-
PDF
- Split View
-
Views
-
Cite
Cite
Renan M. Mauch, Claudio L. Rossi, Talita B. Aiello, José D. Ribeiro, Antônio F. Ribeiro, Niels Høiby, Carlos E. Levy, Secretory IgA response against Pseudomonas aeruginosa in the upper airways and the link with chronic lung infection in cystic fibrosis, Pathogens and Disease, Volume 75, Issue 6, August 2017, ftx069, https://doi.org/10.1093/femspd/ftx069
Close - Share Icon Share
Abstract
We assessed the diagnostic ability of an enzyme-linked immunosorbent assay test for measurement of specific secretory IgA (sIgA) in saliva to identify cystic fibrosis (CF) patients with Pseudomonas aeruginosa chronic lung infection and intermittent lung colonization. A total of 102 Brazilian CF patients and 53 healthy controls were included. Specific serum IgG response was used as a surrogate to distinguish CF patients according to their P. aeruginosa colonization/infection status. The rate of sIgA positivity was 87.1% in CF chronically infected patients (median value = 181.5 U/mL), 48.7% in intermittently colonized patients (median value = 45.8 U/mL) and 21.8% in free of infection patients (median value = 22.1 U/mL). sIgA levels in saliva were significantly associated with serum P. aeruginosa IgG and microbiological culture results. The sensitivity, specificity, PPV and NPV for differentiation between presence and absence of chronic lung infection were 87%, 63%, 51% and 92%, respectively. Measurement of sIgA in saliva may be used for screening patients in risk of developing P. aeruginosa chronic lung infection in CF and possibly also for paranasal sinusitis, and, most importantly, to efficiently rule out chronic P. aeruginosa lung infection.
INTRODUCTION
Pseudomonas aeruginosa chronic lung infection in cystic fibrosis (CF) is preceded by cycles of intermittent colonization, where bacterial strains recovered from the lungs and paranasal sinuses are genotypically and phenotypically similar in most cases, suggesting the paranasal sinuses as a reservoir for subsequent intermittent colonization and chronic infection of the lungs (Aanaes 2013; Ciofu et al.2013; Fothergill et al.2014; Boutin et al.2015). The major problem resides in the difficulty of sampling a specimen from the paranasal sinuses, due to the invasive procedures required for this approach, which may be painful, expensive and time-consuming, thus not being routinely performed in most CF centers (Dosanjh et al.2000; Digoy et al.2012; Fischer et al.2014). On the other hand, measuring the humoral immune response in the upper airways may be a surrogate to predict P. aeruginosa chronic lung infection. In contrast to the systemic IgG inflammatory response in the lungs, a non-phlogistic secretory IgA (sIgA)-mediated humoral response is observed in the CF paranasal sinuses, which attenuates the infectious symptoms so that patients tend not to report sinusitis, despite having sinusitis symptoms (Hansen et al.2012). As a matter of fact, Aanaes et al. (2013) found that sIgA levels in sinus secretion and saliva from CF patients with P. aeruginosa chronic lung infection and intermittent colonization were significantly higher when compared with CF patients without P. aeruginosa infection.
In this study, we assessed the ability of an enzyme-linked immunosorbent assay (ELISA) test for measurement of specific P. aeruginosa sIgA response in saliva to predict chronic infection and intermittent colonization of the lungs in a cohort of Brazilian CF patients.
METHODS
A cross-sectional analysis was made with 102 CF patients [53 males, 49 females; median age = 10.4 years (range: 2.1–30.0)] who attended the reference center of the Campinas University Hospital (Hospital de Clínicas—HC Unicamp) and 53 healthy controls [24 males, 29 females; median age = 10.4 years (range: 7.7–30.0)]. Each CF patient had a minimum of four microbiological sputum/oropharyngeal (OP) swab culture results in the last 12 months prior to the study. Chronic infection definition relied on these results, as well as on specific serum IgG levels measured by a previously standardized ELISA test (Mauch et al.2014) with modifications. The following subgroups were then defined: chronic infection: Pseudomonas aeruginosa isolated in one or more microbiological cultures in the last 12 months prior to the study with IgG levels ≥28.2 U/mL; intermittent colonization: P. aeruginosa isolated in one or more cultures in the last 12 months prior to the study with IgG levels below 28.2 U/mL, or no isolation of P. aeruginosa in the last 12 months but IgG levels ≥28.2 U/mL, and free of colonization/infection: no isolation of P. aeruginosa in the last 12 months with IgG levels below 28.2 U/mL.
Saliva samples were collected with the Salivette® cotton swab (Sarstedt AG&Co, Nümbrecht, Germany). The swab was placed in the patient's mouth for three minutes, and then placed back to the Salivette tube, which was centrifuged for 15 minutes at 1500 rpm for obtainment of clear saliva. SIgA levels were measured with an ELISA test, following the protocol proposed by Aanaes et al. (2013) with minor modifications, and the results were expressed as units per mL (U/mL). A receiver operating characteristic (ROC) analysis was used to determine the cut-off value by using ELISA results from CF patients chronically infected with P. aeruginosa and healthy controls (based on the results of Aanaes et al.2013, the chronically infected patients may also have been colonized in the paranasal sinuses).
The Chi-square test was used to compare the rates of sIgA positivity among CF patients and healthy controls simultaneously. The Kappa-Cohen analysis was used to test the concordance between two different tests. The Mann-Whitney test and the Kruskal-Wallis test were used for comparisons of sIgA concentration between two groups and among more than two groups, respectively. The Spearman test was used to search for correlations. In all tests, a p-value ≤0.05 was considered statistically significant. The statistical analyses were made with SPSS® for Windows version 20 (IBM, New York, USA).
RESULTS AND DISCUSSION
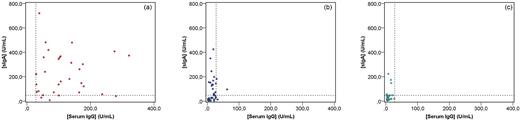
Using the sIgA levels of chronically infected CF patients and healthy controls in the ROC analysis, a value of 44.2 U/mL gave the best sensitivity/specificity ratio. By applying the 6.7% interassay coefficient of variation, a positive result was determined when the sIgA concentration was higher than 47.2 U/mL. Chronically infected patients had both the significantly highest rate of sIgA positivity (87.1%) and sIgA levels in saliva (181.5 U/mL) (Table 1); thus, for this group, the sIgA response seems to well reflect the bacterial presence in the lungs with systemic IgG response to infection (Fig. 1). This was also shown in a global analysis, where we found a significant correlation between sIgA and serum IgG levels (r = 0.595, p < 0.0001) and a significant concordance between sIgA and serum IgG positivity (κ = 0.462, p < 0.0001).
Comparison between P. aeruginosa sIgA levels in saliva serum IgG levels in CF patients separated by their P. aeruginosa lung colonization/infection status: (a) chronic infection, (b) intermittent colonization and (c) free of infection. The vertical dotted line corresponds to the cut-off value for serum IgG (28.2 U/mL) and the horizontal dotted line corresponds to the cut-off value for sIgA in saliva (47.2 U/mL).
Concentration of sIgA to P. aeruginosa (U/mL) in saliva of CF patients and healthy controls, and serum IgG levels in CF patients.
| Pseudomonas aeruginosa lung colonization/infection status . | N . | Median age (range) . | Median [sIgA] in saliva in U/mL (range) . | % sIgA positivity (C.I 95%) . | Median serum IgG (range) . | % IgG positivity* . |
|---|---|---|---|---|---|---|
| CF chronic infectiona | 31 | 17.4 (6.5–30.0) | 181.5 (7.6–719.4)* | 87.1 (70.2–96.4)* | 100.5 (29.2–321.6) | 100 |
| CF intermittent colonizationb | 39 | 7.9 (2.1–25.6) | 45.8 (3.1–424.5)¤ ɤ | 48.7 (32.4–65.22)¤ ¥ | 15.3 (2.1–62.6) | 7.7 |
| CF free of infectionc | 32 | 10.3 (2.7–29.8) | 22.1 (5.2–223.1)¤ | 21.9 (9.3–40.0)¤ | 7.2 (2.8–26.5) | 0 |
| Healthy controls | 53 | 10.4 (7.7–30.0) | 7.7 (2.7–108.7) | 7.5 (2.1–18.2) | - | - |
| Pseudomonas aeruginosa lung colonization/infection status . | N . | Median age (range) . | Median [sIgA] in saliva in U/mL (range) . | % sIgA positivity (C.I 95%) . | Median serum IgG (range) . | % IgG positivity* . |
|---|---|---|---|---|---|---|
| CF chronic infectiona | 31 | 17.4 (6.5–30.0) | 181.5 (7.6–719.4)* | 87.1 (70.2–96.4)* | 100.5 (29.2–321.6) | 100 |
| CF intermittent colonizationb | 39 | 7.9 (2.1–25.6) | 45.8 (3.1–424.5)¤ ɤ | 48.7 (32.4–65.22)¤ ¥ | 15.3 (2.1–62.6) | 7.7 |
| CF free of infectionc | 32 | 10.3 (2.7–29.8) | 22.1 (5.2–223.1)¤ | 21.9 (9.3–40.0)¤ | 7.2 (2.8–26.5) | 0 |
| Healthy controls | 53 | 10.4 (7.7–30.0) | 7.7 (2.7–108.7) | 7.5 (2.1–18.2) | - | - |
Sensitivity = 87%, specificity = 51%, PPV = 59%, NPV = 83%.
sensitivity = 87%, specificity = 63%, PPV = 51%, NPV = 92%
CI 95%: confidence interval 95%.
*p < 0.0001 when compared with all the other groups.
¤p < 0.01 when compared with the healthy controls.
ɤp = 0.124 when compared with the free of infection group.
¥p = 0.019 when compared with the free of infection group.
*IgG was part of the diagnostic criteria for chronic infection (see Methods section).
Concentration of sIgA to P. aeruginosa (U/mL) in saliva of CF patients and healthy controls, and serum IgG levels in CF patients.
| Pseudomonas aeruginosa lung colonization/infection status . | N . | Median age (range) . | Median [sIgA] in saliva in U/mL (range) . | % sIgA positivity (C.I 95%) . | Median serum IgG (range) . | % IgG positivity* . |
|---|---|---|---|---|---|---|
| CF chronic infectiona | 31 | 17.4 (6.5–30.0) | 181.5 (7.6–719.4)* | 87.1 (70.2–96.4)* | 100.5 (29.2–321.6) | 100 |
| CF intermittent colonizationb | 39 | 7.9 (2.1–25.6) | 45.8 (3.1–424.5)¤ ɤ | 48.7 (32.4–65.22)¤ ¥ | 15.3 (2.1–62.6) | 7.7 |
| CF free of infectionc | 32 | 10.3 (2.7–29.8) | 22.1 (5.2–223.1)¤ | 21.9 (9.3–40.0)¤ | 7.2 (2.8–26.5) | 0 |
| Healthy controls | 53 | 10.4 (7.7–30.0) | 7.7 (2.7–108.7) | 7.5 (2.1–18.2) | - | - |
| Pseudomonas aeruginosa lung colonization/infection status . | N . | Median age (range) . | Median [sIgA] in saliva in U/mL (range) . | % sIgA positivity (C.I 95%) . | Median serum IgG (range) . | % IgG positivity* . |
|---|---|---|---|---|---|---|
| CF chronic infectiona | 31 | 17.4 (6.5–30.0) | 181.5 (7.6–719.4)* | 87.1 (70.2–96.4)* | 100.5 (29.2–321.6) | 100 |
| CF intermittent colonizationb | 39 | 7.9 (2.1–25.6) | 45.8 (3.1–424.5)¤ ɤ | 48.7 (32.4–65.22)¤ ¥ | 15.3 (2.1–62.6) | 7.7 |
| CF free of infectionc | 32 | 10.3 (2.7–29.8) | 22.1 (5.2–223.1)¤ | 21.9 (9.3–40.0)¤ | 7.2 (2.8–26.5) | 0 |
| Healthy controls | 53 | 10.4 (7.7–30.0) | 7.7 (2.7–108.7) | 7.5 (2.1–18.2) | - | - |
Sensitivity = 87%, specificity = 51%, PPV = 59%, NPV = 83%.
sensitivity = 87%, specificity = 63%, PPV = 51%, NPV = 92%
CI 95%: confidence interval 95%.
*p < 0.0001 when compared with all the other groups.
¤p < 0.01 when compared with the healthy controls.
ɤp = 0.124 when compared with the free of infection group.
¥p = 0.019 when compared with the free of infection group.
*IgG was part of the diagnostic criteria for chronic infection (see Methods section).
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of the sIgA ELISA test to distinguish chronic lung infection from intermittent lung colonization were 87%, 51%, 59% and 83%, respectively. The specificity and PPV values are low, since 48.7% of the intermittently colonized patients had positive sIgA results (Table 1, Fig. 1), which, however, does not necessarily mean false positive results, but does indicate that this group was probably exposed to Pseudomonas aeruginosa in the upper airways (Aanaes 2013; Aanaes et al.2013), thereby forming a transition group for development of chronic lung infection, especially those with positive specific serum IgG levels, since such levels are good predictors of P. aeruginosa chronic lung infection in CF, even in the absence of positive cultures (Pressler et al.2006; Mauch and Levy 2014). A careful monitoring for changes in microbiological culture and serum IgG results would be very important for this group of patients, which is reinforced by the significantly higher prevalence of sIgA positivity when compared with the free of infection group (48.7 × 21.9%, p = 0.019), although the sIgA levels were not significantly higher (Fig. 1, Table 1). On the other hand, chronically infected patients with negative sIgA levels were probably not exposed to P. aeruginosa in the upper airways, but in their conductive and respiratory lower airways only (Worlitzsch et al.2002; Bjarnsholt et al.2009; Kragh et al.2014).
When comparing the chronically infected group with the intermittently colonized and free of infection groups together, i.e. presence and absence of P. aeruginosa chronic lung infection, the sensitivity, specificity, PPV and NPV values were 87%, 63%, 51% and 92%, respectively (Table 1). Our findings corroborate previous culture-based analyses (Mainz et al.2009), which showed an 88-fold higher likelihood to identify P. aeruginosa in the sinuses of patients with positive microbiological sputum/OP culture, compared to patients with negative culture. Although sputum culture is currently the reference method for diagnosis of P. aeruginosa lung infection, only about 35%–40% of the CF patients is able to expectorate a representative sputum sample (Ramsey et al.1991; Sagel et al.2001) and OP swabs have been shown to poorly reflect the lung microbiota (Goddard et al.2012). Therefore, sIgA measurement in saliva may be very useful to complement microbiological culture results in the CF diagnostic routine, by ruling out chronic lung infection, especially in younger patients who are not able to provide sputum samples.
In the very first report of sIgA as a diagnostic tool for detection of P. aeruginosa in the upper airways, Aanaes et al. (2013) found high sIgA levels against two P. aeruginosa antigens (St-Ag and alginate) in nasal secretions and saliva of both chronically infected and intermittently colonized CF patients, with significant difference when compared with uninfected patients. When the cutoff values for the sIgA response against these two antigens were combined, intermittent colonization could be discriminated from the absence of colonization/infection with the following values of sensitivity, specificity, PPV and NPV: 96%, 81%, 79% and 96% (for nasal secretion) and 72%, 74%, 87% and 52% (for saliva). For clinical use, however, Aanaes’ combined method is rather complicated and dependent on the presence of an otolaryngologist doctor in the CF clinic. Our method for obtaining saliva can be easily used by any physician and nurse or even by the patient or parents, and the sIgA ELISA test can be easily established in any laboratory.
Overall, we present a new and not invasive sampling method, which allows the obtainment of a higher and clearer amount of saliva, thus being more reliable for an ELISA test. The results described herein show that a considerable rate of CF patients have anti-P. aeruginosa sIgA response in saliva, even not having positive P. aeruginosa cultures in lower airway specimen. Therefore, sIgA measurement in saliva may be clinically useful for screening patients in risk of chronic P. aeruginosa lung infection and possibly also to search for paranasal infection. Most importantly, low sIgA response can be used to rule out chronic P. aeruginosa lung infection with a 92% probability. A longitudinal follow-up assessing changes in culture, serum IgG and sIgA findings is currently being carried out so better results may be achieved.
Acknowledgments
The authors are grateful to Ass. Prof. DMS, PhD Oana Ciofu MD, ISIM, University of Copenhagen, for her critical and helpful commentaries.
FUNDING
This study was financed with resources from the São Paulo Research Foundation (FAPESP)—grant number 2014/00007–8 – and from the University of Campinas Funding for Teaching, Research and Community Actions (FAEPEX)—grant number 104489–15/1.
Conflict of interest. None declared.