-
PDF
- Split View
-
Views
-
Cite
Cite
Allen J. Taylor, Todd C. Villines, Eric J. Stanek, Paradoxical progression of atherosclerosis related to low-density lipoprotein reduction and exposure to ezetimibe, European Heart Journal, Volume 33, Issue 23, December 2012, Pages 2939–2945, https://doi.org/10.1093/eurheartj/ehs105
Close - Share Icon Share
Abstract
Ezetimibe reduces low-density lipoprotein cholesterol (LDL-C) but has complex actions on cholesterol transport and metabolism, and thus, LDL-C reduction may not solely define its overall effects. We explored the relationship between treatment effects and cumulative exposure to ezetimibe, with its effects on carotid intima–media thickness (CIMT) in ARBITER 6-HALTS.
This analysis includes the 159 patients randomized to ezetimibe within ARBITER 6-HALTS that completed the final imaging endpoint assessment. Eligibility criteria for ARBITER 6-HALTS included known coronary artery disease (CAD) or high risk for coronary heart disease, and treatment with a statin with LDL-C <100 mg/dL and high-density lipoprotein cholesterol <50 or 55 mg/dL for men and women, respectively. The mean CIMT was measured in the far wall of the distal common carotid artery. We analysed the univariate and multivariate relationships of the change in CIMT with baseline characteristics, on-treatment effects, and cumulative ezetimibe exposure (treatment duration × dose × adherence). Ezetimibe reduced LDL-C from 84 ± 23 to 66 ± 20 mg/dL. No net effect on CIMT was observed (baseline CIMT 0.898 ± 0.151 mm; net change −0.002 mm; P = 0.52). There was an inverse relationship between LDL-C and change in CIMT such that greater reductions in LDL-C were associated with greater CIMT progression (r = −0.266; P < 0.001). Change in CIMT also had univariate associations with baseline LDL-C, triglycerides (TG), high-sensitive C-reactive protein, and systolic blood pressure and was directly associated with the change in TG and inversely associated with the change in high-sensitive C-reactive protein. Multivariable models controlling for change in LDL-C, cumulative ezetimibe exposure, and baseline and on-treatment variables showed that both increased LDL-C reduction (P = 0.005) and cumulative drug exposure (P = 0.02) were associated with ezetimibe-associated CIMT progression.
Among CAD and high-risk patients on statin therapy in the ARBITER-6 trial, ezetimibe leads to paradoxical progression of CIMT in association with both greater LDL-C reduction and cumulative drug exposure. These findings may suggest the presence of off-target actions of ezetimibe.
Trial Registration: ClinicalTrials.gov number: NCT00397657
See page 2892 for the editorial comment on this article (doi:10.1093/eurheartj/ehs183)
Introduction
Pharmacological treatments that reduce the levels of low-density lipoprotein cholesterol (LDL-C) generally lead to slowing of progression or stabilization of atherosclerosis as determined using quantitative arterial measures of atherosclerosis such as carotid intima–media thickness (CIMT) or intravascular ultrasound.1,2 Ezetimibe reduces LDL-C ∼15–20% when added to statin therapy and is a first-in-class compound approved in 2002 by the US Food and Drug Administration for the indication of hypercholesterolaemia either used alone or in combination with a statin. Subsequent to this licensure, the mechanism of action of ezetimibe was found to be related to inhibition of cholesterol transport at the enterocyte.3 However, ezetimibe possesses other actions including inhibition of other cholesterol transport receptors such as the scavenger receptor B1,4–6 and transcriptional down-regulation of key lipid transport genes.7 In the absence of clinical trial outcomes data, surrogate effects of ezetimibe were studied, many demonstrating negative or even adverse findings on the effects of ezetimibe on arterial endothelial function8–18 and structure.19–21 These findings have led to uncertainty regarding the net clinical efficacy of ezetimibe.
We recently conducted a randomized, parallel-group, active control study of either niacin or ezetimibe to compare their effects on CIMT.20,22 After 14 months of treatment among high-risk patients treated with statins, niacin was superior to ezetimibe for the primary endpoint of between-group change in CIMT. Although subjects treated with ezetimibe showed no net change in CIMT, there was a significant inverse relationship between the change in LDL-C and CIMT such that patients with greater LDL-C reduction,20 and greater exposure to ezetimibe,20,22 paradoxically showed a greater extent of progression of CIMT. These effects were not observed with niacin.20,22 Recently, a second group of investigators reported a similar finding among ezetimibe-treated patients with peripheral arterial disease.21 In this post hoc analysis, we further explore the relationship between treatment effects, cumulative exposure to ezetimibe, and the effects on CIMT within subjects enrolled in ARBITER 6-HALTS. The results that ezetimibe leads to paradoxical progression of CIMT in association with both greater LDL reduction and cumulative drug exposure suggest the hypothesis that ezetimibe's complex mechanism of action may lead to adverse consequences on arterial atherosclerosis.
Methods
Patient population
The rationale, design,23 and primary results20,22 of the ARBITER 6-HALTS study have been described. Briefly, inclusion criteria were: (i) patients at least 30 years of age with known atherosclerotic cardiovascular disease or a coronary heart disease (CHD) equivalent, (ii) currently taking statin monotherapy at a consistent dose, (iii) LDL-C <100 mg/dL (2.6 mmol/L), and (iv) high-density lipoprotein cholesterol (HDL-C) <50 mg/dL in men or <55 mg/dL in women (1.3 or 1.4 mmol/L, respectively), documented on a lipid panel within 3 months of enrolment. Baseline characteristics and on-treatment variables have been described previously.20
Eligible patients were randomized to open-label therapy with either ezetimibe (10 mg/day) or extended-release niacin (ERN; target dose 2000 mg/day). This report focuses only on the subjects randomized to treatment with ezetimibe. There were no protocol-directed changes in statin medications or dosage throughout the study. Adherence to study medication was determined through tablet counts. Cumulative exposure to ezetimibe was calculated as the product of drug dose, duration of therapy, and adherence. The primary endpoint in the present analysis was the within-group difference in the change in mean CIMT after 14 months.
Following study termination on 4 June 2009, all actively enrolled patients were contacted and returned for final collection of clinical variables, laboratory data, and blinded CIMT assessment. Among the 176 patients initially randomized to ezetimibe, a total of 161 patients either completed the entire 14-month study (n = 111) or had last observations following the termination of the study (n = 50; mean treatment duration of 7 ± 3 months).
B-mode ultrasonography of the carotid arteries
Carotid ultrasound examinations were performed at baseline, 8, and 14 months. The mean and maximal diastolic CIMT of the distal 1 cm of the far wall of the right and left common carotid arteries was measured by a single-blinded, trained observer utilizing an automated border-detection algorithm. All images were obtained in duplicate and no scans were excluded on the basis of image quality.
Statistical analysis
Continuous variables were evaluated using a t-test for independent variables or a Mann-Whitney U-test, as appropriate. Categorical variables were evaluated using the χ2-test. The bivariate relationships between the change in mean CIMT and baseline, and on-treatment variables were assessed using a Pearson correlation coefficient. Multiple linear regression was performed evaluating the independent relationships between the change in CIMT (dependent variable) and baseline variables, treatment effects, and cumulative exposure to ezetimibe. Variables with a univariate relationship (P < 0.20) to the change in CIMT were entered into the model. A two-sided P-value of ≤0.05 was considered statistically significant. SPSS for Windows version 16 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
The baseline characteristics of the 161 ezetimibe-treated subjects with final CIMT data are shown in Table 1. A majority of the patients were male (80%), hypertensive (88%) with a mean age (±SD) of 65 ± 11 years, and had taken a statin (atorvastatin or simvastatin in 94%) at a mean dose of 40 mg for a median of 4 years (inter-quartile range: 2–10) in duration. Baseline lipid values are also shown in Table 1, with a mean LDL-C of 85 ± 24 mg/dL and a mean HDL-C of 43 ± 9 mg/dL. On-treatment variables included total cholesterol 128 ± 25, LDL-C 66 ± 20, HDL-C 41 ± 9, and triglycerides 121 ± 57 mg/dL. The mean study drug adherence rates over the duration of the study exceeded 80%.
Characteristics of the patients treated with ezetimibe (n = 161)
| . | Ezetimibe (n = 161) . |
|---|---|
| Male gender (n, %) | 129 (80.1) |
| Age, mean ± SD | 65 ± 11 |
| Diabetes mellitus (n, %) | 67 (41.6) |
| Hypertension (n, %) | 142 (88.2) |
| Tobacco use (n, %) | 9 (5.6) |
| Family history of coronary heart disease (n, %) | 60 (37.3) |
| History of coronary heart disease (n, %) | |
| Angina with documented ischemia | 53 (32.9) |
| Angiographic coronary disease | 106 (60.2) |
| Myocardial infarction | 51 (31.7) |
| Percutaneous coronary revascularization | 60 (37.3) |
| Coronary bypass surgery | 37 (23) |
| Medications (n, %) | |
| β-Blocker | 112 (69.6) |
| Aspirin | 139 (86.3) |
| Clopidogrel | 41 (25.5) |
| Angiotensin-converting enzyme inhibitor | 89 (55.3) |
| Statin therapy (n, %) | |
| Simvastatin | 65 (40.3) |
| Atorvastatin | 86 (53.4) |
| Pravastatin | 5 (3.1) |
| Rosuvastatin | 5 (3.1) |
| Lovastatin | 0 (0) |
| Mean daily statin dose (mg) | 42 ± 24 |
| Duration of statin use (years) | 6.5 ± 5.6 |
| BMI (kg/m2) | 30.8 ± 5.6 |
| Waist girth (in.) | 41 ± 5 |
| Systolic blood pressure (mmHg) | 136 ± 17 |
| Diastolic blood pressure (mmHg) | 75 ± 9 |
| Total cholesterol (mg/dL) | 147 ± 29 |
| LDL-C (mg/dL) | 84 ± 24 |
| HDL-C (mg/dL) | 43 ± 9 |
| Triglycerides (mg/dL) | 129 ± 59 |
| Glucose (mg/dL) | 104 ± 27 |
| C-reactive protein (mg/L) | 3.3 ± 8.3 |
| CIMT mean thickness (mm) | 0.895 ± 0.146 |
| . | Ezetimibe (n = 161) . |
|---|---|
| Male gender (n, %) | 129 (80.1) |
| Age, mean ± SD | 65 ± 11 |
| Diabetes mellitus (n, %) | 67 (41.6) |
| Hypertension (n, %) | 142 (88.2) |
| Tobacco use (n, %) | 9 (5.6) |
| Family history of coronary heart disease (n, %) | 60 (37.3) |
| History of coronary heart disease (n, %) | |
| Angina with documented ischemia | 53 (32.9) |
| Angiographic coronary disease | 106 (60.2) |
| Myocardial infarction | 51 (31.7) |
| Percutaneous coronary revascularization | 60 (37.3) |
| Coronary bypass surgery | 37 (23) |
| Medications (n, %) | |
| β-Blocker | 112 (69.6) |
| Aspirin | 139 (86.3) |
| Clopidogrel | 41 (25.5) |
| Angiotensin-converting enzyme inhibitor | 89 (55.3) |
| Statin therapy (n, %) | |
| Simvastatin | 65 (40.3) |
| Atorvastatin | 86 (53.4) |
| Pravastatin | 5 (3.1) |
| Rosuvastatin | 5 (3.1) |
| Lovastatin | 0 (0) |
| Mean daily statin dose (mg) | 42 ± 24 |
| Duration of statin use (years) | 6.5 ± 5.6 |
| BMI (kg/m2) | 30.8 ± 5.6 |
| Waist girth (in.) | 41 ± 5 |
| Systolic blood pressure (mmHg) | 136 ± 17 |
| Diastolic blood pressure (mmHg) | 75 ± 9 |
| Total cholesterol (mg/dL) | 147 ± 29 |
| LDL-C (mg/dL) | 84 ± 24 |
| HDL-C (mg/dL) | 43 ± 9 |
| Triglycerides (mg/dL) | 129 ± 59 |
| Glucose (mg/dL) | 104 ± 27 |
| C-reactive protein (mg/L) | 3.3 ± 8.3 |
| CIMT mean thickness (mm) | 0.895 ± 0.146 |
Characteristics of the patients treated with ezetimibe (n = 161)
| . | Ezetimibe (n = 161) . |
|---|---|
| Male gender (n, %) | 129 (80.1) |
| Age, mean ± SD | 65 ± 11 |
| Diabetes mellitus (n, %) | 67 (41.6) |
| Hypertension (n, %) | 142 (88.2) |
| Tobacco use (n, %) | 9 (5.6) |
| Family history of coronary heart disease (n, %) | 60 (37.3) |
| History of coronary heart disease (n, %) | |
| Angina with documented ischemia | 53 (32.9) |
| Angiographic coronary disease | 106 (60.2) |
| Myocardial infarction | 51 (31.7) |
| Percutaneous coronary revascularization | 60 (37.3) |
| Coronary bypass surgery | 37 (23) |
| Medications (n, %) | |
| β-Blocker | 112 (69.6) |
| Aspirin | 139 (86.3) |
| Clopidogrel | 41 (25.5) |
| Angiotensin-converting enzyme inhibitor | 89 (55.3) |
| Statin therapy (n, %) | |
| Simvastatin | 65 (40.3) |
| Atorvastatin | 86 (53.4) |
| Pravastatin | 5 (3.1) |
| Rosuvastatin | 5 (3.1) |
| Lovastatin | 0 (0) |
| Mean daily statin dose (mg) | 42 ± 24 |
| Duration of statin use (years) | 6.5 ± 5.6 |
| BMI (kg/m2) | 30.8 ± 5.6 |
| Waist girth (in.) | 41 ± 5 |
| Systolic blood pressure (mmHg) | 136 ± 17 |
| Diastolic blood pressure (mmHg) | 75 ± 9 |
| Total cholesterol (mg/dL) | 147 ± 29 |
| LDL-C (mg/dL) | 84 ± 24 |
| HDL-C (mg/dL) | 43 ± 9 |
| Triglycerides (mg/dL) | 129 ± 59 |
| Glucose (mg/dL) | 104 ± 27 |
| C-reactive protein (mg/L) | 3.3 ± 8.3 |
| CIMT mean thickness (mm) | 0.895 ± 0.146 |
| . | Ezetimibe (n = 161) . |
|---|---|
| Male gender (n, %) | 129 (80.1) |
| Age, mean ± SD | 65 ± 11 |
| Diabetes mellitus (n, %) | 67 (41.6) |
| Hypertension (n, %) | 142 (88.2) |
| Tobacco use (n, %) | 9 (5.6) |
| Family history of coronary heart disease (n, %) | 60 (37.3) |
| History of coronary heart disease (n, %) | |
| Angina with documented ischemia | 53 (32.9) |
| Angiographic coronary disease | 106 (60.2) |
| Myocardial infarction | 51 (31.7) |
| Percutaneous coronary revascularization | 60 (37.3) |
| Coronary bypass surgery | 37 (23) |
| Medications (n, %) | |
| β-Blocker | 112 (69.6) |
| Aspirin | 139 (86.3) |
| Clopidogrel | 41 (25.5) |
| Angiotensin-converting enzyme inhibitor | 89 (55.3) |
| Statin therapy (n, %) | |
| Simvastatin | 65 (40.3) |
| Atorvastatin | 86 (53.4) |
| Pravastatin | 5 (3.1) |
| Rosuvastatin | 5 (3.1) |
| Lovastatin | 0 (0) |
| Mean daily statin dose (mg) | 42 ± 24 |
| Duration of statin use (years) | 6.5 ± 5.6 |
| BMI (kg/m2) | 30.8 ± 5.6 |
| Waist girth (in.) | 41 ± 5 |
| Systolic blood pressure (mmHg) | 136 ± 17 |
| Diastolic blood pressure (mmHg) | 75 ± 9 |
| Total cholesterol (mg/dL) | 147 ± 29 |
| LDL-C (mg/dL) | 84 ± 24 |
| HDL-C (mg/dL) | 43 ± 9 |
| Triglycerides (mg/dL) | 129 ± 59 |
| Glucose (mg/dL) | 104 ± 27 |
| C-reactive protein (mg/L) | 3.3 ± 8.3 |
| CIMT mean thickness (mm) | 0.895 ± 0.146 |
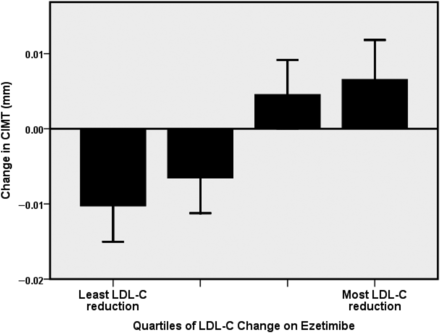
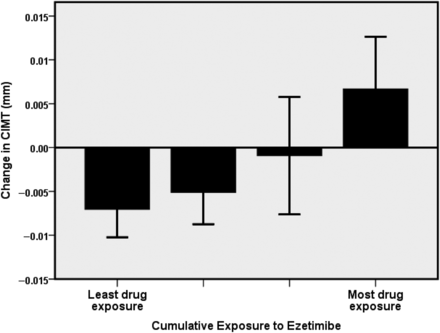
Among baseline and on-treatment variables, ezetimibe-induced reductions in LDL-C were associated with baseline total cholesterol and LDL-C (Table 2). There was a significant, graded increase in the progression of CIMT observed across quartiles of increasing LDL-C reduction (P < 0.001; Figure 1). There was an inverse bivariate relationship between the change in LDL-C and the change in CIMT, r = −0.27; P = 0.001). Cumulative exposure to ezetimibe was associated with the progression of CIMT (Table 2 and Figure 2). There was a bivariate relationship of borderline significance between cumulative drug exposure and the change in CIMT such that greater drug exposure was related to CIMT progression (r = 0.16; P = 0.051). Non-significant relationships with CIMT change were found between baseline LDL-C (r = 0.12, P = 0.14) and achieved LDL-C (r = −0.11, P = 0.18).
Baseline laboratory parameters and changes observed among quartiles of ezetimibe-induced low-density lipoprotein cholesterol change and ezetimibe exposure
| . | Quartiles of LDL-C change on ezetimibe . | ||||
|---|---|---|---|---|---|
| Less than −30 mg/dL . | −30 to −19 mg/dL . | −18 to −8 mg/dL . | Greater than −8 mg/dL . | P-value . | |
| Age (years) | 64 ± 12 | 66 ± 11 | 64 ± 12 | 66 ± 10 | 0.64 |
| Waist girth (in.) | 42 ± 5 | 41 ± 6 | 41 ± 4 | 40 ± 5 | 0.69 |
| Systolic blood pressure (mmHg) | 136 ± 19 | 136 ± 18 | 138 ± 17 | 135 ± 15 | 0.84 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 74 ± 10 | 76 ± 9 | 73 ± 9 | 0.58 |
| Total cholesterol (mg/dL) | 171 ± 27 | 147 ± 15 | 139 ± 22 | 129 ± 21 | <0.001 |
| Triglycerides (mg/dL) | 128 (IQR 100–178) | 122 (IQR 83–155) | 110 (IQR 89–148) | 103 (IQR 67–140) | 0.18 |
| HDL-C (mg/dL) | 44 ± 8 | 44 ± 8 | 42 ± 9 | 42 ± 10 | 0.58 |
| LDL-C (mg/dL) | 106 ± 22 | 83 ± 13 | 78 ± 19 | 69 ± 17 | <0.001 |
| Glucose (mg/dL) | 108 ± 31 | 102 ± 24 | 102 ± 19 | 102 ± 29 | 0.71 |
| Carotid IMT (mm) | 0.885 ± 0.143 | 0.911 ± 0.151 | 0.884 ± 0.154 | 0.883 ± 0.130 | 0.79 |
| Change in carotid IMT | 0.007 ± 0.005 | 0.005 ± 0.005 | −0.007 ± 0.005 | −0.010 ± 0.005 | 0.03 |
| Change in LDL-C (mg/dL) | −42 ± 10 | −23 ± 3 | −13 ± 3 | 4.5 ± 13 | |
| Change in HDL-C (mg/dL) | −5.2 ± 6.2 | −2.5 ± 5.8 | −2.0 ± 4.9 | −1.8 ± 5.5 | 0.11 |
| Change in C-reactive protein | −0.05 (−0.77, 1.69) | −0.14 (−0.66, 0.22) | −0.36 (−0.83, 0.38) | −0.28 (−2.10, 0.74) | 0.60 |
| Quartiles of ezetimibe exposure (dose × duration × adherence) | |||||
| Lowest | Quartile 2 | Quartile 3 | Highest | ||
| Change in LDL-C (mg/dL) | −20 ± 22 | −24 ± 23 | −21 ± 25 | −14 ± 30 | 0.34 |
| Change in HDL-C (mg/dL) | −0.2 ± 5.2 | −2.4 ± 6.0 | −3.8 ± 5.5 | −1.8 ± 5.4 | 0.25 |
| Change in C-reactive protein | −0.08 (−1.0, 0.12) | −0.2 (−1.24, 0.66) | −0.28 (−1.14, 0.23) | 0.01 (−0.63, 1.72) | 0.43 |
| Change in carotid IMT (mm) | −0.007 ± 0.003 | −0.005 ± 0.004 | −0.001 ± 0.007 | 0.007 ± 0.006 | 0.05 |
| . | Quartiles of LDL-C change on ezetimibe . | ||||
|---|---|---|---|---|---|
| Less than −30 mg/dL . | −30 to −19 mg/dL . | −18 to −8 mg/dL . | Greater than −8 mg/dL . | P-value . | |
| Age (years) | 64 ± 12 | 66 ± 11 | 64 ± 12 | 66 ± 10 | 0.64 |
| Waist girth (in.) | 42 ± 5 | 41 ± 6 | 41 ± 4 | 40 ± 5 | 0.69 |
| Systolic blood pressure (mmHg) | 136 ± 19 | 136 ± 18 | 138 ± 17 | 135 ± 15 | 0.84 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 74 ± 10 | 76 ± 9 | 73 ± 9 | 0.58 |
| Total cholesterol (mg/dL) | 171 ± 27 | 147 ± 15 | 139 ± 22 | 129 ± 21 | <0.001 |
| Triglycerides (mg/dL) | 128 (IQR 100–178) | 122 (IQR 83–155) | 110 (IQR 89–148) | 103 (IQR 67–140) | 0.18 |
| HDL-C (mg/dL) | 44 ± 8 | 44 ± 8 | 42 ± 9 | 42 ± 10 | 0.58 |
| LDL-C (mg/dL) | 106 ± 22 | 83 ± 13 | 78 ± 19 | 69 ± 17 | <0.001 |
| Glucose (mg/dL) | 108 ± 31 | 102 ± 24 | 102 ± 19 | 102 ± 29 | 0.71 |
| Carotid IMT (mm) | 0.885 ± 0.143 | 0.911 ± 0.151 | 0.884 ± 0.154 | 0.883 ± 0.130 | 0.79 |
| Change in carotid IMT | 0.007 ± 0.005 | 0.005 ± 0.005 | −0.007 ± 0.005 | −0.010 ± 0.005 | 0.03 |
| Change in LDL-C (mg/dL) | −42 ± 10 | −23 ± 3 | −13 ± 3 | 4.5 ± 13 | |
| Change in HDL-C (mg/dL) | −5.2 ± 6.2 | −2.5 ± 5.8 | −2.0 ± 4.9 | −1.8 ± 5.5 | 0.11 |
| Change in C-reactive protein | −0.05 (−0.77, 1.69) | −0.14 (−0.66, 0.22) | −0.36 (−0.83, 0.38) | −0.28 (−2.10, 0.74) | 0.60 |
| Quartiles of ezetimibe exposure (dose × duration × adherence) | |||||
| Lowest | Quartile 2 | Quartile 3 | Highest | ||
| Change in LDL-C (mg/dL) | −20 ± 22 | −24 ± 23 | −21 ± 25 | −14 ± 30 | 0.34 |
| Change in HDL-C (mg/dL) | −0.2 ± 5.2 | −2.4 ± 6.0 | −3.8 ± 5.5 | −1.8 ± 5.4 | 0.25 |
| Change in C-reactive protein | −0.08 (−1.0, 0.12) | −0.2 (−1.24, 0.66) | −0.28 (−1.14, 0.23) | 0.01 (−0.63, 1.72) | 0.43 |
| Change in carotid IMT (mm) | −0.007 ± 0.003 | −0.005 ± 0.004 | −0.001 ± 0.007 | 0.007 ± 0.006 | 0.05 |
Baseline laboratory parameters and changes observed among quartiles of ezetimibe-induced low-density lipoprotein cholesterol change and ezetimibe exposure
| . | Quartiles of LDL-C change on ezetimibe . | ||||
|---|---|---|---|---|---|
| Less than −30 mg/dL . | −30 to −19 mg/dL . | −18 to −8 mg/dL . | Greater than −8 mg/dL . | P-value . | |
| Age (years) | 64 ± 12 | 66 ± 11 | 64 ± 12 | 66 ± 10 | 0.64 |
| Waist girth (in.) | 42 ± 5 | 41 ± 6 | 41 ± 4 | 40 ± 5 | 0.69 |
| Systolic blood pressure (mmHg) | 136 ± 19 | 136 ± 18 | 138 ± 17 | 135 ± 15 | 0.84 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 74 ± 10 | 76 ± 9 | 73 ± 9 | 0.58 |
| Total cholesterol (mg/dL) | 171 ± 27 | 147 ± 15 | 139 ± 22 | 129 ± 21 | <0.001 |
| Triglycerides (mg/dL) | 128 (IQR 100–178) | 122 (IQR 83–155) | 110 (IQR 89–148) | 103 (IQR 67–140) | 0.18 |
| HDL-C (mg/dL) | 44 ± 8 | 44 ± 8 | 42 ± 9 | 42 ± 10 | 0.58 |
| LDL-C (mg/dL) | 106 ± 22 | 83 ± 13 | 78 ± 19 | 69 ± 17 | <0.001 |
| Glucose (mg/dL) | 108 ± 31 | 102 ± 24 | 102 ± 19 | 102 ± 29 | 0.71 |
| Carotid IMT (mm) | 0.885 ± 0.143 | 0.911 ± 0.151 | 0.884 ± 0.154 | 0.883 ± 0.130 | 0.79 |
| Change in carotid IMT | 0.007 ± 0.005 | 0.005 ± 0.005 | −0.007 ± 0.005 | −0.010 ± 0.005 | 0.03 |
| Change in LDL-C (mg/dL) | −42 ± 10 | −23 ± 3 | −13 ± 3 | 4.5 ± 13 | |
| Change in HDL-C (mg/dL) | −5.2 ± 6.2 | −2.5 ± 5.8 | −2.0 ± 4.9 | −1.8 ± 5.5 | 0.11 |
| Change in C-reactive protein | −0.05 (−0.77, 1.69) | −0.14 (−0.66, 0.22) | −0.36 (−0.83, 0.38) | −0.28 (−2.10, 0.74) | 0.60 |
| Quartiles of ezetimibe exposure (dose × duration × adherence) | |||||
| Lowest | Quartile 2 | Quartile 3 | Highest | ||
| Change in LDL-C (mg/dL) | −20 ± 22 | −24 ± 23 | −21 ± 25 | −14 ± 30 | 0.34 |
| Change in HDL-C (mg/dL) | −0.2 ± 5.2 | −2.4 ± 6.0 | −3.8 ± 5.5 | −1.8 ± 5.4 | 0.25 |
| Change in C-reactive protein | −0.08 (−1.0, 0.12) | −0.2 (−1.24, 0.66) | −0.28 (−1.14, 0.23) | 0.01 (−0.63, 1.72) | 0.43 |
| Change in carotid IMT (mm) | −0.007 ± 0.003 | −0.005 ± 0.004 | −0.001 ± 0.007 | 0.007 ± 0.006 | 0.05 |
| . | Quartiles of LDL-C change on ezetimibe . | ||||
|---|---|---|---|---|---|
| Less than −30 mg/dL . | −30 to −19 mg/dL . | −18 to −8 mg/dL . | Greater than −8 mg/dL . | P-value . | |
| Age (years) | 64 ± 12 | 66 ± 11 | 64 ± 12 | 66 ± 10 | 0.64 |
| Waist girth (in.) | 42 ± 5 | 41 ± 6 | 41 ± 4 | 40 ± 5 | 0.69 |
| Systolic blood pressure (mmHg) | 136 ± 19 | 136 ± 18 | 138 ± 17 | 135 ± 15 | 0.84 |
| Diastolic blood pressure (mmHg) | 76 ± 9 | 74 ± 10 | 76 ± 9 | 73 ± 9 | 0.58 |
| Total cholesterol (mg/dL) | 171 ± 27 | 147 ± 15 | 139 ± 22 | 129 ± 21 | <0.001 |
| Triglycerides (mg/dL) | 128 (IQR 100–178) | 122 (IQR 83–155) | 110 (IQR 89–148) | 103 (IQR 67–140) | 0.18 |
| HDL-C (mg/dL) | 44 ± 8 | 44 ± 8 | 42 ± 9 | 42 ± 10 | 0.58 |
| LDL-C (mg/dL) | 106 ± 22 | 83 ± 13 | 78 ± 19 | 69 ± 17 | <0.001 |
| Glucose (mg/dL) | 108 ± 31 | 102 ± 24 | 102 ± 19 | 102 ± 29 | 0.71 |
| Carotid IMT (mm) | 0.885 ± 0.143 | 0.911 ± 0.151 | 0.884 ± 0.154 | 0.883 ± 0.130 | 0.79 |
| Change in carotid IMT | 0.007 ± 0.005 | 0.005 ± 0.005 | −0.007 ± 0.005 | −0.010 ± 0.005 | 0.03 |
| Change in LDL-C (mg/dL) | −42 ± 10 | −23 ± 3 | −13 ± 3 | 4.5 ± 13 | |
| Change in HDL-C (mg/dL) | −5.2 ± 6.2 | −2.5 ± 5.8 | −2.0 ± 4.9 | −1.8 ± 5.5 | 0.11 |
| Change in C-reactive protein | −0.05 (−0.77, 1.69) | −0.14 (−0.66, 0.22) | −0.36 (−0.83, 0.38) | −0.28 (−2.10, 0.74) | 0.60 |
| Quartiles of ezetimibe exposure (dose × duration × adherence) | |||||
| Lowest | Quartile 2 | Quartile 3 | Highest | ||
| Change in LDL-C (mg/dL) | −20 ± 22 | −24 ± 23 | −21 ± 25 | −14 ± 30 | 0.34 |
| Change in HDL-C (mg/dL) | −0.2 ± 5.2 | −2.4 ± 6.0 | −3.8 ± 5.5 | −1.8 ± 5.4 | 0.25 |
| Change in C-reactive protein | −0.08 (−1.0, 0.12) | −0.2 (−1.24, 0.66) | −0.28 (−1.14, 0.23) | 0.01 (−0.63, 1.72) | 0.43 |
| Change in carotid IMT (mm) | −0.007 ± 0.003 | −0.005 ± 0.004 | −0.001 ± 0.007 | 0.007 ± 0.006 | 0.05 |
Relationship between quartiles of low-density lipoprotein cholesterol reduction during ezetimibe treatment and the change in mean carotid intima–media thickness.
Relationship between the extent of cumulative exposure to ezetimibe treatment and the change in mean carotid intima–media thickness.
A series of multivariable linear models evaluated the change in CIMT controlling for covariates with univariate significance (Table 3). Controlling for the change in LDL-C, baseline LDL-C, and cumulative drug exposure showed that CIMT progression on ezetimibe was independently related to greater LDL-C reduction (P = 0.042) and greater cumulative drug exposure (P = 0.036). Similar models controlling for achieved LDL-C were unchanged; however, in these models, lower achieved LDL-C was significantly associated with CIMT progression. Additional exploratory models further controlling for baseline CIMT, glucose, and changes in C-reactive protein and HDL-C showed that the relationships between change in LDL-C and cumulative drug exposure were not attenuated. Reductions in C-reactive protein were also paradoxically associated with CIMT progression (P = 0.036).
Linear regression models for change in carotid intima–media thickness during ezetimibe treatment
| Covariate . | Model 1 . | Model 2 . | Model 3 . | |||
|---|---|---|---|---|---|---|
| Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | |
| Change in LDL-C | −0.308 | 0.001 | −0.306 | 0.001 | −0.292 | 0.003 |
| Cumulative drug exposure | 0.183 | 0.02 | 0.187 | 0.02 | 0.181 | 0.02 |
| Baseline LDL-C | −0.032 | 0.74 | −0.054 | 0.56 | −0.05 | 0.60 |
| Change in C-reactive protein | −0.172 | 0.03 | −0.169 | 0.04 | ||
| Change in HDL-C | −0.0436 | 0.61 | ||||
| Baseline CIMT | −0.078 | 0.32 | ||||
| Covariate . | Model 1 . | Model 2 . | Model 3 . | |||
|---|---|---|---|---|---|---|
| Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | |
| Change in LDL-C | −0.308 | 0.001 | −0.306 | 0.001 | −0.292 | 0.003 |
| Cumulative drug exposure | 0.183 | 0.02 | 0.187 | 0.02 | 0.181 | 0.02 |
| Baseline LDL-C | −0.032 | 0.74 | −0.054 | 0.56 | −0.05 | 0.60 |
| Change in C-reactive protein | −0.172 | 0.03 | −0.169 | 0.04 | ||
| Change in HDL-C | −0.0436 | 0.61 | ||||
| Baseline CIMT | −0.078 | 0.32 | ||||
Linear regression models for change in carotid intima–media thickness during ezetimibe treatment
| Covariate . | Model 1 . | Model 2 . | Model 3 . | |||
|---|---|---|---|---|---|---|
| Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | |
| Change in LDL-C | −0.308 | 0.001 | −0.306 | 0.001 | −0.292 | 0.003 |
| Cumulative drug exposure | 0.183 | 0.02 | 0.187 | 0.02 | 0.181 | 0.02 |
| Baseline LDL-C | −0.032 | 0.74 | −0.054 | 0.56 | −0.05 | 0.60 |
| Change in C-reactive protein | −0.172 | 0.03 | −0.169 | 0.04 | ||
| Change in HDL-C | −0.0436 | 0.61 | ||||
| Baseline CIMT | −0.078 | 0.32 | ||||
| Covariate . | Model 1 . | Model 2 . | Model 3 . | |||
|---|---|---|---|---|---|---|
| Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | Standardized β coefficient . | P-value . | |
| Change in LDL-C | −0.308 | 0.001 | −0.306 | 0.001 | −0.292 | 0.003 |
| Cumulative drug exposure | 0.183 | 0.02 | 0.187 | 0.02 | 0.181 | 0.02 |
| Baseline LDL-C | −0.032 | 0.74 | −0.054 | 0.56 | −0.05 | 0.60 |
| Change in C-reactive protein | −0.172 | 0.03 | −0.169 | 0.04 | ||
| Change in HDL-C | −0.0436 | 0.61 | ||||
| Baseline CIMT | −0.078 | 0.32 | ||||
Discussion
Ezetimibe had no overall effect on CIMT in ARBITER 6-HALTS; however, we previously identified a paradoxical univariate relationship between greater ezetimibe-induced reductions in LDL-C and progression of CIMT. In this report, we further explore this finding in a post hoc analysis controlling for baseline and on-treatment effects of ezetimibe. The findings show that although LDL-C reductions with ezetimibe are statistically significant, treatment with ezetimibe paradoxically led to greater progression of CIMT in the setting of greater LDL-C reductions and greater cumulative drug exposure. These results suggest that the net effect of ezetimibe is not modelled by sole consideration of its effect on LDL-C and raises concerns that ezetimibe may have effects that adversely impact the progression of atherosclerosis.
Cholesterol treatments that lower LDL-C generally lead to favourable effects on the extent of atherosclerosis.1,2 Treatments with either statins or cholesterol-binding resins lead to a slowing of atherosclerosis progression and can potentially lead to regression of atherosclerosis in the setting of large reductions in LDL-C and an LDL/HDL ratio of ∼1.5 or less. These effects of LDL-C treatment support the hypothesis that lipid deposition contributes to atherosclerosis formation and progression. However, these data also specifically relate to the pharmacological treatment studied and do not represent a class effect of drugs that reduce LDL-C, as other properties of drugs may potentiate or interfere with their overall net effect on atherosclerosis.
Ezetimibe lowers LDL-C ∼10–18%, although the drug's original FDA licensure came amidst uncertainty regarding its mechanism of action. The compound was originally developed as an acyl-coenzyme A: cholesterol acyltransferase inhibitor and now a demonstrated failed approach to pharmacotherapy of lipids. However, its effect in this regard was weak; yet, developers noted its ability to lower LDL-C leading them to seek, and the FDA to grant, a licence for clinical use. Subsequently, investigators identified that ezetimibe inhibits the uptake of cholesterol in the enterocyte.3 Originally described as inhibition of the Niemann–Pick C1 Like 1 receptor, work by independent investigators report that the drug predominately inhibits the scavenger receptor B1,4–6 involved in intracellular translocation of cholesterol. However, absorbed ezetimibe may lead to an off-target consequence as this same receptor binds to the ligand apoprotein A1, the principal apoprotein component of HDL-C in the process of reverse cholesterol transport. Absorption of ezetimibe creates the potential that these effects are active at the hepatocyte, in that ∼18% of orally administered ezetimibe is absorbed and then glucuronidated, after which it is recirculated in a more potent form. Further basic data suggesting that ezetimibe could have effects beyond LDL-C reduction include transcriptional down-regulation of key lipid transport proteins including the ATP-binding cassette transporter (ABCA1) and SRB1. Following significant inhibition of cholesterol absorption, effects at the hepatocyte include not just an increase in LDL receptors, but also changes in the proportion of sterols such as lathosterol, and an increase in hepatic cholesterol synthesis.24,25 Recent studies suggest that the effect on the lipid particle profile is an absolute or relative increase in the proportion of small dense LDL-C.26,27 Taken in sum, it becomes clear that ezetimibe possesses effects on cholesterol transport mechanisms, and off-target and counter-regulatory mechanisms that putatively could offset or even over-ride some of the anticipated benefit mediated by LDL-C reduction.
Animal models have applied ezetimibe for its experimental effects on atherosclerosis. In a hypercholesterolaemic rabbit model of atherosclerosis, ezetimibe, at doses nine-fold those used in humans, inhibited atherosclerosis formation. However, animal models poorly predict human effects on cholesterol transport receptors and have not proven to be a reliable indicator of treatment effects as shown by the ACAT inhibitors and torcetrapib. Thus, at issue is the net effect in humans treated with ezetimibe, which represents a summation of ezetimibe effects which may include a diverse array of potentially favourable and unfavourable actions. Measures of net clinical effects include effects on endothelial function, arterial structure and atherosclerosis, and clinical CHD outcomes.
Studies of endothelial function have evaluated whether treatment with ezetimibe improves arterial function. Treatment with statins has uniformly been associated with improved endothelial function. Among 11 published, controlled studies evaluating endothelial function or endothelial cell effects during ezetimibe treatment,8–18 8 have shown the combination of ezetimibe and statin to be worse than a statin alone with regard to its effect on endothelial function. In the two largest and most rigorous randomized controlled studies,10,15 ezetimibe was found to be comparable to placebo for its effects on endothelial function, despite equipotent reductions in LDL compared with statins. Four of these showed other negative findings on biomarkers of endothelial cell function.12,14,15,17 None of the available studies on endothelial function have associated their findings with clinical outcomes; thus, the clinical interpretation of these results remains uncertain.
Three studies have examined the effect of ezetimibe on arterial structure in humans.19–21 In the ENHANCE study, subjects with familial hypercholesterolaemia on statin therapy were randomized to treatment with either ezetimibe or placebo.19 Ezetimibe had a major LDL-C-lowering effect, with on-treatment LDL-C reduced from 200 to 150 mg/dL. However, after 2 years, there was no difference in the rate of CIMT progression, and a similar rate of cardiovascular events in the ezetimibe (3%) and placebo groups (2.5%). The strict interpretation of this study is that ezetimibe was comparable to placebo for its effect on CIMT. However, alternative hypotheses for the negative trial result include the unique nature of patients with familial hypercholesterolaemia in terms of their abnormalities in lipid transport, their general absence of co-morbidities, and prior statin treatment. Also noted is the generally low baseline CIMT values which were ∼0.70 mm; however, these represent values above the 75th percentile for age and gender. Similarly, ARBITER 6-HALTS compared ezetimibe with niacin in an active control trial and found no net effect of ezetimibe on CIMT, and found that ezetimibe was inferior to niacin for the primary endpoint of CIMT and the secondary endpoint of clinical cardiovascular events.20,22 Most recently, a study compared ezetimibe with placebo in statin-naïve patients with peripheral arterial disease.21 In this study, in repudiation of the ENHANCE criticism, ezetimibe showed no difference with placebo when added to statin therapy. Moreover, the study found a significant bivariate inverse relationship between the extent of LDL reduction and peripheral atherosclerosis progression (r = −0.38), of a comparable magnitude to that described in ARBITER 6. This effect remains counterintuitive to the prevailing understanding that a greater reduction in LDL-C yields superior clinical efficacy and requires explanation. Could the extent to which ezetimibe inhibits SRB1-related cholesterol absorption,28 reflected in the measurable extent of LDL-C reduction, be more than offset by an unmeasurable ezetimibe-induced inhibition of other transport mechanisms known to be important in HDL-related reverse cholesterol transport? Which other off-target mechanisms may contribute? Torcetrapib stands as the most recent reminder that the net effects of a lipid drug, and particularly a cholesterol transport inhibitor, cannot be measured solely through its effects on circulating cholesterol concentrations.29,30
The present study raises significant concerns on the mechanism of action of ezetimibe and its potential effect on cardiovascular outcomes. We found that two factors were independently associated with a greater progression of CIMT during ezetimibe treatment, a greater degree of LDL-C reduction, and the cumulative exposure to the drug. A paradoxical relationship between LDL-C reduction and CIMT progression is in direct conflict with findings from statins, binding resins, or lifestyle-induced LDL-C reductions. The implication is that the surrogate basis upon which ezetimibe was licensed (LDL-C reduction) is not linked with an expected favourable effect on CIMT. Also of concern is the finding that controlling for LDL-C reduction, simply the intensity of ezetimibe exposure was associated with CIMT progression, independent of its LDL-C-lowering effect. Taken together with concerns on the diverse mechanisms of ezetimibe within cholesterol transport and homeostasis, and findings on other surrogate effects such as endothelial function, the data suggest that ezetimibe has effects not modelled through its effect on LDL-C.
Study limitations
The findings from this study are relevant to higher risk patients with controlled LDL-C on statin monotherapy with levels of HDL-C below the population median. This analysis is a post hoc analysis intended to explore the overall net negative effect of ezetimibe in ARBITER 6-HALTS. As such, it should be regarded as hypothesis-generating. We conducted a number of analyses among possible endpoints, thus a conservative statistical interpretation is warranted. The study utilized CIMT as a surrogate endpoint for the net clinical effects of ezetimibe. Clinical outcome studies are required; however, taken along with other concerns regarding ezetimibe's clinical effects leads to reasonable uncertainty regarding the efficacy and safety of ezetimibe. Although a recent clinical study in patients with advanced kidney disease suggested that a combination of simvastatin with ezetimibe, in comparison to a placebo, reduced cardiovascular outcomes in the subgroup subjects not yet on haemodialysis, the study design lacked a statin control arm and therefore cannot discern the independent effect of ezetimibe.31 Additional clinical studies are needed.
Conclusions
Among CHD and high-risk patients on statin therapy in the ARBITER-6 trial, ezetimibe leads to paradoxical progression of CIMT in association with both greater LDL reduction and cumulative drug exposure. These findings may suggest the presence of other effects of ezetimibe, which appear to have adverse consequences on arterial atherosclerosis.
Funding
Abbott Pharmaceuticals funded the study via an unrestricted, investigator-initiated research grant administered by the Henry M. Jackson Foundation for the Advancement of Military Medicine, Rockville, MD, USA. Dr. Taylor has received lecture honoraria from Abbott.
Conflict of interest: The opinions or assertions herein are the private views of the authors and are not to be construed as reflecting the views of the Department of the Army or the Department of Defense.




Comments
Dear Editor,
The article by Taylor and associates on progression of atherosclerosis during ezetemibe therapy (1) was of great interest to me. In 159 patients with coronary artery disease treated with a statin and randomized to ezetimibe within ARBITER 6-HALTS trial, carotid intima-media thickness (CIMT) was paradoxically found to be progressed in association with both greater low-density cholesterol (LDL-C) reduction and cumulative exposure to ezetimibe. It was considered that these were off-target actions of ezetemibe. Characteristics of this patient group included predominance of middle-aged and elderly non-smoking male adults, with a high prevalence of abdominal obesity, impaired fasting glucose and diabetes. We find these observations less surprising and paradoxical, in view of the knowledge of autoimmune activation (consisting of aggregation of a damaged protein such as lipoprotein(a) and a protective protein such as apolipipoprotein A-I) being a driver of cardio-renal-metabolic disorders (2,3), a mechanism that probably underlay the patient sample. The fact that change in CIMT increased linearly with greater LDL-C reduction suggests that ezetimibe and/or statins induced a mechanism similar to that observed in patients with rheumatoid arthritis preceding the clinical onset (4,5). Statins have been documented in meta-analyses to slightly increase the risk of development of diabetes (6) and in our experience that of incident coronary disease in primary prevention of a population prone to impaired glucose tolerance (IGT) (as yet unpublished). Though reasons have remained unclear, long-term usage of statin/ezetimibe might possibly induce apo(a) proteolysis with fragmentation. The critical issue in the unexpected effect is that the sample population is characterized by susceptibility to IGT. Much further research is sorely needed to delineate the role of enhanced pro-inflammatory state and autoimmune activation in atherosclerotic vascular disease as well as in other chronic diseases.
References
1. Taylor AJ, Villines TC, Stanek VJ. Paradoxical progression of atherosclerosis related to low-density lipoprotein reduction and exposure to ezetemibe. Eur Heart J 2012; 33:2939-45
2. Onat A, Can G, Y?ksel H. Dysfunction of high-density lipoprotein and its apolipoproteins: New mechanisms underlying cardiometabolic risk in the population at large. Arch Turk Soc Cardiol 2012; 40:368-85
3. Onat A, Can G, Ademo?lu E, ?elik E, Karag?z A, ?rnek E. Coronary disease risk curve of serum creatinine is linear in Turkish men, U-shaped in women. J Investig Med 2012 Nov 15 [Epub] doi: 10.231/JIM.0b013e318276de59
4. Myasoedova E, Crowson CS, Kremers HM, et al. Total cholesterol and LDL levels decrease before rheumatoid arthritis. Ann Rheum Dis. 2010; 69:310-4
5. Onat A, Direskeneli H. Excess cardiovascular risk in inflammatory rheumatic diseases: pathophysiology and targeted therapy. Curr Pharmaceut Design 2012; 18:1465-77
6. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomized statin trials. Lancet 2010; 375:738-42
Conflict of Interest:
None declared
doi:10.1093/eurheartj/ehs105 Online publish-ahead-of-print December 2012.
Paradoxical progression of atherosclerosis related to low-density lipoprotein reduction and exposure to ezitimibe.
We read with interest the recently published paper by AJ Taylor et al(1)concerning the paradoxical progression of atherosclerosis in relation to low-density lipoprotein reduction and ezitimibe The authors used the non-invasive screenin test,the ultrasound scanning of common carotid arteries with measurement of intima-media thickness(IMT)for assessment and presence of plaques and progression of atherosclrosis process.
The the non-invasive screening test such as carotid IMT has been shown to identify the abnormal structure and function of larges arteries in patients with atherosclerosis risk facors(2) Nevertheless the location of this screening test should be chosen with great caution.
The choice of common carotid arteies only, for evaluation of atherosclerosis process cannot be admited on the basis of a number of published studies.
Even if the relationship between carotid IMT and occurrence of cardiovascular and cerebrovascular diseases is continuous, the ultrasound scanning of isolated common carotid arteries,as in this study, is not appropriate choice for this screening.
The common carotid arteries are an infrequent sites of atherosclerosis plaque deposition and progression,but are likely to measure vascular hypertrophy more sensitive to calcium channel antagonist than lipid lowering drugs such as ezitimibe.
Conversly scanning of the carotids bifurcation and internal carotids arteries ,where the plaques develop first, should be the site for followup of progression or regression of the disease, under therapeutic medication(3-4). The presence of a plaque can be identified by an IMT of more than 1.3 mm to 1.5 mm or by focal increase in thickness of 0.5mm or 50?/? of the surrounding IMT.
In conclusion we should first look on the "right side of the coin" before taking stand in our interpretation of the events.
References
1.Taylor Aj,Villines Tc and Stanek Ej. Paradoxical progression of atherosclerosis related to low-densty lipoprotein reduction and exposure to ezitimibe.
2.Salonen JT,Salonen R.Ultra sound B-mode imaging in observational studies of atherosclerosis progression Circulation 1993;87(supplII):II56-II65.OS
3.Zanchetti A,Bond MG,Henning M et al. Europeen Lacidipine study on atherosclerosis investigators. calcium antagonist lacidipine slow down progression of asymptomatic atherosclerosis:principal results of European Lacidipine study of atheroslerosis(ELSA),a randomized double blind long term trial.Circulation 2002;106:2422-2427-RT.
4.Zanchetti A,Bod MG,Henning M et al.ELSA investiga- tors.Absolute and relative changes carotid intima- media thickness and atherosclerosis plaques during long term antihypertensive treatement:further results of the European Lacidipine Study on atherosclerosis (ELSA);J.Hypertens 2004;22:1201-1212.RT H.ACHRAFI Former Professor Of Cardiology. Villa Achrafi 92 Avenue de La Republuique 28600 Luisant France Email:hachrafi002@yahoo.fr
Conflict of Interest:
None declared