with OPA (one-phor-all buffer) and FMOC (methoxy-oxymethyl-phenoxymethylated amino acid ester) using 5.7 N HCl for 2 h at 166 °C. The amino acid sequences were compared with gene sequences in GenBank and aligned with full-length deduced amino acid sequences of LTP homologues using Clustal W, multiple sequence alignment software, and a phylogenetic tree was generated from the aligned sequences.
PG enzyme assays
For determination of PG activity, two different methods were used. PG activity in fractions eluting from the columns was measured using the method of Gross (1982). This method determines the increase in reducing groups following hydrolysis of a polygalacturonic acid (PGA) substrate, using 2-cyanoacetamide. In experiments where the effect of extracts (containing the 8 kDa protein or recombinant LTPs) on PG activity were tested, a modified ferricyanide method was used (Rozie et al., 1988). In both methods, D-galacturonic acid was used as a standard. Although both methods gave essentially similar results, the method of Rozie was found more appropriate as the results obtained were more reproducible (data not shown).
PGA was used as a substrate in all assays; if pectin (Serva, Mr 150 000–300 000 Da) was used in comparison, this invariably yielded similar results. All assays were carried out in duplicate. All extractions and experiments were repeated at least once, and representative data are shown.
Effect of the 8 kDa protein on PG2 activity
The effect of the 8 kDa protein was determined by monitoring its influence on the kinetics of PG2-mediated depolymerization of PGA, resulting in formation of reducing sugars. The reaction mixture contained an enzyme solution of purified PG2, with or without the addition of the 8 kDa protein, adjusted to 250 μl with sodium acetate buffer containing 100 mM NaAc and 200 mM NaCl, pH 4.5. The reaction mixtures were preincubated for 40 min at 37 °C to allow association between the proteins and, thereafter, some were heated for 5 min at 65 °C to destroy free PG2. After the preincubation, the reaction mixtures were cooled on ice. A 1 ml aliquot of 0.25% PGA, dissolved in NaAc buffer pH 4.5, was added. Directly after adding the pectin solution, a sample of 100 μl (t=0) was taken and stored on ice. Thereafter the reaction mixture was placed in a shaking water bath at 37 °C and every 15 min samples of 100 μl were taken and stored on ice. When all samples had been collected, 900 μl of a freshly prepared yellow-coloured mixture of a cyanide solution [0.125% KCN, 0.04% Fe(CN)6, 1% Na2CO3] was added to each sample. The samples were incubated at room temperature for 20 min and boiled for 10 min in a shaking water bath and immediately cooled on ice. After 1 h, the discoloration was measured spectrophotometrically at 420 nm. PG activity was expressed as the number of moles of galacturonic acid-reducing group units (GA-units) produced per minute. The assay was carried out in duplicate; all extractions and experiments were repeated at least once, and representative data are shown.
Effect of recombinant apple and peach LTPs on activity of purified Aspergillus niger PG
To verify that LTPs and PG2 interact with each other to increase the PG activity, purified PG (EC 3.2.1.15; 1.8 U mg−1, Fluka) from Aspergillus niger, and different recombinant LTPs, i.e. recombinant apple mature LTP [fast protein liquid chromatography (FPLC) purified], recombinant apple pro-LTP (FPLC purified), recombinant apple pro-LTP (monoclonal 6B5 affinity purified), and recombinant peach mature-LTP (FPLC purified) (Riccardo Asero et al., 2000) were obtained. The effect of the different LTPs on the activity of purified A. niger PG was determined as described above. The reaction mixture contained 5 μl of purified A. niger PG (0.4 mg ml−1), with or without the addition of 50 μl of recombinant LTP (60 μg ml−1), adjusted to 125 μl with sodium acetate buffer containing 100 mM NaAc and 200 mM NaCl, pH 4.5.
In situ tissue degradation determined by scanning electron microscopy (SEM)
The effect of the 8 kDa protein on tissue deterioration was illustrated using discs (12 mm diameter) of pericarp tissue. The discs were prepared with a cork borer and trimmed to 2 mm thickness with a razor blade. The discs were treated with 25 μl of NaAc buffer (100 mM NaAc, 200 mM NaCl) at pH 4.5 and NaAc buffer with purified 8 kDa protein. Discs were incubated in a covered Petri dish with moist filter paper for 2 h at 37 °C and dried with paper tissue. The discs were processed for cryoscanning microscopy. Briefly, sections of tissue were fixed in an SEM holder, frozen in melting nitrogen, and sputter-coated with gold (Oxford CT1500 cryostation). All these steps took place under cryogenic conditions. The surface of the disc was observed, and SEM images were made using a Jeol 5600 LV cryoscanning electron microscope at −193 °C.
Results
Different PG isoenzymes from tomato fruit
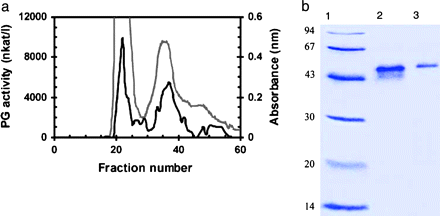
Red-ripe tomato fruit was selected as the starting material for the isolation of PG isoenzymes because PG activity is highest in ripe fruit. After salt extraction and gel filtration chromatography (Sephadex G100), the supernatant was resolved into two major peaks of PG activity (Fig. 1a). The first activity eluted in fractions 20–25 (corresponding to a protein of ∼100 kDa), while the second eluted in fractions 30–45 (corresponding to a protein of ∼43 kDa). Following SDS-PAGE of fraction 22, two bands (∼44–45 kDa) appeared (Fig. 1b), possibly representing PG2 and the β-subunit (Zheng, 1992). SDS-PAGE of fraction 37 showed only one major band, at ∼45 kDa (Fig. 1b), representing PG2 (DellaPenna et al., 1996). Fractions 20–25 and 37 were used in further experiments and are referred to as tomato PG1 and PG2, respectively.
Isolation of PG isoenzymes from extracts of red tomatoes. (a) The protein elution profile (grey line) and activity analysis (black line) of PG on a Sephadex G100 column (molecular weights were calculated from the standards blue dextran, bovine serum albumin, trypsin, and cytochrome c). (b) SDS-PAGE of low molecular weight standards (LMW Pharmacia) (lane 1), peak 1 fraction 22 (lane 2), and peak 2 fraction 37 (lane 3) eluted from the Sephadex G100 column.
The PG2 fraction was further tested for possible impurities and was found not to contain any PME activity (data not shown).
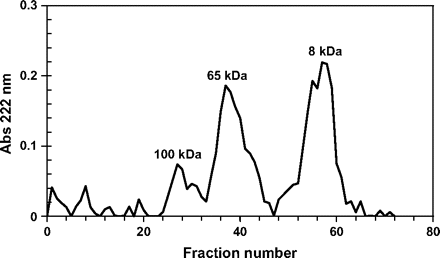
A solution containing tomato PG1 was heated from 65 °C to 100 °C in a 5 min period, cooled, and separated on a Sephacryl S300 gel filtration column. Several protein peaks appeared (Fig. 2), indicating that tomato PG1 consists of at least three proteins or protein complexes with distinct molecular weights. The recovery of the proteins was too low for visualization on an SDS-polyacrylamide gel.
Elution profile of heat-treated peak 1 of the elution profile of Sephadex G100 (fractions 20–25, Fig. 1a) on a Sephacryl S300 gel filtration column. Molecular weights were calculated from the standards blue dextran, bovine serum albumin, trypsin, and cytochrome c.
The relative molecular weight of the various protein fractions was determined and compared with molecular weight markers. The first protein (fractions 22–32) had a molecular weight of ∼100 kDa, corresponding to the molecular weight of PG1 (Pressey and Avants, 1973). The PG activity of fraction 28 was 82 nkat l−1. The protein of the second peak (fractions 34–45) was ∼65 kDa (Fig. 2), and the PG activities of fractions 34 and 38 were 493 nkat l−1 and 3893 nkat l−1, respectively. This activity was assumed to correspond to PGx (Knegt et al., 1991). The last peak (fractions 53–61) had a molecular weight of ∼8 kDa and fractions from this peak showed no PG activity at all. This suggests that the PG1 protein complex consists of distinct proteins, among them an 8 kDa protein with no PG activity of its own.
Isolation of the 8 kDa protein from green tomato fruit
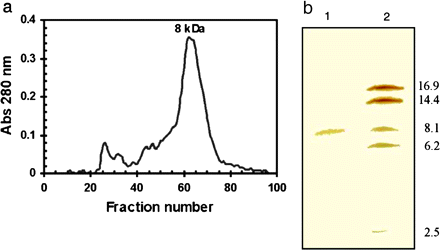
Green tomato fruit was selected as the starting material for direct isolation of the 8 kDa protein. PG2 is low or non-existent in green tomato fruit and the 8 kDa protein may, therefore, be present in a free form in mature green tomatoes, which would facilitate its isolation. To ensure that any traces of free PG2 were denatured, the pericarp tissue was heated for 5 min at 65 °C prior to isolation of the 8 kDa protein. Gel filtration of the extract from heat-treated pericarp on Sephacryl S300 was effective in the isolation of one single major protein peak of ∼8 kDa (Fig. 3a). Figure 3b illustrates a silver-stained protein from fraction 63 with an Mr of 8.1 kDa (lane 1).
Isolation of an 8 kDa protein from heat-treated green tomato pericarp. (a) The separation of an 8 kDa protein on a Sephacryl S300 column. Molecular weights were calculated from the standards blue dextran, bovine serum albumin, trypsin, and cytochrome c. (b) Identification by SDS-PAGE on a high density gel (silver-stained) of the 8 kDa protein in fraction 63 from the Sephacryl S300 column (lane 1) and molecular weight markers, myoglobin, (Pharmacia) (lane 2).
The purity of the fraction containing the 8 kDa protein was further tested, and it was found not to contain any PME activity (data not shown).
Identity of the 8 kDA protein
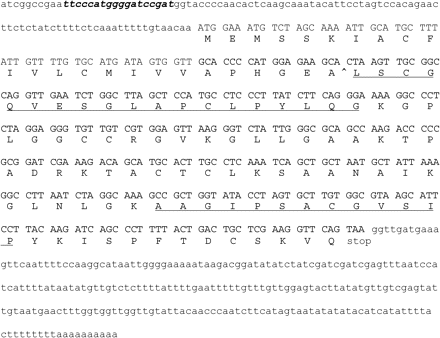
N-terminal and internal peptide sequences of the 8 kDa protein isolated from green tomato fruit (fraction 63, Sephacryl S300 column) were determined. This revealed the following N-terminal amino acid sequence: Leu-Ser-Xxx-Gly-Gln-Xxx-Glu-Ser-Gly-Leu-Ala-Pro-Xxx-Leu-Pro-Tyr-Leu-Gln-Gly (Xxx indicates uncertainty about the identity). After pyridylethylation, trypsin digestion, and preparative HPLC of the 8 kDa protein, the amino acid sequences of two peptides (the N-terminus and an internal peptide) were obtained: N-terminus, Leu-Ser-Cys-Gly-Gln-Val-Glu-Ser-Gly-Leu-Ala-Pro-Cys; internal peptide sequence, Ala-Ala-Gly-Ile-Pro-Ser-Ala-Xxx-Gly-Val-Ser-Ile-Pro. NCBI BLAST analysis using tBLASTN on the expressed sequence tag (EST) database revealed several EST clones matching the determined amino acid sequence (Fig. 4). The amino acid sequences of both the N-terminus and the internal peptide of the 8 kDa protein are 100% identical to the deduced amino acid sequence of the EST clones. From the full-length EST clones, an open reading frame (ORF) encoding a 114 amino acid protein was deduced (Fig. 4). The predicted signal sequence (SignalP, http:www.cbs.dtu.dk) is in lower case; a most probable cleavage site (P=0.849) was predicted between amino acids 24 and 25. The start of the mature protein sequence is in line with the deduced sequence of the N-terminus. The predicted molecular weight of the mature protein (http://www.expasy.org/cgi-bin/) of 9.8 kDa is slightly bigger than the estimated molecular weight of the 8 kDa protein on SDS-PAGE, indicating that additional processing of the primary gene product may take place.
Nucleotide and deduced amino acid sequence of the 8 kDa protein based on several full-length ESTs from Lycopersicon esculentum Micro-Tom fruit (FC04BB09, FC08DF03, FC17AD03, FC05DE06, FC04DB01, FC17AD01, FC26DE02, and FC09BF12), submitted in April 2005 by Kazusa DNA Research Institute, Kisarazu, Chiba, Japan. The coding region is shown in upper-case letters and the flanking regions in lower-case letters. A putative intron in the leader sequence is shown in bold italics. The amino acid sequence of the obtained peptide sequences is underlined. The predicted cleavage site in the mature protein is indicated by an arrowhead between amino acids 24 and 25.
BLAST analysis of this ORF revealed significant homology to the class of nsLTPs as identified in many species, including Lycopersicon species. The cysteine and proline residues in the 8 kDa protein are at the highly conserved positions as found in all other nsLTPs. Clustal W analysis of the presently identified LTPs of Lycopersicon species, namely LpLTP1 (AAB07486.1), Le-nsLTP2 [P27056 and identical to CAA39512.1 (TSW12) and S20862], Le-nsLTP1 [P93224 (LE16) and identical to TO7626, AAB42069 and CAJ19706.1], LpLTP2 (AAB07487.1), and Le-nsLTP (CAJ19705.1), revealed the highest homology (up to 88% identity) between the 8 kDa protein and Le-nsLTP (Fig. 5). The deduced amino acid sequence of the purified 8 kDa protein is underlined, illustrating its unique sequence (Fig. 5a). The phylogenetic tree of LTPs groups into three major classes (Fig. 5b). The branch length of the tree reflects the extent of amino acid divergence. Collectively, this analysis shows that the 8 kDa protein is an nsLTP.
Clustal W (1.83) multiple sequence alignment and phylogenetic tree of all presently identified LTP proteins of Lycopersicon species in the public database. (a) Sequence alignment of LpLTP1 (AAB07486.1), Le-nsLTP2 [P27056 and identical to CAA39512.1 (TSW12) and S20862], Le-nsLTP1 [P93224 (LE16) and identical to TO7626, AAB42069, and CAJ19706.1], LpLTP2 (AAB07487.1), and Le-nsLTP (CAJ19705.1). The deduced amino acid sequence of the purified 8 kDa protein is underlined, illustrating its unique sequence. (b) Phylogenetic tree of the aligned amino acid sequences of tomato LTPs.
Effect of the 8 kDa protein and apple and peach LTPs on in vitro PG2 activity and heat stability
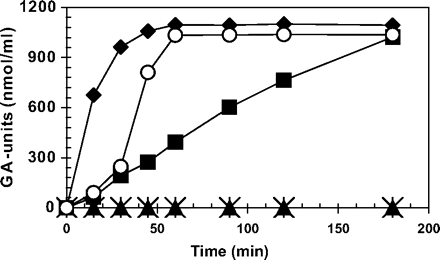
The effect of the 8 kDa protein from green tomato on the kinetics of PG-mediated PGA degradation was studied. The 8 kDa protein was incubated with or without tomato PG2 and the concentration of GA-units produced through PGA degradation was determined. If PGA was incubated with purified PG2 alone, degradation took place at an almost linear rate (Fig. 6, filled squares). After heating PG2 for 5 min at 65 °C, no degradation was observed (Fig. 6, filled triangles), confirming that this heat treatment destroys PG2. If an aliquot of the 8 kDa protein was added to the PG assay mixture after 30 min, an immediate increase in PGA degradation was observed (Fig. 6, open circles), indicating that the 8 kDa protein stimulates PG2 activity. When the 8 kDa protein was incubated with PG2 for 30 min and thereafter heated for 5 min at 65 °C (Fig. 6, filled diamonds), the heat-treated ‘8 kDa protein–PG2’ complex was considerably more active than unheated PG2 alone, indicating that the 8 kDa protein stimulates PG2 activity and, in addition, greatly increases its heat stability. The 8 kDa protein alone showed no PGA-degrading activity (Fig. 6, asterisks). Addition of extra PGA to the assay mixture containing non-heated PG2 after 90 min induced an immediate increase in activity (data not shown), which clearly shows that PG2 was still present and active, and that the 8 kDa protein specifically stimulates PG2 activity and does not merely act to prevent proteolytic degradation of PG2.
Effect of the 8 kDa protein on the activity of purified PG2. Formation of galacturonic acid units from pectin was measured following addition of PG2 alone (filled squares), PG2 heated for 5 min at 65 °C (filled trangles), unheated PG2 (alone for the first 30 min), and with 8 kDa protein, added after 30 min (open circles). PG2 and the 8 kDa protein following 30 min incubation and subsequent heat treatment (5 min at 65 °C) (filled diamonds), and 8 kDa protein alone (asterisks).
Similar experiments peformed with the 8 kDa protein isolated from the tomato PG1 multiprotein complex confirmed the effect of the 8 kDa protein on both activity and heat stability of tomato PG2 (data not shown).
This shows that the 8 kDa protein derived from PG1 and from green tomato are similar proteins. The observation that it is possible to confer heat stability to PG2 using an nsLTP derived from the (heat-stable) PG1 complex implies that an nsLTP apparently is a ‘natural’ component of the multiprotein PG complex.
When either extracts containing apple or peach LTPs were incubated with A. niger PG2, a significant increase in PG activity was observed (Fig. 7). Compared with PG alone, addition of apple or peach LTPs caused a >2-fold increase in activity. In addition, an increase in heat stability was observed (Fig. 7). Addition of 8 kDa protein to tomato PG2 generally caused a 4-fold to 5-fold increase in activity (Fig. 6). The interaction between the recombinant LTPs and the A. niger PG, however, was studied under the conditions optimal for tomato PG2, and this may have caused the weaker effect. Although these proteins originate from quite diverse systems, they apparently have the ability to interact with each other, resulting in modification of the activity of PG2.
Effect of apple mature LTP (apple mat-LTP,), FPLC-purified apple pro-LTP (apple pro-LTP), monoclonal-purified apple pro-LTP [apple pro (mono)-LTP], and peach LTP on the activity of A. niger PG2 with out (grey bars) or with (black bars) heat treatment (5 min at 65 °C). Formation of galacturonic acid units during PGA degradation is expressed relative to PG activity without addition of LTPs.
Effect of the 8 kDa protein on tissue degradation in tomato pericarp
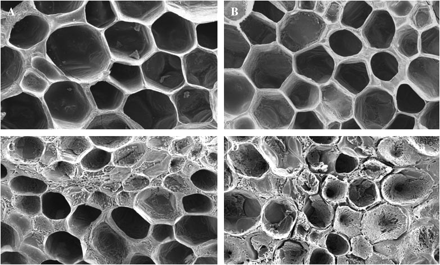
SEM was used to investigate whether the 8 kDa protein modifies tissue degradation in vivo. Chun and Huber (2000) used electron microscopy to illustrate the effects of PG2 and the β-subunit on tomato pericarp discs. In the present study, freshly excised pericarp discs from mature green and breaker tomato fruit were treated with NaAc buffer alone or with NaAc buffer containing the 8 kDa protein (fraction 63, Fig. 3). Treatment of mature green tomato pericarp discs with the 8 kDa protein did not visibly change tissue structure (Fig. 8A, B). Breaker fruit tomato discs showed less tissue integrity than mature green tomato discs (Fig. 8C). Treatment of breaker tomato pericarp discs with NaAc buffer containing the 8 kDa protein showed increased tissue degradation (Fig. 8D). It may be hypothesized that the lack of effect of the ACT in mature green tissue is due to the absence of free PG2; the increased tissue degradation in breaker fruit treated with ACT may be due to stimulation of existing PG2. These observations, however, should be interpreted with care as there are no clues about the processes responsible for the observed increased tissue degradation.
Effect of the 8 kDa protein on in vivo cell wall properties analysed by SEM (×100). Cut surfaces of mature green fruit pericarp discs were treated with buffer (A), or buffer containing purified 8 kDa protein (B). Breaker tomato fruit pericarp discs were also treated with buffer (C) or with buffer containing purified 8 kDa protein (D).
Discussion
The expression and activity of the tomato fruit cell wall enzyme PG have been studied for many years (Pressey and Avants, 1973; Chun and Huber, 2000). The presence of multiple PG forms (PG1, PGx, PG2a, and PG2b) with different properties has been demonstrated. The possible existence of additional proteins (e.g. β-subunit and CV) that associate with PG2 to form the more active and stable PG1 and/or PGx has been suggested by several authors (Giovannoni et al., 1990; Knegt et al., 1991). The β-subunit protein that associates with PG was previously shown to assist in maintaining the integrity of the cell wall during tomato fruit ripening and apparently does not play a role in the stimulation of PG activity (Chun and Huber, 2000). Studies on the hypothetical CV (Knegt et al., 1988, 1991) have never revealed the identity of this protein. Although all previous studies have implied that there is an additional protein involved in the regulation of PG-mediated pectin degradation during fruit ripening, this protein has not yet been identified.
An 8 kDa protein capable of influencing pectin metabolism in vivo and cell wall degradation in vitro was isolated and characterized. Because of its effect on PG-mediated pectin degradation, the protein was designated ‘activator’ (ACT). The fact that it proved difficult to isolate this protein (Knegt et al., 1991) is at least partly due to its tight association with other proteins (e.g. PG2, β-subunit, and possibly others). The use of a modified procedure for isolation of this protein may have stimulated the occurrence of the unbound form in tomato extracts. To isolate the ACT from ripening tomato fruit, the breakdown products of purified PG1 were analysed. Knegt et al. (1988) heated the PG1 solution for 5 min at 100 °C and then found two proteins, PG1 (100 kDa) and PGx (71 kDa). The present data show that dissociation of the PG1 protein complex is more efficient when the extract is slowly heated from 65 °C to 100 °C. The complex is then separated into three proteins or protein complexes (Fig. 2). PG2 was totally denaturated at these temperatures. The first protein or protein complex that eluted from the Sephacryl S300 column corresponded to the molecular weight of PG1 (Tucker et al., 1980) and shows PG activity. The second protein or protein complex has a molecular weight of 65 kDa and also shows PG activity. This size is less than the reported size of PGx, 71 kDa (Knegt et al., 1991; van Loon and Bruinsma, 1992). It is assumed that this protein or protein complex is the ACT associated with either the β-subunit or PG2. The last, smaller protein (∼8 kDa) was not found by Knegt et al. (1988, 1991) and is the ACT that was characterized further here. The ACT shows no PG activity.
Part of the amino acid sequence of ACT was obtained, and this showed that ACT belongs to the nsLTPs. nsLTPs are small (8–10 kDa), mostly extracellularly located secretory proteins, and most plant species contain small families of them. nsLTPs have been recognized as major allergens in plant-derived food products. The biological functions of LTPs in plants are currently not well understood. Although LTPs are able to load and transfer hydrophobic molecules such as fatty acids and phospholipids in vitro, there is no indication that they confer such a role in vivo. It is generally believed that LTPs may exert a function in plant defence against fungal and bacterial pathogens in an as yet unknown fashion (Molina and Garcia-Olmedo, 1997; Buhot et al., 2001; Lindorff-Larsen et al., 2001).
The ACT was shown to associate with tomato PG2, stimulating in vitro PG-mediated PGA and pectin metabolism and increasing PG heat stability (Fig. 6). As these experiments were performed with purified fractions, the possibility that the observed effect of ACT may be due to traces of other hydrolytic enzymes present in the fractions was considered. Several additional tests showed that the fractions containing ACT did not have any PG activity towards either PGA or pectin, and that both PG2 and ACT fractions did not exhibit any PME activity. In addition, tomato PME did not show any activity towards either PGA or pectin under the conditions used for PG activity that were employed (data not shown). This rules out the possibility that other proteins present in the ACT fraction may have facilitated the increased breakdown of PGA or pectin by PG. To verify further that LTPs and PG2 can interact and to rule out the possibility that the present findings are artefactual, it was shown that purified recombinant apple and peach LTPs increase the activity of purified A. niger PG towards PGA (Fig. 7).
Experiments in which tomato fruit pericarp was treated with ACT showed that tissue degradation in breaker pericarp, but not in mature green pericarp, was markedly stimulated by addition of ACT, supporting the view that the ACT may stimulate the activity of ‘free’ PG2 present in breaker pericarp (Fig. 8).
The current finding that an nsLTP is a functional part of the pectin-degrading protein complex suggests a new, not previously identified, function for nsLTPs. Apart from cell wall degradation during fruit softening, nsLTPs may exert a regulatory role in other PG-mediated processes such as abscission and xylogenesis, and may similarly function in modifying the activity of other wall-bound enzymes. The connection between PG2 and one or more units of ACT is most probably the previously described PGx, which is thought to be responsible for pectin degradation in vivo. It is proposed that PGx activity could be limited by the β-subunit. Two mechanisms can be suggested. First, the β-subunit may interact with free PG2 and would then restrict PG2 association with ACT. Secondly, when the β-subunit interacts with pectin, as suggested by DellaPenna et al. (1996), PGx (ACT–PG2) may not be able to interact at the same position. If PGx associates with the β-subunit, PG1 may be formed. This association may only occur in extracts of tomato tissue, because in planta the β-subunit is reported to be associated with the cell wall (DellaPenna et al., 1996).
Further experiments are being performed to characterize the ACT at the molecular level and to unravel its putative mode of action during stimulation and stabilization of PG2.
In conclusion, an 8 kDa tomato protein was isolated and named activator (ACT). ACT is part of the PG multiprotein complex and stimulates both the activity and heat stability of PG2 in vivo. ACT itself has no pectin-degrading activity. This protein belongs to the class of nsLTPs, indicating a new, not previously identified role for nsLTPs as modulators of the activity and stability of cell wall-based enzymes. ACT exhibits some of the putative properties of the hypothetical PG convertor and is thought to play an important role in pectin degradation during softening of tomato fruits.
Abbreviations
- ACT
activator
- CV
convertor
- EST
expressed sequence tag
- FPLC
fast protein liquid chromatography
- GA
galacturonic acid
- HPLC
high-pressure liquid chromatography
- LPT
lipid transfer protein
- nsLTP
non-specific lipid transfer protein
- ORF
open reading frame
- PG
polygalacturonase
- PGA
polygalacturonic acid
- PME
pectin methylesterase
- SEM
scanning electron microscopy
The authors are grateful to Jacqueline Donkers for assistance with SEM, to Patrick Vivet for assistance with PG measurements, to Jurriaan Mes for sequence alignments, and to Ronald van Ree and Laurian Zuidmeer for providing the recombinant apple and peach LTPs.



![Clustal W (1.83) multiple sequence alignment and phylogenetic tree of all presently identified LTP proteins of Lycopersicon species in the public database. (a) Sequence alignment of LpLTP1 (AAB07486.1), Le-nsLTP2 [P27056 and identical to CAA39512.1 (TSW12) and S20862], Le-nsLTP1 [P93224 (LE16) and identical to TO7626, AAB42069, and CAJ19706.1], LpLTP2 (AAB07487.1), and Le-nsLTP (CAJ19705.1). The deduced amino acid sequence of the purified 8 kDa protein is underlined, illustrating its unique sequence. (b) Phylogenetic tree of the aligned amino acid sequences of tomato LTPs.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/58/5/10.1093/jxb/erl288/2/m_jexboterl288f05_lw.gif?Expires=1716432167&Signature=d8XU9cLRqwqtv1lAzU8bZwW8VSePowFOcgYgrThBp~diadBYff~OyKbHwBNdjUl1unYzxGvB0IKj94~C1unHvrns6AFCoXwol8QheoP0oLlLiCHvbMmVrlpa625Djk8Rb0J3uX8faUL3GFoszScMow2E8PtjEAdUHBG~ppmoNk-gFGBtNaSjeH61z3zqHREpfPvHj~GRUe1T1BNC7PKdvorrvZWG4nlCfkdJm9ts178rWD5xttSCgwu~GSMyMbywCr7pwjSaV99ptrpNJArHjZn6SZTKJdfM8JnFGR822~JbDzIMtqncY-4KBeoYtPNTQXxJgOHxtYsdma-tqnn0AA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Effect of apple mature LTP (apple mat-LTP,), FPLC-purified apple pro-LTP (apple pro-LTP), monoclonal-purified apple pro-LTP [apple pro (mono)-LTP], and peach LTP on the activity of A. niger PG2 with out (grey bars) or with (black bars) heat treatment (5 min at 65 °C). Formation of galacturonic acid units during PGA degradation is expressed relative to PG activity without addition of LTPs.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/58/5/10.1093/jxb/erl288/2/m_jexboterl288f07_ht.gif?Expires=1716432167&Signature=1NweI8II4tDNyNDzKyisdqITT3D3CuAkZclpmXzYG6cJMJynhR1nYa5Qnkj8EPmm50ayIvfw684SKgKsBT-T7KG2MPaS1vDp657uiTTLnyMEDiEw49NxOT-xlllZJDbYfLGNMPIjk0X3-OVwdcJMPRSOmgrFy1K1iCLC2EsJwY3w2BjSikq6U1vPgduN52iaONysBNV87tBANQfFmJ61c~0Br6HNMEQcpRZ39v6h68FEB5FGW49EH9E-9UiO83Ff-7qgIdNWgEDC-mIFPOgGm8kwn8~~ERkE03kWlkDgd5j7Zz0yfIck6NMIbGJuZsYzwxT7K9h~p2OGEsmOkUCL7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments