-
PDF
- Split View
-
Views
-
Cite
Cite
Neil Swanson, Martin Goddard, Gerry McCann, G. André Ng, Sarcoidosis presenting with tachy- and brady-arrhythmias, EP Europace, Volume 9, Issue 2, February 2007, Pages 134–136, https://doi.org/10.1093/europace/eul173
Close - Share Icon Share
Abstract
We describe a case report of a 49-year-old man admitted to a cardiology unit with a series of arrhythmias with no initially obvious aetiology. Further assessment and the use of cardiac magnetic resonance imaging and histology allowed a diagnosis of cardiac sarcoidosis to be made.
Cardiac sarcoidosis is a major cause of death in patients with systemic sarcoidosis. Cardiac magnetic resonance imaging is an additional diagnostic tool for this condition without ionizing radiation exposure.
Case report
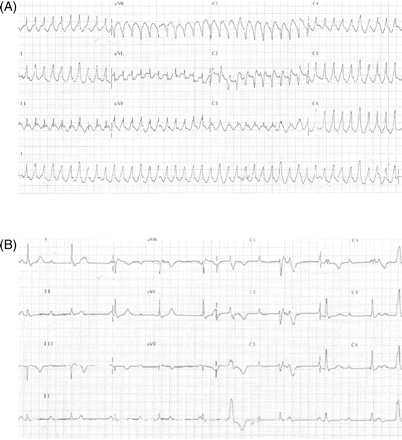
A 49-year-old man was admitted with an episode of palpitation and clamminess. Electrocardiography showed a broad complex tachycardia (ventricular rate of 260 bpm, Figure 1A), (mis)diagnosed as supraventricular tachycardia with aberrancy and treated with amiodarone. Adenosine was not given. The rhythm persisted, with unrecordable blood pressure. Single DC shock resulted immediately in persistent complete heart block with a different broad complex QRS morphology (Figure 1B).
12-lead ECG (A) on admission and (B) after DC cardioversion. (B) Complete heart block is seen with an escape rhythm conducted with a typical right bundle-branch block morphology.
Old records showed investigations for paroxysmal atrial fibrillation (PAF) 10 years earlier, with normal coronary angiography and echocardiography. Holter monitoring had recorded PAF and non-sustained ventricular tachycardia (VT). He was diagnosed as having ‘early cardiomyopathy’, declined medications and was lost to follow-up.
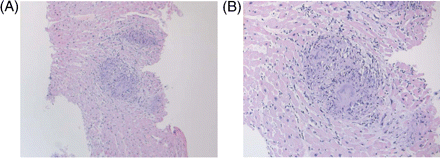
At the second presentation, he was otherwise well; in particular, no respiratory or skin problems. Clinical examination and chest X-ray were normal. Echocardiography demonstrated mild left ventricular (LV) hypertrophy, normal LV systolic function but severe right ventricular (RV) and atrial dilatation, moderate tricuspid incompetence and severe impairment of RV systolic function, suggestive of arrhythmogenic right ventricular dysplasia (ARVD). Coronary angiography was normal. Right heart catheterization and ventriculography showed low right-sided pressures. Endomyocardial biopsy was performed at the interventricular septum. Cardiac biopsy showed non-caseating granulomata, highly suggestive of a diagnosis of cardiac sarcoidosis (Figure 2).
Histology (haematoxylin and eosin stain) showing non-caseating granuloma with multinucleate giant cells at (A) low (×100) and (B) high (×200) magnification.
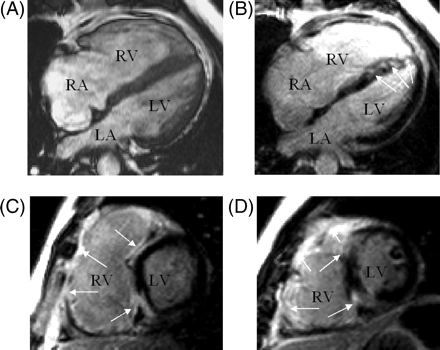
Cardiac magnetic resonance (CMR) imaging confirmed severe RV dilatation and systolic dysfunction [ejection fraction (EF) 24%, end diastolic volume 115 mL/m2, Figure 2A] without fat infiltration or aneurysms. There was mild hypokinesis of the interventricular septum and inferior wall of the LV with mildly impaired systolic function (EF 49%, end diastolic volume 64 mL/m2). Mediastinal lymphadenopathy was noted. There was extensive hyperenhancement of the RV free wall, interventricular septum, inferior wall, and focal enhancement in the LV anterior walls 10 min after administration of gadolinium-DTPA (Figure 3B–D). A diagnosis of cardiac sarcoid was made on the basis of these MRI findings, histology, and the clinical picture. The marked anteroseptal enhancement on MRI was likely to explain complete heart block and RBBB.
Cardiac magnetic resonance imaging (A) four-chamber end-diastolic gradient echo. (B–D) Inversion recovery gradient echo delayed contrast images. Normal myocardium black. Abnormal contrast enhancement arrowed. The septum is diffusely enhanced and shows marked anteroseptal enhancement, which is likely to cause complete heart block ±RBBB. (B): Four-chamber; (C): basal short axis; (D): mid ventricular short axis. RA, right atrium; LA, left atrium.
Programmed stimulation using two extrastimuli (coupling interval 600/260/200 ms) at the RV apex induced sustained VT (cycle length 230–240 ms) with left bundle-branch block morphology and left axis deviation. It caused haemodynamic compromise requiring cardioversion. No His bundle signal was recorded, suggesting the level of block was at and below the AV node.
The original arrhythmia must have been of ventricular origin—AV dissociation was evident in the initial ECG. The patient declined systemic steroid therapy. A dual chamber cardiac defibrillator was implanted (ruling out further MRI evaluation). He remains well. Informed consent of the patient has been obtained for the publication of this report.
Discussion
Sarcoidosis is a granulomatous disorder of unknown aetiology, affecting the heart in ∼25% of cases at autopsy.1 Clinically, the incidence of cardiac sarcoidosis in patients with systemic disease is ∼5%, but is the most common cause of (sudden) death. Survival with cardiac sarcoidosis has historically been poor. Such studies, however predate extensive use of implantable defibrillators. Heart block is the most common arrhythmia,2 though many others are documented, including RV tachycardias suggestive of ARVD.3,4 Atrial involvement alone is rare,5 making it uncertain whether the episode of AF years earlier was related to sarcoidosis or not. Cardiac sarcoidosis can be difficult to diagnose. Biopsy (which may miss patchy involvement of the myocardium) or myocardial scintigraphy (which entails radioisotope exposure) have been used, with ECGs, but CMR imaging may detect small areas of oedema and/or fibrosis6 representing subclinical cardiac involvement.7 The published sensitivity and specificity of CMR imaging were 100% (95% confidence interval, 78–100%) and 78% (64–89%), respectively.5 Steroid therapy has been shown to diminish CMR imaging abnormalities in cardiac sarcoidosis.8
Summary
Cardiac involvement is common in sarcoidosis, but may be clinically silent. Cardiac sarcoidosis may be the only clinical feature, in this case for many years. Arrhythmias are common, and cardiac sarcoidosis may present with life-threatening arrhythmia and screening by CMR imaging should be considered in all patients with sarcoidosis. Unexplained arrhythmias may indeed represent ‘early cardiomyopathy’, including cardiac sarcoidosis and this should be assiduously sought.