-
PDF
- Split View
-
Views
-
Cite
Cite
Cristina Basso, Federico Ronco, Frank Marcus, Aierken Abudureheman, Stefania Rizzo, Anna Chiara Frigo, Barbara Bauce, Francesco Maddalena, Andrea Nava, Domenico Corrado, Francesco Grigoletto, Gaetano Thiene, Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria, European Heart Journal, Volume 29, Issue 22, November 2008, Pages 2760–2771, https://doi.org/10.1093/eurheartj/ehn415
Close - Share Icon Share
Abstract
To provide a standardized endomyocardial biopsy (EMB) protocol and diagnostic quantitative parameters for arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D). The Task Force criteria for the in vivo diagnosis of ARVC/D include tissue characterization by EMB as a major criterion.
EMBs were simulated in vitro with a Cordis bioptome in explanted hearts from six groups: diffuse (n = 10) and segmental (n = 10) ARVC/D, dilated cardiomyopathy (DC) (n = 10), controls (n = 10), adipositas cordis (n = 10), elderly >80 years (n = 10). Sampling sites were the RV inferior-subtricuspid, antero-apical, and mid-outflow tract (RVOT), the septum, and the left ventricle (LV). Histomorphometry was performed to evaluate the amount of myocardium and fibrous and fatty tissues. Myocyte diameters and abnormalities were also assessed. By selecting a 95% specificity, the ARVC/D diagnostic cut-offs on cumulative RV EMB samples are myocardium <59%, fibrosis >31% and fat >22% (80, 50, and 50% sensitivity, respectively). By excluding elderly and obese people groups a lower cut-off for fat was found (>9%). A high variability between different RV sampling sites was observed; the antero-apical was the most informative region although fat at this level is non-specific. No useful diagnostic cut-off for fatty tissue was identified at the antero-apical and RVOT area. No significant difference was found for any tissue parameter either in septal or in LV EMB. Increased RV myocyte diameters and cytological changes were detected in ARVC/D and DC.
The residual myocardium is the main diagnostic morphometric parameter in ARVC/D, whereas fat at the apex is non-specific. Sensitivity and specificity vary according to the RV region. Target sampling of the triangle of dysplasia is required, although only a single region is often informative, emphasizing the usefulness of imaging-guided EMB. There is no diagnostic value of either septal or LV EMB. Cardiomyopathic changes of the myocytes also appear important for establishing a pathological diagnosis.
Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) is a genetically determined heart muscle disease characterized by progressive fibro-fatty replacement of ventricular myocardium.1–4 In 1994, standardized diagnostic criteria were proposed by the Study Group on ARVC/D of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies.5 The diagnosis of ARVC/D is based on the presence of major and minor criteria that encompass structural, histological, electrocardiographic, arrhythmic, and family history factors. On the basis of this classification, the diagnosis is fulfilled by the presence of two major criteria or one major plus two minor criteria or four minor criteria from different categories. The identification of fibro-fatty replacement of myocardium on endomyocardial biopsy (EMB) is listed as a major criterion. However, no accurate and objective methods to assess this abnormality were provided. In particular, there is a need to quantify the location and extent of the RV wall tissue changes in ARVC/D in comparison with normal subjects.
The aim of the present study was to identify the most important morphometric parameters of myocardial atrophy and fibrous and fatty tissue replacement in different sites of the RV and left ventricle (LV) and to define their sensitivity, specificity, and diagnostic cut-off values.
Methods
Sixty heart specimens belonging to the Archives of the Cardiovascular Registry, Institute of Pathologic Anatomy of the University of Padua constituted our study population. They were classified into six groups according to the autopsy diagnosis and/or clinical data: ARVC/D diffuse forms (more than two RV areas involved) (group 1, n = 10), ARVC/D segmental (two or less RV areas involved) (group 2, n = 10), dilated cardiomyopathy (DC) (group 3, n = 10), normal controls (group 4, n = 10), adipositas cordis from obese people (group 5, n = 10), hearts from people >80 years (group 6, n = 10). ARVC/D hearts were obtained either from autopsy of individuals with sudden death (n = 14) or heart failure death (n = 1) or from cardiac transplantation (n = 5). ARVC/D diagnosis fulfilled the current pathological criteria, i.e. transmural fibro-fatty replacement of the atrophic myocardium in at least one RV region.3 Adipositas cordis (also known as adipomatosis cordis, cor adiposum, and lipomatosis cordis) was an incidental finding at autopsy and was diagnosed in the setting of increased epicardial fat on visual inspection associated with fatty infiltration of the underlying myocardium with adipocytes interposed between myocytes, in the absence of replacement fibrosis.6 A patient with a body mass index above 30 was considered obese.
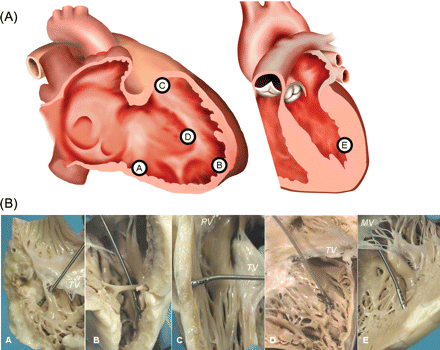
Three standard sampling sites were selected in the RV, the inferior-subtricuspid, antero-apical, and mid-outflow tract (RVOT), since these are the regions most frequently involved in the pathological process of ARVC/D (‘triangle of dysplasia’).1 EMBs were also taken from the right side of the septum and the LV free wall, for a total of five samples per heart as illustrated in Figure 1.
Endomyocardial biopsy sampling sites in the explanted hearts. (A) Graphical representation of the sampling sites in the right ventricular inferior-subtricuspid (A), right ventricular antero-apical (B), right ventricular outflow tract (C), right-sided ventricular septum (D) and the left ventricular free wall (E). (B) Simulated endomyocardial biopsy in vitro in explanted hearts in the corresponding sampling sites (A–E as in Figure 1A). MV, mitral valve; PV, pulmonary valve; TV, tricuspid valve.
EMBs were performed in vitro with disposable Cordis bioptome (Miami, FL, USA). EMB samples were fixed in 10% phosphate-buffered formalin (pH 7.35) and samples were processed for histological examination. Seven microns thick, paraffin-embedded sections were serially cut and stained by haematoxylin–eosin and Heidenhain trichrome. To calculate the total surface area, myocardium, and fibrous and fatty tissues, histomorphometric analysis was performed on digitally acquired trichrome-stained slides at ×25 magnification using an image analyzer system and commercially available software (Image-Pro Plus Version 4.0, Media Cybernetics, MD, USA) as previously described.7,8
The presence of apoptotic myocytes and myocarditis (i.e. inflammatory infiltrates associated with myocyte necrosis) was also evaluated according to a previously described method.9 The diameter of at least 30 cardiomyocytes per site was determined at a ×400 magnification and the value in microns was expressed as mean ± SD for the RV, the septum, and the LV in each group. Cardiomyocytes were selected if the nucleus was centrally located in a cross-section and the measurements were along the short axis of the myocytes at the level of the nucleus.
Measurements were made independently by two observers (F.R. and C.B.), blinded to clinical and pathological characteristics. The difference between the measures of the two observers related to the value of the more experienced observer was always <5%. Data of the more experienced observer were considered for analyses.
Statistical analysis
For the parameters considered, we compared the means of the different groups by non-parametric ANOVA (Kruskal–Wallis test). Diagnostic cut-offs and sensitivity were determined by receiver operating characteristic curves, by selecting a standard specificity of 95%. In defining diagnostic cut-offs, we preferred a high specificity because patients candidates to EMB have been already screened by other tests and this technique is a third-level invasive exam which is usually requested to confirm the diagnosis. A multivariable logistic regression analysis was performed to assess which histological variable is the most useful for ARVC/D diagnosis in RV cumulative EMB samples. The model considered the groups classified as ARVC/D and controls as response variable, whereas the predictors were the percentages of myocardium and fibrous and fatty tissues. No selection criteria for independent variables were established. Following the criteria recommended by Hosmer and Lemeshow,10 linearity assumption was checked graphically by plotting logits vs. the covariate values.
Because of the fact that cut-off estimates and the sensitivity were based on the same data, the results obtained may be too optimistic. To evaluate the degree of over-optimism, we calculated the cut-off and sensitivity distributions from bootstrap samples and provided 95% confidence limits on the basis of bootstrap percentiles.11 We generated 1000 bootstrap samples with the same size of the original data and estimated for each sample the logistic regression coefficients to calculate sensitivity and specificity. We derived for each bootstrap sample the value for which a specificity of 95% attains maximum sensitivity and calculated the corresponding cut-off.
All statistical tests were two-sided. In the case of multiple testing, no adjustment was performed to account for the inflation of the experiment-wise Type I error and the individual P-values were reported. Data were analysed by SAS 9.1.3 for Windows (SAS Institute Inc., Cary, NC, USA).
Results
A total of 300 Heidenhain trichrome and 300 haematoxylin–eosin-stained slides were analysed for histomorphometric evaluation of tissue parameters and myocyte characteristics, respectively. The mean area of the 300 EMB samples was 3.8 ± 0.2 mm2 (range 2–4).
Right ventricular data
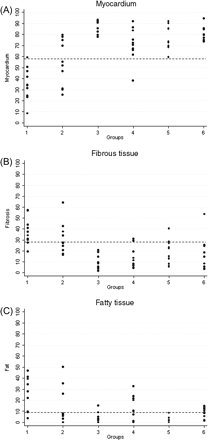
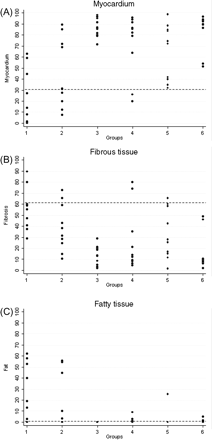
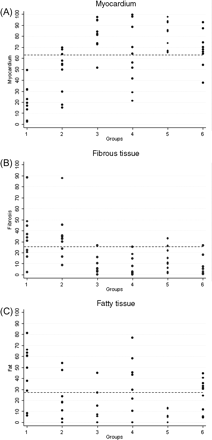
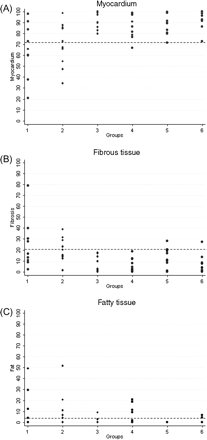
Quantitative data of the RV myocardium and fibrous and fatty tissues are shown in Table 1 and Figures 234–5. Representative EMB samples from the three RV sampling sites in the six groups are illustrated in Figure 6.
Cumulative right ventricular endomyocardial biopsy: histomorphometric data and cut-off values of myocardium (A), fibrous (B) and fatty tissue (C) in the six groups. Group 1, arrhythmogenic right ventricular cardiomyopathy/dysplasia diffuse; Group 2, arrhythmogenic right ventricular cardiomyopathy/dysplasia segmental; Group 3, dilated cardiomyopathy; Group 4, normal; Group 5, adipositas cordis; Group 6, elderly.
Right ventricular inferior wall endomyocardial biopsy: histomorphometric data and cut-off values of myocardium (A), fibrous tissue (B), and fatty tissue (C) in the six groups. (see legend, Figure 2).
Right ventricular antero-apical wall endomyocardial biopsy: histomorphometric data and cut-off values of myocardium (A), fibrous tissue (B) and fatty tissue (C) in the six groups. (see legend, Figure 2).
Right ventricular outflow tract endomyocardial biopsy: histomorphometric data and cut-off values of myocardium (A), fibrous tissue (B) and fatty tissue (C) in the six groups. (see legend, Figure 2).
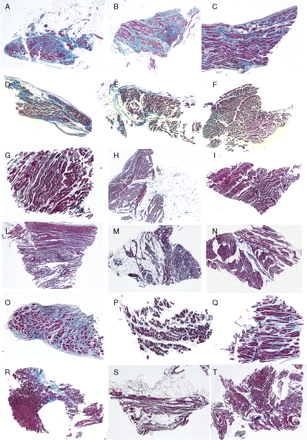
Representative right ventricular (inferior-subtricuspid, apex, right ventricular outflow tract) endomyocardial biopsy samples in the six groups: arrhythmogenic right ventricular cardiomyopathy/dysplasia diffuse forms (A–C); arrhythmogenic right ventricular cardiomyopathy/dysplasia segmental forms (D–F); controls (G–I); adipositas cordis (L–N); dilated cardiomyopathy (O–Q); elderly (R–T).
Demographic data and endomyocardial biopsy histomorphometric results in the six groups
| Disease . | Group 1 (n = 10), ARVC/D diffuse . | Group 2 (n = 10), ARVC/D segmental . | Group 3 (n = 10), DC . | Group 4 (n = 10), controls . | Group 5 (n = 10), adipositas . | Group 6 (n = 10), elderly . | P-values . |
|---|---|---|---|---|---|---|---|
| Gender (M/F) | 7/3 | 9/1 | 7/3 | 4/6 | 6/4 | 7/3 | |
| Age (mean ± SD) | 33.9 ± 17.6 | 29.6 ± 6.0 | 56.1 ± 11.3 | 30.2 ± 14.8 | 45.5 ± 23.4 | 83.8 ± 4.5 | |
| Cause of death/failure | CT (4), SCD (5), HF (1) | SCD (9), CT (1) | CT (5), HF (3), SCD (2) | Trauma (10) | PE (5), stroke (3), haemorrhagic shock (2) | Pneumonia (3), stroke (3), aortic rupture (2), PE (2) | |
| RV myocardium | 35.4 ± 14.8 | 54.1 ± 20.8 | 76.5 ± 10.8 | 87.1 ± 5.8 | 71.2 ± 15.1 | 80.8 ± 6.5 | <0.0001 |
| RV fibrosis | 37.8 ± 12.2 | 31.2 ± 14.4 | 19.0 ± 11.4 | 9.0 ± 6.8 | 13.4 ± 10.0 | 16.1 ± 15.6 | <0.0001 |
| RV fat | 26.8 ± 15,1 | 14.8 ± 16.5 | 2.2 ± 2.7 | 3.7 ± 4.9 | 13.4 ± 10.6 | 9.3 ± 4.9 | 0.0001 |
| RV inferior myocardium | 21.8 ± 25.1 | 44.5 ± 31.0 | 65.0 ± 25.3 | 86.5 ± 8.8 | 72.3 ± 27.6 | 83.9 ± 16.7 | <0.0001 |
| RV inferior fibrosis | 53.5 ± 19.5 | 38.7 ± 21.1 | 32.4 ± 22.7 | 13.4 ± 8.8 | 26.4 ± 28.2 | 15.5 ± 17.0 | 0.0006 |
| RV inferior fat | 24.7 ± 25.8 | 16.8 ± 24.3 | 2.5 ± 8.0 | 0.0 ± 0.0a | 1.3 ± 2.8 | 0.7 ± 1.6 | 0.0174 |
| RV apex myocardium | 19.3 ± 15.2 | 48.0 ± 20.1 | 83.0 ± 11.0 | 82.6 ± 14.2 | 61.9 ± 27.4 | 66.7 ± 16.1 | <0.0001 |
| RV apex fibrosis | 34.5 ± 23.5 | 34.8 ± 21.3 | 12.9 ± 11.1 | 7.4 ± 8.3 | 7.4 ± 9.1 | 7.1 ± 8.6 | <0.0001 |
| RV apex fat | 46.2 ± 25.3 | 17.5 ± 19.5 | 4.1 ± 5.1 | 10.0 ± 15.1 | 30.8 ± 25.3 | 26.2 ± 14.3 | 0.0004 |
| RVOT myocardium | 65.0 ± 23.4 | 69.9 ± 20.1 | 88.4 ± 9.6 | 92.2 ± 7.5 | 85.2 ± 10.9 | 91.9 ± 7.8 | 0.0017 |
| RVOT fibrosis | 25.6 ± 22.1 | 20.0 ± 11.0 | 11.6 ± 9.6 | 6.6 ± 7.2 | 6.6 ± 5.9 | 7.0 ± 8.2 | 0.0037 |
| RVOT fat | 9.5 ± 16.9 | 10.1 ± 16.0 | 0.0 ± 0.0a | 1.2 ± 2.9 | 8.2 ± 8.8 | 1.1 ± 2.4 | 0.5449 |
| Septal myocardium | 81.4 ± 24.0 | 86.3 ± 12.9 | 80.4 ± 14.7 | 92.5 ± 9.6 | 91.2 ± 10.6 | 90.4 ± 8.0 | 0.2562 |
| Septal fibrosis | 15.8 ± 17.1 | 12.13 ± 12.9 | 19.2 ± 14.4 | 7.5 ± 9.6 | 6.4 ± 5.6 | 8.7 ± 7.7 | 0.2089 |
| Septal fat | 2.8 ± 7.3 | 1.6 ± 5.1 | 0.4 ± 1.3 | 0.0 ± 0.0a | 2.4 ± 5.4 | 0.9 ± 2.7 | 0.5218 |
| LV myocardium | 86.5 ± 13.9 | 88.0 ± 13.4 | 70.9 ± 29.9 | 97.0 ± 4.1 | 94.1 ± 6.6 | 89.9 ± 11.9 | 0.0337 |
| LV fibrosis | 13.2 ± 13.4 | 12.0 ± 13.4 | 29.0 ± 29.8 | 3.0 ± 4.1 | 5.9 ± 6.6 | 8.2 ± 7.9 | 0.0315 |
| LV fat | 0.2 ± 0.8 | 0.0 ± 0.1 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 1.8 ± 5.9 | 0.9953 |
| Disease . | Group 1 (n = 10), ARVC/D diffuse . | Group 2 (n = 10), ARVC/D segmental . | Group 3 (n = 10), DC . | Group 4 (n = 10), controls . | Group 5 (n = 10), adipositas . | Group 6 (n = 10), elderly . | P-values . |
|---|---|---|---|---|---|---|---|
| Gender (M/F) | 7/3 | 9/1 | 7/3 | 4/6 | 6/4 | 7/3 | |
| Age (mean ± SD) | 33.9 ± 17.6 | 29.6 ± 6.0 | 56.1 ± 11.3 | 30.2 ± 14.8 | 45.5 ± 23.4 | 83.8 ± 4.5 | |
| Cause of death/failure | CT (4), SCD (5), HF (1) | SCD (9), CT (1) | CT (5), HF (3), SCD (2) | Trauma (10) | PE (5), stroke (3), haemorrhagic shock (2) | Pneumonia (3), stroke (3), aortic rupture (2), PE (2) | |
| RV myocardium | 35.4 ± 14.8 | 54.1 ± 20.8 | 76.5 ± 10.8 | 87.1 ± 5.8 | 71.2 ± 15.1 | 80.8 ± 6.5 | <0.0001 |
| RV fibrosis | 37.8 ± 12.2 | 31.2 ± 14.4 | 19.0 ± 11.4 | 9.0 ± 6.8 | 13.4 ± 10.0 | 16.1 ± 15.6 | <0.0001 |
| RV fat | 26.8 ± 15,1 | 14.8 ± 16.5 | 2.2 ± 2.7 | 3.7 ± 4.9 | 13.4 ± 10.6 | 9.3 ± 4.9 | 0.0001 |
| RV inferior myocardium | 21.8 ± 25.1 | 44.5 ± 31.0 | 65.0 ± 25.3 | 86.5 ± 8.8 | 72.3 ± 27.6 | 83.9 ± 16.7 | <0.0001 |
| RV inferior fibrosis | 53.5 ± 19.5 | 38.7 ± 21.1 | 32.4 ± 22.7 | 13.4 ± 8.8 | 26.4 ± 28.2 | 15.5 ± 17.0 | 0.0006 |
| RV inferior fat | 24.7 ± 25.8 | 16.8 ± 24.3 | 2.5 ± 8.0 | 0.0 ± 0.0a | 1.3 ± 2.8 | 0.7 ± 1.6 | 0.0174 |
| RV apex myocardium | 19.3 ± 15.2 | 48.0 ± 20.1 | 83.0 ± 11.0 | 82.6 ± 14.2 | 61.9 ± 27.4 | 66.7 ± 16.1 | <0.0001 |
| RV apex fibrosis | 34.5 ± 23.5 | 34.8 ± 21.3 | 12.9 ± 11.1 | 7.4 ± 8.3 | 7.4 ± 9.1 | 7.1 ± 8.6 | <0.0001 |
| RV apex fat | 46.2 ± 25.3 | 17.5 ± 19.5 | 4.1 ± 5.1 | 10.0 ± 15.1 | 30.8 ± 25.3 | 26.2 ± 14.3 | 0.0004 |
| RVOT myocardium | 65.0 ± 23.4 | 69.9 ± 20.1 | 88.4 ± 9.6 | 92.2 ± 7.5 | 85.2 ± 10.9 | 91.9 ± 7.8 | 0.0017 |
| RVOT fibrosis | 25.6 ± 22.1 | 20.0 ± 11.0 | 11.6 ± 9.6 | 6.6 ± 7.2 | 6.6 ± 5.9 | 7.0 ± 8.2 | 0.0037 |
| RVOT fat | 9.5 ± 16.9 | 10.1 ± 16.0 | 0.0 ± 0.0a | 1.2 ± 2.9 | 8.2 ± 8.8 | 1.1 ± 2.4 | 0.5449 |
| Septal myocardium | 81.4 ± 24.0 | 86.3 ± 12.9 | 80.4 ± 14.7 | 92.5 ± 9.6 | 91.2 ± 10.6 | 90.4 ± 8.0 | 0.2562 |
| Septal fibrosis | 15.8 ± 17.1 | 12.13 ± 12.9 | 19.2 ± 14.4 | 7.5 ± 9.6 | 6.4 ± 5.6 | 8.7 ± 7.7 | 0.2089 |
| Septal fat | 2.8 ± 7.3 | 1.6 ± 5.1 | 0.4 ± 1.3 | 0.0 ± 0.0a | 2.4 ± 5.4 | 0.9 ± 2.7 | 0.5218 |
| LV myocardium | 86.5 ± 13.9 | 88.0 ± 13.4 | 70.9 ± 29.9 | 97.0 ± 4.1 | 94.1 ± 6.6 | 89.9 ± 11.9 | 0.0337 |
| LV fibrosis | 13.2 ± 13.4 | 12.0 ± 13.4 | 29.0 ± 29.8 | 3.0 ± 4.1 | 5.9 ± 6.6 | 8.2 ± 7.9 | 0.0315 |
| LV fat | 0.2 ± 0.8 | 0.0 ± 0.1 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 1.8 ± 5.9 | 0.9953 |
ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; CT, cardiac transplantation; HF, heart failure; LV, left ventricular, PE, pulmonary embolism; RV, right ventricular; RVOT, right ventricular outflow tract; SCD, sudden cardiac death; SD, standard deviation.
aExcluded from the analysis because all data were null.
Demographic data and endomyocardial biopsy histomorphometric results in the six groups
| Disease . | Group 1 (n = 10), ARVC/D diffuse . | Group 2 (n = 10), ARVC/D segmental . | Group 3 (n = 10), DC . | Group 4 (n = 10), controls . | Group 5 (n = 10), adipositas . | Group 6 (n = 10), elderly . | P-values . |
|---|---|---|---|---|---|---|---|
| Gender (M/F) | 7/3 | 9/1 | 7/3 | 4/6 | 6/4 | 7/3 | |
| Age (mean ± SD) | 33.9 ± 17.6 | 29.6 ± 6.0 | 56.1 ± 11.3 | 30.2 ± 14.8 | 45.5 ± 23.4 | 83.8 ± 4.5 | |
| Cause of death/failure | CT (4), SCD (5), HF (1) | SCD (9), CT (1) | CT (5), HF (3), SCD (2) | Trauma (10) | PE (5), stroke (3), haemorrhagic shock (2) | Pneumonia (3), stroke (3), aortic rupture (2), PE (2) | |
| RV myocardium | 35.4 ± 14.8 | 54.1 ± 20.8 | 76.5 ± 10.8 | 87.1 ± 5.8 | 71.2 ± 15.1 | 80.8 ± 6.5 | <0.0001 |
| RV fibrosis | 37.8 ± 12.2 | 31.2 ± 14.4 | 19.0 ± 11.4 | 9.0 ± 6.8 | 13.4 ± 10.0 | 16.1 ± 15.6 | <0.0001 |
| RV fat | 26.8 ± 15,1 | 14.8 ± 16.5 | 2.2 ± 2.7 | 3.7 ± 4.9 | 13.4 ± 10.6 | 9.3 ± 4.9 | 0.0001 |
| RV inferior myocardium | 21.8 ± 25.1 | 44.5 ± 31.0 | 65.0 ± 25.3 | 86.5 ± 8.8 | 72.3 ± 27.6 | 83.9 ± 16.7 | <0.0001 |
| RV inferior fibrosis | 53.5 ± 19.5 | 38.7 ± 21.1 | 32.4 ± 22.7 | 13.4 ± 8.8 | 26.4 ± 28.2 | 15.5 ± 17.0 | 0.0006 |
| RV inferior fat | 24.7 ± 25.8 | 16.8 ± 24.3 | 2.5 ± 8.0 | 0.0 ± 0.0a | 1.3 ± 2.8 | 0.7 ± 1.6 | 0.0174 |
| RV apex myocardium | 19.3 ± 15.2 | 48.0 ± 20.1 | 83.0 ± 11.0 | 82.6 ± 14.2 | 61.9 ± 27.4 | 66.7 ± 16.1 | <0.0001 |
| RV apex fibrosis | 34.5 ± 23.5 | 34.8 ± 21.3 | 12.9 ± 11.1 | 7.4 ± 8.3 | 7.4 ± 9.1 | 7.1 ± 8.6 | <0.0001 |
| RV apex fat | 46.2 ± 25.3 | 17.5 ± 19.5 | 4.1 ± 5.1 | 10.0 ± 15.1 | 30.8 ± 25.3 | 26.2 ± 14.3 | 0.0004 |
| RVOT myocardium | 65.0 ± 23.4 | 69.9 ± 20.1 | 88.4 ± 9.6 | 92.2 ± 7.5 | 85.2 ± 10.9 | 91.9 ± 7.8 | 0.0017 |
| RVOT fibrosis | 25.6 ± 22.1 | 20.0 ± 11.0 | 11.6 ± 9.6 | 6.6 ± 7.2 | 6.6 ± 5.9 | 7.0 ± 8.2 | 0.0037 |
| RVOT fat | 9.5 ± 16.9 | 10.1 ± 16.0 | 0.0 ± 0.0a | 1.2 ± 2.9 | 8.2 ± 8.8 | 1.1 ± 2.4 | 0.5449 |
| Septal myocardium | 81.4 ± 24.0 | 86.3 ± 12.9 | 80.4 ± 14.7 | 92.5 ± 9.6 | 91.2 ± 10.6 | 90.4 ± 8.0 | 0.2562 |
| Septal fibrosis | 15.8 ± 17.1 | 12.13 ± 12.9 | 19.2 ± 14.4 | 7.5 ± 9.6 | 6.4 ± 5.6 | 8.7 ± 7.7 | 0.2089 |
| Septal fat | 2.8 ± 7.3 | 1.6 ± 5.1 | 0.4 ± 1.3 | 0.0 ± 0.0a | 2.4 ± 5.4 | 0.9 ± 2.7 | 0.5218 |
| LV myocardium | 86.5 ± 13.9 | 88.0 ± 13.4 | 70.9 ± 29.9 | 97.0 ± 4.1 | 94.1 ± 6.6 | 89.9 ± 11.9 | 0.0337 |
| LV fibrosis | 13.2 ± 13.4 | 12.0 ± 13.4 | 29.0 ± 29.8 | 3.0 ± 4.1 | 5.9 ± 6.6 | 8.2 ± 7.9 | 0.0315 |
| LV fat | 0.2 ± 0.8 | 0.0 ± 0.1 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 1.8 ± 5.9 | 0.9953 |
| Disease . | Group 1 (n = 10), ARVC/D diffuse . | Group 2 (n = 10), ARVC/D segmental . | Group 3 (n = 10), DC . | Group 4 (n = 10), controls . | Group 5 (n = 10), adipositas . | Group 6 (n = 10), elderly . | P-values . |
|---|---|---|---|---|---|---|---|
| Gender (M/F) | 7/3 | 9/1 | 7/3 | 4/6 | 6/4 | 7/3 | |
| Age (mean ± SD) | 33.9 ± 17.6 | 29.6 ± 6.0 | 56.1 ± 11.3 | 30.2 ± 14.8 | 45.5 ± 23.4 | 83.8 ± 4.5 | |
| Cause of death/failure | CT (4), SCD (5), HF (1) | SCD (9), CT (1) | CT (5), HF (3), SCD (2) | Trauma (10) | PE (5), stroke (3), haemorrhagic shock (2) | Pneumonia (3), stroke (3), aortic rupture (2), PE (2) | |
| RV myocardium | 35.4 ± 14.8 | 54.1 ± 20.8 | 76.5 ± 10.8 | 87.1 ± 5.8 | 71.2 ± 15.1 | 80.8 ± 6.5 | <0.0001 |
| RV fibrosis | 37.8 ± 12.2 | 31.2 ± 14.4 | 19.0 ± 11.4 | 9.0 ± 6.8 | 13.4 ± 10.0 | 16.1 ± 15.6 | <0.0001 |
| RV fat | 26.8 ± 15,1 | 14.8 ± 16.5 | 2.2 ± 2.7 | 3.7 ± 4.9 | 13.4 ± 10.6 | 9.3 ± 4.9 | 0.0001 |
| RV inferior myocardium | 21.8 ± 25.1 | 44.5 ± 31.0 | 65.0 ± 25.3 | 86.5 ± 8.8 | 72.3 ± 27.6 | 83.9 ± 16.7 | <0.0001 |
| RV inferior fibrosis | 53.5 ± 19.5 | 38.7 ± 21.1 | 32.4 ± 22.7 | 13.4 ± 8.8 | 26.4 ± 28.2 | 15.5 ± 17.0 | 0.0006 |
| RV inferior fat | 24.7 ± 25.8 | 16.8 ± 24.3 | 2.5 ± 8.0 | 0.0 ± 0.0a | 1.3 ± 2.8 | 0.7 ± 1.6 | 0.0174 |
| RV apex myocardium | 19.3 ± 15.2 | 48.0 ± 20.1 | 83.0 ± 11.0 | 82.6 ± 14.2 | 61.9 ± 27.4 | 66.7 ± 16.1 | <0.0001 |
| RV apex fibrosis | 34.5 ± 23.5 | 34.8 ± 21.3 | 12.9 ± 11.1 | 7.4 ± 8.3 | 7.4 ± 9.1 | 7.1 ± 8.6 | <0.0001 |
| RV apex fat | 46.2 ± 25.3 | 17.5 ± 19.5 | 4.1 ± 5.1 | 10.0 ± 15.1 | 30.8 ± 25.3 | 26.2 ± 14.3 | 0.0004 |
| RVOT myocardium | 65.0 ± 23.4 | 69.9 ± 20.1 | 88.4 ± 9.6 | 92.2 ± 7.5 | 85.2 ± 10.9 | 91.9 ± 7.8 | 0.0017 |
| RVOT fibrosis | 25.6 ± 22.1 | 20.0 ± 11.0 | 11.6 ± 9.6 | 6.6 ± 7.2 | 6.6 ± 5.9 | 7.0 ± 8.2 | 0.0037 |
| RVOT fat | 9.5 ± 16.9 | 10.1 ± 16.0 | 0.0 ± 0.0a | 1.2 ± 2.9 | 8.2 ± 8.8 | 1.1 ± 2.4 | 0.5449 |
| Septal myocardium | 81.4 ± 24.0 | 86.3 ± 12.9 | 80.4 ± 14.7 | 92.5 ± 9.6 | 91.2 ± 10.6 | 90.4 ± 8.0 | 0.2562 |
| Septal fibrosis | 15.8 ± 17.1 | 12.13 ± 12.9 | 19.2 ± 14.4 | 7.5 ± 9.6 | 6.4 ± 5.6 | 8.7 ± 7.7 | 0.2089 |
| Septal fat | 2.8 ± 7.3 | 1.6 ± 5.1 | 0.4 ± 1.3 | 0.0 ± 0.0a | 2.4 ± 5.4 | 0.9 ± 2.7 | 0.5218 |
| LV myocardium | 86.5 ± 13.9 | 88.0 ± 13.4 | 70.9 ± 29.9 | 97.0 ± 4.1 | 94.1 ± 6.6 | 89.9 ± 11.9 | 0.0337 |
| LV fibrosis | 13.2 ± 13.4 | 12.0 ± 13.4 | 29.0 ± 29.8 | 3.0 ± 4.1 | 5.9 ± 6.6 | 8.2 ± 7.9 | 0.0315 |
| LV fat | 0.2 ± 0.8 | 0.0 ± 0.1 | 0.0 ± 0.0a | 0.0 ± 0.0a | 0.0 ± 0.0a | 1.8 ± 5.9 | 0.9953 |
ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; CT, cardiac transplantation; HF, heart failure; LV, left ventricular, PE, pulmonary embolism; RV, right ventricular; RVOT, right ventricular outflow tract; SCD, sudden cardiac death; SD, standard deviation.
aExcluded from the analysis because all data were null.
Table 2 reports the diagnostic cut-offs and sensitivity for ARVC/D, obtained by selecting a 95% specificity, both calculated from original data and by bootstrapping. These measures are related to cumulative RV and site-by-site EMB.
Diagnostic cut-offs (% endomyocardial biopsy area) and sensitivity (%) for arrhythmogenic right ventricular cardiomyopathy/dysplasia with 95% specificity
| Sampling site/tissue parameters . | ARVC/D vs. all other groups . | ARVC/D vs. DC and controls . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (%) . | Sensitivity (%) . | Cut-off (%) . | Sensitivity (%) . | |||||||||
| Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | |
| RV myocardium | <59.4 | <59.9 | 50.4–69.5 | 80 | 80 | 60–95 | <59.4 | <63.7 | 59.4–74.8 | 80 | 82 | 65–100 |
| RV fibrosis | >30.9 | >33.2 | 25.3–43.9 | 50 | 54 | 20–85 | >28.5 | >28.3 | 22.3–33.8 | 60 | 69 | 45–95 |
| RV fat | >21.6 | >23.0 | 14.9–32.6 | 50 | 49 | 25–70 | >9.0 | >8.6 | 5.1–14.9 | 60 | 68 | 40–95 |
| RV inferior myocardium | <31.4 | <29.9 | 19.9–44.7 | 65 | 61 | 30–85 | <31.9 | <40.2 | 32.0–71.9 | 65 | 69 | 45–95 |
| RV inferior fibrosis | >65.4 | >67.9 | 47.0–80.1 | 15 | 24 | 0–60 | >60.1 | >55.7 | 28.1–65.4 | 20 | 39 | 5–90 |
| RV inferior fat | >4.9 | >6.7 | 1.6–18.9 | 50 | 56 | 30–80 | >1.0 | >3.5 | 2.7–9.9 | 60 | 60 | 35–80 |
| RV apex myocardium | <31.7 | <34.6 | 21.3–53.9 | 60 | 60 | 30–85 | <63.6 | <64.0 | 53.9–69.9 | 90 | 94 | 75–100 |
| RV apex fibrosis | >26.5 | >27.5 | 20.5–33.1 | 65 | 67 | 45–90 | >26.5 | >27.0 | 16.4–33.1 | 65 | 70 | 45–95 |
| RV apex fat | >45.6 | >49.9 | 43.4–63.3 | 40 | 38 | 15–60 | >27.1 | >24.1 | 8.3–37.6 | 50 | 60 | 35–85 |
| RVOT myocardium | <71.5 | <71.1 | 66.8–77.0 | 55 | 59 | 35–80 | <72.9 | <74.7 | 71.5–80.7 | 65 | 65 | 45–85 |
| RVOT fibrosis | >20.4 | >23.3 | 17.5 –28.5 | 45 | 47 | 25–70 | >20.4 | >22.7 | 17.5–28.1 | 45 | 48 | 30–70 |
| RVOT fat | >18.6 | >16.2 | 6.2–20.9 | 20 | 25 | 5–50 | >2.8 | >2.7 | 0.04–6.8 | 50 | 53 | 30–75 |
| Sampling site/tissue parameters . | ARVC/D vs. all other groups . | ARVC/D vs. DC and controls . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (%) . | Sensitivity (%) . | Cut-off (%) . | Sensitivity (%) . | |||||||||
| Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | |
| RV myocardium | <59.4 | <59.9 | 50.4–69.5 | 80 | 80 | 60–95 | <59.4 | <63.7 | 59.4–74.8 | 80 | 82 | 65–100 |
| RV fibrosis | >30.9 | >33.2 | 25.3–43.9 | 50 | 54 | 20–85 | >28.5 | >28.3 | 22.3–33.8 | 60 | 69 | 45–95 |
| RV fat | >21.6 | >23.0 | 14.9–32.6 | 50 | 49 | 25–70 | >9.0 | >8.6 | 5.1–14.9 | 60 | 68 | 40–95 |
| RV inferior myocardium | <31.4 | <29.9 | 19.9–44.7 | 65 | 61 | 30–85 | <31.9 | <40.2 | 32.0–71.9 | 65 | 69 | 45–95 |
| RV inferior fibrosis | >65.4 | >67.9 | 47.0–80.1 | 15 | 24 | 0–60 | >60.1 | >55.7 | 28.1–65.4 | 20 | 39 | 5–90 |
| RV inferior fat | >4.9 | >6.7 | 1.6–18.9 | 50 | 56 | 30–80 | >1.0 | >3.5 | 2.7–9.9 | 60 | 60 | 35–80 |
| RV apex myocardium | <31.7 | <34.6 | 21.3–53.9 | 60 | 60 | 30–85 | <63.6 | <64.0 | 53.9–69.9 | 90 | 94 | 75–100 |
| RV apex fibrosis | >26.5 | >27.5 | 20.5–33.1 | 65 | 67 | 45–90 | >26.5 | >27.0 | 16.4–33.1 | 65 | 70 | 45–95 |
| RV apex fat | >45.6 | >49.9 | 43.4–63.3 | 40 | 38 | 15–60 | >27.1 | >24.1 | 8.3–37.6 | 50 | 60 | 35–85 |
| RVOT myocardium | <71.5 | <71.1 | 66.8–77.0 | 55 | 59 | 35–80 | <72.9 | <74.7 | 71.5–80.7 | 65 | 65 | 45–85 |
| RVOT fibrosis | >20.4 | >23.3 | 17.5 –28.5 | 45 | 47 | 25–70 | >20.4 | >22.7 | 17.5–28.1 | 45 | 48 | 30–70 |
| RVOT fat | >18.6 | >16.2 | 6.2–20.9 | 20 | 25 | 5–50 | >2.8 | >2.7 | 0.04–6.8 | 50 | 53 | 30–75 |
ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; CI, confidence interval; RV, right ventricle; RVOT, right ventricular outflow tract.
Diagnostic cut-offs (% endomyocardial biopsy area) and sensitivity (%) for arrhythmogenic right ventricular cardiomyopathy/dysplasia with 95% specificity
| Sampling site/tissue parameters . | ARVC/D vs. all other groups . | ARVC/D vs. DC and controls . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (%) . | Sensitivity (%) . | Cut-off (%) . | Sensitivity (%) . | |||||||||
| Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | |
| RV myocardium | <59.4 | <59.9 | 50.4–69.5 | 80 | 80 | 60–95 | <59.4 | <63.7 | 59.4–74.8 | 80 | 82 | 65–100 |
| RV fibrosis | >30.9 | >33.2 | 25.3–43.9 | 50 | 54 | 20–85 | >28.5 | >28.3 | 22.3–33.8 | 60 | 69 | 45–95 |
| RV fat | >21.6 | >23.0 | 14.9–32.6 | 50 | 49 | 25–70 | >9.0 | >8.6 | 5.1–14.9 | 60 | 68 | 40–95 |
| RV inferior myocardium | <31.4 | <29.9 | 19.9–44.7 | 65 | 61 | 30–85 | <31.9 | <40.2 | 32.0–71.9 | 65 | 69 | 45–95 |
| RV inferior fibrosis | >65.4 | >67.9 | 47.0–80.1 | 15 | 24 | 0–60 | >60.1 | >55.7 | 28.1–65.4 | 20 | 39 | 5–90 |
| RV inferior fat | >4.9 | >6.7 | 1.6–18.9 | 50 | 56 | 30–80 | >1.0 | >3.5 | 2.7–9.9 | 60 | 60 | 35–80 |
| RV apex myocardium | <31.7 | <34.6 | 21.3–53.9 | 60 | 60 | 30–85 | <63.6 | <64.0 | 53.9–69.9 | 90 | 94 | 75–100 |
| RV apex fibrosis | >26.5 | >27.5 | 20.5–33.1 | 65 | 67 | 45–90 | >26.5 | >27.0 | 16.4–33.1 | 65 | 70 | 45–95 |
| RV apex fat | >45.6 | >49.9 | 43.4–63.3 | 40 | 38 | 15–60 | >27.1 | >24.1 | 8.3–37.6 | 50 | 60 | 35–85 |
| RVOT myocardium | <71.5 | <71.1 | 66.8–77.0 | 55 | 59 | 35–80 | <72.9 | <74.7 | 71.5–80.7 | 65 | 65 | 45–85 |
| RVOT fibrosis | >20.4 | >23.3 | 17.5 –28.5 | 45 | 47 | 25–70 | >20.4 | >22.7 | 17.5–28.1 | 45 | 48 | 30–70 |
| RVOT fat | >18.6 | >16.2 | 6.2–20.9 | 20 | 25 | 5–50 | >2.8 | >2.7 | 0.04–6.8 | 50 | 53 | 30–75 |
| Sampling site/tissue parameters . | ARVC/D vs. all other groups . | ARVC/D vs. DC and controls . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut-off (%) . | Sensitivity (%) . | Cut-off (%) . | Sensitivity (%) . | |||||||||
| Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | Data-based estimate . | Bootstrap-based estimate . | Bootstrap 95% CI . | |
| RV myocardium | <59.4 | <59.9 | 50.4–69.5 | 80 | 80 | 60–95 | <59.4 | <63.7 | 59.4–74.8 | 80 | 82 | 65–100 |
| RV fibrosis | >30.9 | >33.2 | 25.3–43.9 | 50 | 54 | 20–85 | >28.5 | >28.3 | 22.3–33.8 | 60 | 69 | 45–95 |
| RV fat | >21.6 | >23.0 | 14.9–32.6 | 50 | 49 | 25–70 | >9.0 | >8.6 | 5.1–14.9 | 60 | 68 | 40–95 |
| RV inferior myocardium | <31.4 | <29.9 | 19.9–44.7 | 65 | 61 | 30–85 | <31.9 | <40.2 | 32.0–71.9 | 65 | 69 | 45–95 |
| RV inferior fibrosis | >65.4 | >67.9 | 47.0–80.1 | 15 | 24 | 0–60 | >60.1 | >55.7 | 28.1–65.4 | 20 | 39 | 5–90 |
| RV inferior fat | >4.9 | >6.7 | 1.6–18.9 | 50 | 56 | 30–80 | >1.0 | >3.5 | 2.7–9.9 | 60 | 60 | 35–80 |
| RV apex myocardium | <31.7 | <34.6 | 21.3–53.9 | 60 | 60 | 30–85 | <63.6 | <64.0 | 53.9–69.9 | 90 | 94 | 75–100 |
| RV apex fibrosis | >26.5 | >27.5 | 20.5–33.1 | 65 | 67 | 45–90 | >26.5 | >27.0 | 16.4–33.1 | 65 | 70 | 45–95 |
| RV apex fat | >45.6 | >49.9 | 43.4–63.3 | 40 | 38 | 15–60 | >27.1 | >24.1 | 8.3–37.6 | 50 | 60 | 35–85 |
| RVOT myocardium | <71.5 | <71.1 | 66.8–77.0 | 55 | 59 | 35–80 | <72.9 | <74.7 | 71.5–80.7 | 65 | 65 | 45–85 |
| RVOT fibrosis | >20.4 | >23.3 | 17.5 –28.5 | 45 | 47 | 25–70 | >20.4 | >22.7 | 17.5–28.1 | 45 | 48 | 30–70 |
| RVOT fat | >18.6 | >16.2 | 6.2–20.9 | 20 | 25 | 5–50 | >2.8 | >2.7 | 0.04–6.8 | 50 | 53 | 30–75 |
ARVC/D, arrhythmogenic right ventricular cardiomyopathy/dysplasia; CI, confidence interval; RV, right ventricle; RVOT, right ventricular outflow tract.
No useful diagnostic cut-off for fatty tissue was identified at the antero-apical and RVOT sites.
By applying the calculated histomorphometric cut-offs, a diagnostic EMB in at least one RV sampling site was obtained in 18 ARVC/D hearts (90%), including all diffuse forms (100%) and eight segmental forms (80%). Diagnostic tissue parameters were found for all three RV sampling sites in nine (45%), for two RV sampling sites in seven (35%) and for one RV sampling site in two (10%). EMB was not diagnostic in two ARVC/D hearts (10%), both characterized by a segmental form affecting the distal RVOT. Diagnostic EMB samples were obtained from the antero-apical area in 19 (95%), from the inferior-subtricuspid area in 13 (65%), and from the RVOT in 12 (60%).
The logistic regression analysis confirmed that the residual myocardium is the discriminating tissue parameter between ARVC/D and the other groups, though the presence of fibrosis seems to be important [model tested with Hosmer–Lemeshow χ2 (8 df) = 6.90; P = 0.5478; the logistic model correctly classified 92.4% of cases]. In fact, 14 of 20 ARVC/D cases had more fibrous than fatty tissue, and even in those cases where fat was prevalent, fibrosis was always at least 20%. In three segmental ARVC/D forms, fatty tissue was poorly represented (≤4%), whereas fibrosis was greatly increased (≥16%).
Septal and left ventricular data
No diagnostic cut-off for any tissue parameter (myocardium, fatty tissue, and fibrosis) was found in these sampling sites (Table 1).
Additional histological features
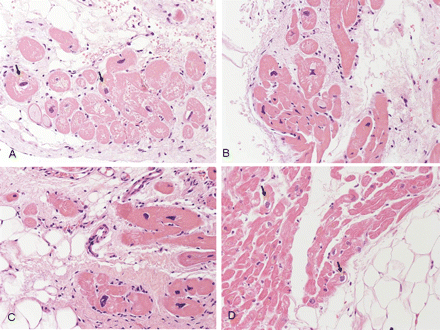
Patchy, focal myocarditis was detected in 3 (15%) and apoptotic myocytes in 7 (35%) of 20 ARVC/D cases. Myocyte abnormalities, dysmetric and dysmorphic nuclei, and cytoplasm vacuolization were a constant finding in both ARVC/D and DC EMB, when compared with the other groups. Moreover, small empty cells with peripheral nucleus in keeping with early adipogenesis were observed only in ARVC/D (Fig. 7).
Myocyte abnormalities in right ventricular endomyocardial biopsy samples. Note the presence of hypertrophic myocytes with dysmetric and dysmorphic nuclei, cytoplasmatic vacuolization and perinuclear halo (arrows); small adipocytes and fibroblasts are seen in arrhythmogenic right ventricular cardiomyopathy/dysplasia (A–C). Perinuclear halo (arrows) is also evident in dilated cardiomyopathy: note the focal fatty infiltration (D).
The mean RV free wall cardiomyocyte diameter was 29.9 ± 1.9 in ARVC/D vs. 17.3 ± 2.3 in controls (P < 0.0001), 26.3 ± 1.38 in adipositas cordis (P = 0.0001), 28.6 ± 1.5 in DC (P = 0.04), and 23.4 ± 1.5 in the elderly (P = 0.0002). The mean septal cardiomyocyte diameter was 29.8 ± 2.3 in ARVC/D vs. 18.8 ± 1.7 in controls (P = 0.01), 22.5 ± 2 in adipositas cordis (P = 0.08), 24.6 ± 1.5 in DC (P = 0.16) and 24.3 ± 1.9 in the elderly (P = 0.10).
The mean LV cardiomyocyte diameter was 28.3 ± 1.9 in ARVC/D vs. 20.6 ± 1.3 in controls (P = 0.008), 24.1 ± 1.5 in adipositas cordis (P = 0.16), 38.0 ± 1.8 in DC (P = 0.03) and 26.1 ± 1.3 in the elderly (P = 0.34).
Discussion
Myocardial atrophy should be regarded as the most important morphological parameter for the in vivo diagnosis of ARVC/D by EMB, with a diagnostic cut-off of cumulative RV residual myocardium <59% (80% sensitivity and 95% specificity), ranging from <31% in the RV inferior wall to <72% in the RVOT. The three tissue parameters are interdependent, i.e. the higher the fibro-fatty replacement, the lower the residual myocardium. Fibrous tissue is increased in both ARVC/D and DC and it is always considerable in ARVC/D (at least 16% in RV biopsy samples). Fatty tissue is a less reliable diagnostic parameter, particularly at the antero-apical RV wall, since there are no statistically significant differences when comparing ARVC/D with adipositas cordis and the elderly, and it may be not highly represented in some segmental forms.
EMB is a well-established procedure for the diagnosis of heart muscle disease and is particularly helpful in ARVC/D, because of the peculiar topographic and histological features of the disease with fibro-fatty replacement reaching the subendocardium.1–4 However, it must be recognized that the histopathological finding of fatty or fibrous tissue in the myocardium is not specific.12 Several authors have reported that fat is a normal finding in the heart, particularly in older and obese people, and it was concluded that fat replacement alone is a different entity from ARVC/D.6,13–15 Also, fibrosis can be observed in many cardiomyopathic and non-cardiomyopathic conditions.16–20
EMB sensitivity in ARVC/D is low if samples are taken from the septum, a region uncommonly involved by the disease. Although this is already well known on the basis of previous pathology investigations,3,4 the present study definitely demonstrates that a biopsy performed on the right-sided septum is unlikely to be diagnostic.
Quantitative diagnostic histomorphometric parameters have been previously reported in vivo by our group, i.e. a myocardial atrophy with residual myocytes <45%, fibrous tissue >40%, and fatty tissue >3%, with a 67% sensitivity and a 92% specificity for at least one parameter.7,8 In that study, there were no elderly or obese people for comparison. Morphometric data were calculated from a mean of the different sites of the RV and control EMBs were usually performed in the septum. A high variability among the different EMB specimens is frequently observed, since the disease is often segmental and there may be only one region that can provide informative samples. Moreover, there is still no agreement on which criteria should be adopted for the histopathological diagnosis of ARVC/D and the presence of fat on EMB is still considered the most suggestive pathological finding. In some series, a proposed diagnostic criterion was a mean amount of fatty tissue >3.5% of all the specimens drawn from different sites of the RV.21 Other investigators considered fibro-fatty or fatty tissue exceeding 25% of the EMB area in at least one sample.22
It is important to note that, for each analysed tissue parameter, both sensitivity and specificity are different according to the sampling sites. In nearly half of ARVC/D cases, only one or two EMB samples were diagnostic, and the antero-apical region was the most informative and involved in 90% of cases. This finding emphasizes the need to identify the various EMB samples, since the final interpretation requires knowledge of their precise location. This was also found at post-mortem investigation by Burke et al.,14 who stated that, although up to 15% fatty replacement is distinctly abnormal in the inferior wall, it is probably normal in the anterior wall near the apex.
Our data are in agreement with the recommendations of the EMB protocol of the NIH Multidisciplinary Study of ARVC/D, which requires target sampling of areas likely to be affected (inferior, antero-apical, RVOT) or of abnormal areas of the RV.23 The latter is particularly important in segmental forms of ARVC/D, where EMB could be guided by cardiac imaging techniques able to identify RV regions with myocardial loss, such as late-enhancement magnetic resonance and three-dimensional electro-anatomic voltage mapping.24 As a consequence, a greater diagnostic sensitivity should be expected from a limited number of samples, when targeting a focal pathological substrate.
Our recommendation in the clinical practice is to perform multiple sampling from different RV regions, moving the bioptome from one area to another, providing an adequate (at least 2 mm2) sample per each sampling site. This increases the probability to catch the disease particularly in segmental forms, thus increasing the sensitivity of the procedure. Of course, when a guided EMB targeting the diseased area is feasible, a single sampling area may be sufficient and this should be the preferred option.
A multidisciplinary group of experts convened by the American Heart Association, the American College of Cardiology, and the European Society of Cardiology recently emphasized that the histopathological findings from EMB may be diagnostic of ARVC/D if it is performed in the appropriate RV location.25 Although clinically relevant complications from EMB are rare and estimated at 1–2%, with death an exceptionally rare event at <0.2%, we must recognize the potential risk of perforation during the procedure in ARVC/D due to a thin and fragile RV free wall. However, this risk has been probably overemphasized since the few studies on EMB in ARVC/D do not report a high rate of complications. In particular, Chimenti et al.21 found no EMB-related complications in a consecutive series of 30 patients and findings in keeping with cardiac perforation were never observed. No major complications were reported by Avella et al.24 in 22 consecutive patients who underwent voltage-mapping-guided EMB in the RV free wall; only a minimal asymptomatic pericardial effusion was detected in two cases by routine post-procedural echocardiography. In the original series of 47 patients by Wichter et al.,22 as well as in our recent series of 31 and 27 patients,26,27 no major and minor complications have been reported. Finally, among the 64 NIH registry probands who underwent EMB, only one had a small pericardial effusion that resolved spontaneously (unpublished results).
Anyway, RV free wall EMB should be performed only in interventional cardiology Labs where a fair amount of EMB procedures per year are carried out by experienced operators. Precautions to prevent myocardial perforation include gently withdrawing the biopsy sample. If there is resistance, it may indicate a thin fibrotic area and another site should be selected. If the biopsy is guided by Carto mapping, the border of the low-voltage area should be targeted.24
We did not find significant differences among the groups when considering LV EMB samples, confirming previous data suggesting that LV EMB is not useful for the diagnosis of ARVC/D. In the series by Pinamonti et al.,28 no fat was detected in six LV EMBs, but there was some fibrosis associated with hypertrophy and myocyte attenuation. In the Mayo Clinic series, no fatty infiltration was evident, and only myopathic changes were present in four patients who had LV EMB.29 More recently, Chimenti et al.21 reported normal LV EMB samples in all ARVC/D patients. The fact that LV EMB is not informative in terms of tissue characterization, even in the setting of biventricular involvement, is not surprising, since the dystrophic process in the LV is usually segmental and restricted to the subepicardial or mid-mural layers of the thick free wall, which are not accessible by the EMB approach.
Finally, it should be recognized that the disease phenotype, although frequently genetically determined, is acquired and progressive. In the early stages, histological abnormalities may consist of myocyte death and degenerative changes rather than mature fatty and fibrous tissue deposition,30 and may be confined to the subepicardial regions and not be transmural. Thus, although histomorphometric evaluation of tissue parameters is mandatory when evaluating EMB, careful cytological assessment of residual myocytes may assist or be crucial to the correct diagnosis. In fact, ARVC/D is commonly characterized at histology by myocyte hypertrophy, cytoplasmic vacuolization, and nuclear abnormalities, and sometimes by cell death and reactive inflammatory infiltrates.
Apoptosis and necrosis, as well as inflammatory infiltrates, represent important features of ARVC/D, particularly during the acute ‘hot phases’ of the disease and are an almost consistent finding at post-mortem when evaluating multiple transmural sections of both ventricles.3,4 However, they are not commonly observed in EMB samples,9 as confirmed by this investigation. This is not surprising due to their focal distribution and possibly their episodic nature. Thus, even though their detection in association with fibro-fatty tissue can be of additional value, apoptosis and myocarditis cannot be regarded as essential features for the diagnosis of ARVC/D on EMB.
On the other hand, ‘cardiomyopathic’ changes of the residual myocytes resembling DC were common, and they have been previously observed in vivo. For example, in the original Mayo Clinic series,29 whereas 54% of the affected patients had abnormal amounts of adipose tissue, the remaining 46% had only myocyte hypertrophy and fibrosis. Thus, the pathologist should be aware that, in addition to myocardial atrophy with fibro-fatty replacement, degenerative changes of the myocytes should be searched for.6
There is also a role for EMB for research purposes, such as molecular pathology (viral genomes),31 histochemistry and immunohistochemistry,9 and ultrastructural analysis.32 Moreover, in sporadic forms, EMB can help in the differential diagnosis with other entities mimicking ARVC/D, with and without structural abnormalities, such as myocarditis,21,26 sarcoidosis,33,34 and idiopathic RVOT tachycardia.27
Limitations of the study
Quantitative results of EMB obtained from explanted hearts, either at autopsy or cardiac transplantation, might not be representative of in vivo EMB because of alleged differences in severity due to ‘end-stage’ disease. To overcome this potential limitation, we included both diffuse forms of ARVC/D, in which the main clinical challenge is differential diagnosis with DC, and segmental forms (involving two or less RV areas), in which sudden death may be the first clinical manifestation in apparently healthy individuals.
Of course, we cannot recommend routine histomorphometric investigation of EMB. On the basis of our findings, in the setting of an RV EMB with replacement fibrosis ± fatty tissue, our proposal is to consider an amount of residual myocytes <60% by morphometric analysis a major diagnostic criterion; and of 60–75% a minor diagnostic criterion. However, since histomorphometric investigation of EMB cannot be routinely available, at least a visual estimation at a glance of the amount of tissue parameters on EMB should be provided, taking into account that tissue characterization is considered a diagnostic criterion for ARVC/D. Thus, to make these data useful for the clinical pathologist in the real world, an amount of residual myocytes <50% by visual estimation could be regarded as a major criterion. Nevertheless, prospective validation of these quantitative criteria by serial assessment of EMBs performed in living patients with suspected ARVC/D is needed and ongoing international registries will be useful to this purpose.23,35
Conclusion
Our data clarify the diagnostic value of EMB in ARVC/D and emphasize that myocardial atrophy (<59% of the overall RV samples) should be considered the main morphometric parameter. While fibrosis is always present and may be even isolated, fatty tissue alone is not a diagnostic finding of ARVC/D. EMB should not be performed in the ventricular septum or LV free wall for diagnostic purposes. For each analysed tissue parameter, both sensitivity and specificity change according to the RV region, thus underlying the need of identifying the sampling site. Although EMB's diagnostic accuracy is higher in diffuse than in segmental ARVC/D forms, in the latter it could be increased by targeting sampling through cardiac imaging-guided EMB. Besides histomorphometric tissue characterization, cardiomyopathic changes of the residual myocytes appear crucial for the final diagnosis on EMB.
Funding
This study was supported by Ministry of Health and Telethon, Rome; Fondazione Cariparo, Padova and Rovigo; Registry of Cardio- cerebro-vascular Pathology, Veneto Region, Venice; and ARVC/D Project, QLG1-CT-2000-01091 5th Framework Programme European Commission, Bruxelles.
Acknowledgements
The authors thank Alessandra Dubrovich, Giovanna Mattiazzo and Anna Saracino for their technical assistance. The authors are also indebted to Claudio Bellini for the graphical support and to Chiara Carturan for secretariat assistance.
Conflict of interest: none declared.