-
PDF
- Split View
-
Views
-
Cite
Cite
Noam Sobel, Rehan M. Khan, Catherine A. Hartley, Edith V. Sullivan, John D.E. Gabrieli, Sniffing Longer rather than Stronger to Maintain Olfactory Detection Threshold, Chemical Senses, Volume 25, Issue 1, February 2000, Pages 1–8, https://doi.org/10.1093/chemse/25.1.1
Close - Share Icon Share
Abstract
Air flow-rate is usually higher in one nostril in comparison to the other. Also, within bounds, higher nasal flow-rate improves odorant detection. It follows from the above that odorant detection should be better in the nostril with higher flow-rate in comparison to the nostril with lower flow-rate. Paradoxically, previous research has shown that odorant detection thresholds are equal for the high and low flow-rate nostrils. Here we resolve this apparent paradox by showing that when detecting through the nostril with lower air flow-rate, humans sniffed longer than when detecting through the nostril with higher air flow-rate, thus equalizing performance between the nostrils. When this compensatory mechanism was blocked, a pronounced advantage in odorant detection was seen for the nostril with higher air flow-rate over the nostril with lower air flow-rate. Finally, we show that normal birhinal sniff duration may enable only one nostril to reach optimal threshold. This finding implies that during each sniff, each nostril conveys to the brain a slightly different image of the olfactory world. It remains to be shown how the brain combines these images into a single olfactory percept.
Introduction
Because of unilateral nasal turbinate swelling, resistance to air flow is usually greater in one nostril than in the other, resulting in different air flow-rates in the two nostrils (Kayser, 1895; Principato and Ozenberger, 1970; Hasegawa and Kern, 1977). The side with higher air flow-rate alternates on an ultradian rhythm, of which the periodicity is unclear (Gilbert and Rosenwasser, 1987; Gilbert, 1989; Mirza et al., 1997).
Flow-rate in the nostrils affects odorant detection thresholds (LeMagnen, 1945; Rehn, 1978; Laing, 1983). Given this, one expects odorant detection thresholds to differ between the nostril with higher flow-rate (HFR nostril) and the nostril with lower flow-rate (LFR nostril). Previous research, however, has found that odorant detection thresholds in the HFR nostril and the LFR nostril are equal (Eccles et al., 1989; Frye, 1995). Considering the expected effects of flow-rate on detection thresholds, this equivalence is paradoxical. Whereas comparisons of the LFR versus the HFR nostrils have revealed symmetry in detection, comparisons of the left versus the right nostril have yielded conflicting results. Whereas some studies reported left/ right symmetry (Schneider and Wolf, 1960; Koelega, 1979; Bellas et al., 1988; Eskenazi et al., 1988; Zatorre and Jones-Gotman, 1990; Shimomura and Motokizawa, 1995; Betchen and Doty, 1998), others have reported asymmetric detection that may be related to general neural asymmetry as reflected in handedness (Toulouse and Vaschide, 1899; Koelega, 1979; Youngentob et al., 1982).
In previous studies comparing olfactory thresholds between the HFR and LFR nostrils, nasal airflow was assessed only before or after, but not during, the detection task. Here we measure nasal airflow before, after and during detection in order to address two behavioral models that could account for the previously described paradox: a vigor model predicts that when forced to detect through the LFR nostril, subjects compensate by sniffing with greater vigor, i.e. increase flow-rate during the task in comparison to baseline in that nostril. A duration model predicts that when forced to detect through the LFR nostril, subjects compensate by sniffing longer, i.e. increase duration in the LFR as compared to the HFR nostril during the task. Either or both of these in-task behavioral adjustments, if present, may equalize performance between the nostrils and thus account for the paradoxical observed equivalence in detection.
Materials and methods
Subjects
Forty right-handed healthy subjects, 23 women and 17 men, mean age 23 years, participated in the study after giving informed consent. Twenty subjects participated in parts 1 and 2, and twenty additional subjects participated in the ensuing control experiments.
Experimental design
Part 1: baseline measurements
For each subject, baseline airflow characteristics (flow-rate, duration and volume) and odorant detection thresholds were determined for each nostril independently, following occlusion of the other nostril with Microfoam tape (Betchen and Doty, 1998). All airflow measurements were obtained by anterior rhinomenometry performed with an ICS medical (Schaumburg, IL) Master Nasal Function digital recorder. Subjects were instructed to take five consecutive strong sniffs from a nasal mask coupled to the rhinometer. The measurement was repeated for each nostril, once at the beginning of the experimental session and once at the end of all experimentation. Mean peak flow of the ten sniffs was set as baseline for that nostril.
Monorhinal detection thresholds were determined using the two-alternative, forced-choice ascending staircase method (Cain et al., 1988). Threshold was determined as the lowest concentration at which five consecutive hits were achieved. The odorants vanillin and propionic acid were diluted in deionized water on a decismel (DS) scale dilution series [Odorant level in DS = 20log10 (test vapor concentration/reference concentration)] (Amoore, 1992), such that average detection threshold for 30 previously tested subjects = 0 DS. For each odorant, 24 dilutions were available, starting at –45 DS up to 65 DS (5 DS increments). In all measurements, a 40 s inter-trial-interval was used, and all experimental factors were randomized and counterbalanced.
Part 2: forced-choice detection
After baseline measurements were obtained, airflow characteristics were redetermined separately for each nostril during (rather than before and after) performance of an odorant forced-choice detection task. In each trial, the subject took one sniff through the odorant mask coupled to the rhinometer (Youngentob et al., 1986), and then judged whether an odorant was present or not. Each subject performed ten trials, four with no odorant, five with a low suprathreshold concentration of propionic acid (10 DS), and one with a high suprathreshold concentration of propionic acid (80 DS). To measure airflow parameters during performance of the task, methods were used according to Youngentob et al. (Youngentob et al., 1986). In brief, subjects sniffed through a nasal mask that was coupled to the rhinometer. At the coupling point was an interchangeable unit that consisted of a concentric tube with an inner perforated tube. The space between the tubes was filled with silica gel into which a given amount of the odorant dilution was absorbed. Three such separate interchangeable units plus mask were used, one for each concentration tested. In all tests, a 40 s inter-trial interval was used, and all experimental factors were randomized and counterbalanced.
Results
Part 1: baseline airflow and detection thresholds in each nostril
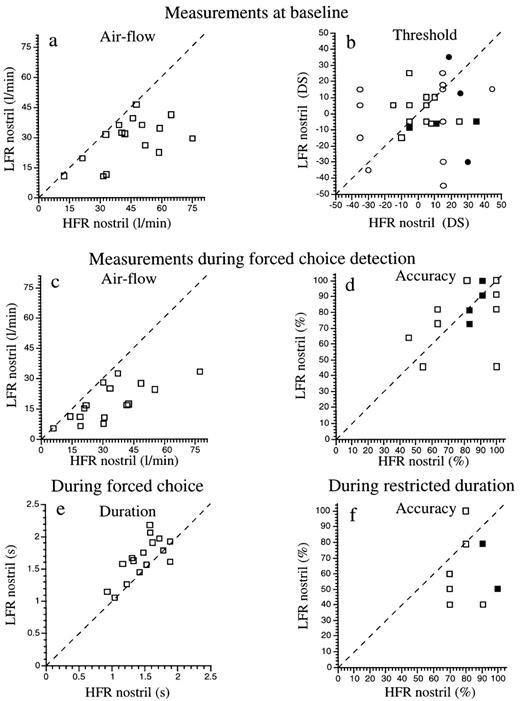
The results from three of the volunteers were discarded from analysis because the side of greater air flow-rate in these volunteers switched from one nostril to the other during the experiment. Of the remaining 17 volunteers, eight had greater air flow through the left nostril and nine had greater air flow through the right nostril. For these 17 subjects combined, air flow-rate was not greater overall in either the left or right nostril [left mean = 37 l/min, right mean = 46 l/min, t(16) = 1.46, P = 0.16], but was (by definition) significantly greater in the HFR in comparison to LFR nostril [HFR mean = 51 l/min, LFR mean = 32 l/min, t(16) = 4.7, P = 0.0002; Figure 1a]. Detection thresholds did not differ significantly for either odorant between the HFR and LFR [vanillin: HFR = 5.9 DS, LFR = –0.3 DS, t(16) = 1.35, P = 0.19; propionic acid: HFR = 7.6 DS, LFR = 0 DS, t(16) = 0.95, P = 0.35; Figure 1b], or the left and right [vanillin: left = 3 DS, right = 2.6 DS, t(16) = 0.06, P = 0.95; propionic acid: left = 4 DS, right = 3.6 DS, t(16) = 0.007, P = 0.99] nostrils.
Part 2: airflow and detection accuracy during the forced-choice detection task
One subject repeatedly violated the instructions by taking more than one sniff and was therefore discarded from further analysis. As at baseline, flow-rate remained significantly greater in the HFR nostril in comparison to the LFR nostril during performance of the task [mean HFR = 38 l/min, mean LFR = 22.5 l/min, t(15) = 3.53, P = 0.003; Figure 1c]. The difference in flow-rate between the nostrils during performance of the task was no different from that measured at baseline [compare Figure 1a to Figure 1c, t(15) = 0.6, P = 0.55]. Also, detection accuracy did not differ overall between the left and right [left mean = 86%, right mean = 74%, t(16) = 1.18, P = 0.25], or HFR and LFR [HFR mean = 81%, LFR mean = 79%, t(16) = 0.3, P = 0.7] nostrils in this task (Figure 1d). Sniff duration did not differ overall for the left or right nostril [t(15) = 1, P = 0.32], but was significantly longer when sniffing through the LFR as compared to the HFR nostril [t(15) = 2.35, P = 0.03; Figure 1e]. This increase in sniff duration from an average 1.66 s in the HFR nostril to an average 1.93 s in the LFR nostril (16%) was evident in 14 of the 16 subjects (binomial, P = 0.002). Larger differences in flow-rate between the nostrils correlated positively with larger differences in sniff duration between the nostrils during performance of the task (r = 0.59, P = 0.01; Figure 2). The increase in sniff duration in the LFR nostril versus the HFR nostril was evident for the entire data set when analyzed as a whole, as well as for the low concentration, high concentration and no-odorant conditions, when analyzed separately. In terms of volume, the increased duration of sniffing through the LFR nostril did not fully compensate for the difference in sniff flow-rate, resulting in an overall greater sniff volume in the HFR nostril during the task [HFR volume = 1340 cm3, LFR volume = 939 cm3, t(15) = 3.12, P = 0.007].
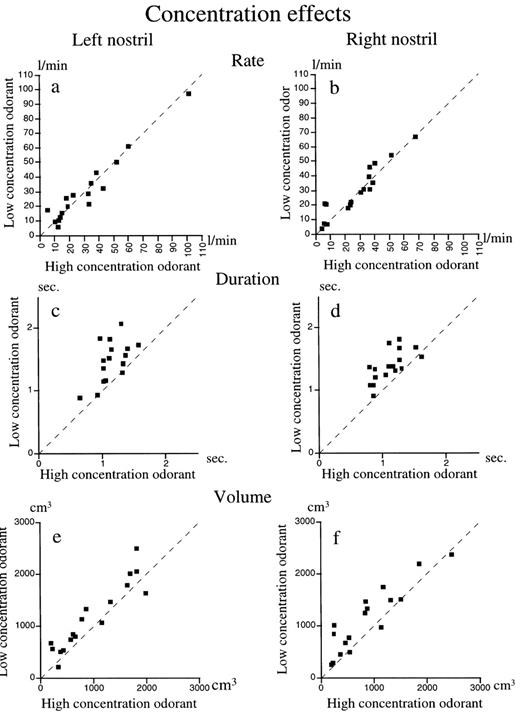
Odor concentration affected sniffing within each nostril in a predicted manner (Laing, 1983), regardless of HFR or LFR classification: sniff flow-rate did not change for either high or low concentration odorants [left, mean high concentration = 30.7 l/min, mean low concentration = 30.1 l/min, t(15) = 0.29, P = 0.77; right, mean high concentration = 28 l/min, mean low concentration = 29.8 l/min, t(15) = 1.18, P = 0.25]. In contrast, both sniff duration and volume decreased when sniffing the higher concentration odorant in comparison to the lower concentration odorant [duration: left, mean high concentration = 1.53 s, mean low concentration = 2 s, t(15) = 2.17, P = 0.04; right, mean high concentration = 1.45 s, mean low concentration = 1.82 s, t(15) = 4.46, P = 0.0005; volume: left, mean high concentration = 923 cm3, mean low concentration = 1121 cm3, t(15) = 3.17, P = 0.006; right, mean high concentration = 905 cm3, mean low concentration = 1158 cm3, t(15) = 3.56, P = 0.002; Figure 3].
Control experiments
Performance following limited duration
The above findings reject the ‘vigor’ model that predicted sniff flow-rate would change when sniffing through the LFR nostril during a detection task, but support the ‘duration’ model that predicted sniff duration would change when sniffing through the LFR nostril during a detection task.
To test whether elimination of this compensatory mechanism of change in sniff duration would hamper detection in the LFR nostril, ten subjects who did not participate in the previous tasks were tested on a two-alternative, forced-choice detection paradigm in an experimental design that restricted sniff duration to the same value for both nostrils. To choose sniff duration for each subject, subjects first performed the forced-choice detection task as described above (part 2). Sniff duration was then set to the average value for that subject’s HFR nostril (i.e. the shorter of the two durations). In this control experiment, methods were used as in Teghtsoonian et al. (Teghtsoonian et al., 1978). In brief, subjects were trained to initiate and terminate sniffs in response to an on-screen computer display. Simultaneous rhinomenometry testing showed that subjects were highly accurate at controlling sniff duration, with no subject reaching the exclusion criteria that was set at 5% of the sniff duration. In these trials where sniff duration was limited, each subject was tested using the odorant concentration that was determined as threshold for that subject in previous odorant detection threshold testing. Each subject performed five odorant trials in each nostril, that were randomly dispersed within 40 trials, of which 30 were not restricted in duration, thus rendering the limited duration sniffs a surprise occurrence.
Equalizing sniff duration between the nostrils to the value used by the HFR nostril led to a significant advantage in performance for the HFR in comparison to the LFR nostril [HFR mean accuracy = 83%, LFR mean = 65%, t(9) = 2.79, P = 0.02; Figure 1f]. A concentration that was reliably detected by both nostrils under conditions of unrestricted sniff duration, was reliably detected only by the HFR nostril when sniff duration was restricted to the same value. In other words, the compensatory mechanism we have described is necessary for maintaining optimum performance.
Duration of birhinal sniff
Under natural conditions, humans do not sniff one nostril at a time, and both nostrils sniff for the same duration during a given sniff. To test whether the duration of a natural birhinal sniff (BNS) is determined by the preferred duration of the HFR or LFR nostril, ten subjects who did not participate in the previous tasks were tested in the HFR, LFR and BNS. BNS duration was almost identical to the HFR nostril duration [BNS = 2.299 s, HFR = 2.275 s, t(9) = 0.23, P = 0.82] and both were significantly shorter than the LFR nostril duration [LFR = 2.512 s, LFR versus HFR: t(9) = 3.85, P = 0.004; LFR versus BNS: t(9) = 2.36, P = 0.04].
Discussion
Functional asymmetry between the nostrils can be addressed vis-à-vis differences between the left and right nostrils or between the HFR and LFR nostrils. That the HFR and LFR nostrils were symmetric, i.e. displayed equal detection thresholds in previous studies (Eccles et al., 1989), was a paradox in olfaction. Here we solved the paradox by revealing a compensatory mechanism employed by subjects in these tasks: subjects increased the duration of sniffs when using the LFR nostril, in order to achieve equivalent thresholds in both nostrils. Blocking this compensatory mechanism revealed a pronounced advantage in performance for the HFR nostril. Future comparisons of performance between the left and right or LFR and HFR nostrils in olfactory tasks, should either control for this compensatory mechanism (by equalizing sniff duration for both nostrils), or consider it in the interpretation of findings.
Regarding left/right nostril asymmetry, whereas this and the majority of other studies found no functional asymmetry in detection threshold (Schneider and Wolf, 1960; Koelega, 1979; Bellas et al., 1988; Eskenazi et al., 1988; Zatorre and Jones-Gotman, 1990; Shimomura and Motokizawa, 1995; Betchen and Doty, 1998), still other studies have reported such an asymmetry (Toulouse and Vaschide, 1899; Koelega, 1979; Youngentob et al., 1982). The reasons for these differences between studies remain unclear.
The mechanism revealed in this study is one of many used by the brain to cope with one of the greatest complexities of sensory perception, namely constancy. The brain must assess the external environment as constant using sensory apparatus that is constantly changing due to internal physiological changes. In the case of olfaction, air flow-rate is not only different across nostrils, but also changes overall constantly over time. For the olfactory system to continue to perceive a constant olfactory world in spite of these changes, compensatory mechanisms are needed. Here we have shown such a compensatory mechanism: when air flow-rate is reduced, sniff duration increases.
The olfactory system could theoretically compensate for reduced air flow-rate by either sniffing longer or sniffing stronger. Models of odorant transport in the nose suggest that ∼10% of nasal air flow is directed to the olfactory portion of the nasal passage, regardless of air flow-rate (Keyhani et al., 1995, 1997). This suggests that equal compensation may have been achieved by either increasing sniff duration or increasing sniff flow-rate. One may ask then why did the olfactory system opt for the former rather than the latter compensatory mechanism? One possibility is that this preference is odorant specific. Increasing flow-rate is expected to increase the perceived intensity of highly soluble odorants and decrease the perceived intensity of poorly soluble odorants (Keyhani et al., 1997). Therefore, and in contrast to subjects’ behavior in this study, compensation may be achieved by sniffing stronger rather than longer when using odorants of even higher solubility than those used here.
In a series of studies by Teghtsoonian and colleagues (Teghtsoonian et al., 1978; Teghtsoonian and Teghtsoonian, 1982, 1984) it was suggested that humans combine the information about sniff content with the information about sniff vigor to produce an invariant precept of odorant strength. In order accurately to estimate concentration, the olfactory system must know exactly what quantity it was that gave rise to a specific level of neural discharge. An equal duration high-flow-rate sniff of a low concentration or a low-flow-rate sniff of a high concentration may transport similar quantities of odorant to the olfactory receptors. Therefore, information regarding sniff vigor is essential in order to distinguish between such concentrations. The Teghtsoonian model was further supported by results from Youngentob et al. (Youngentob et al., 1986) and Hornung et al. (Hornung et al., 1997). Youngentob et al. tested the effects of odorant concentration, airway resistance, and the interaction of the two, on five sniff measures: (i) volume, (ii) duration, (iii) average flow-rate, (iv) peak flow-rate and (v) sniffing bout duration. Although none of the above interactions reached statistical significance, the only interaction that approached significance was that of airway resistance and sniff duration (F = 2.55). The latter is in agreement with the findings here. The findings of Teghtsoonian, Youngentob, Hornung and colleagues combine to suggest that information on sniff vigor is used to maintain olfactory constancy. The latter was demonstrated, however, by artificially modulating vigor only, either by altering subjects’ volitional sniffing behavior (Teghtsoonian) or by altering the airway resistance encountered by subjects (Youngentob). Here we allowed subjects to employ their own compensatory strategy and found that subjects prefer to modulate duration over vigor. This is not to say that sniff vigor is not a parameter of olfactory constancy, as it is the combination of the two—vigor and duration—that ultimately determines volume. Finally in this regard, when comparing our findings to previous studies on sniffing and olfactory constancy, one should bear in mind that whereas previous studies consisted of suprathreshold magnitude estimation tasks, our study consisted of detection tasks. It is possible that the compensatory mechanisms employed by subjects in a detection task are different from those potentially employed in a magnitude estimation task.
Our results combine with previous psychophysical and physiological findings in demonstrating that the sniff is a major component of the olfactory percept (Tucker, 1963; Teghtsoonian et al., 1978; Rehn, 1978; Teghtsoonian and Teghtsoonian, 1982, 1984; Laing, 1983; Tatchell et al., 1985; Youngentob et al., 1986, 1987; Mozell et al., 1984, 1991; Hornung et al., 1997). This is further evident in that information regarding air flow is represented in the olfactory cortex: recent functional imaging studies with humans have shown that both the somatosensory stimulation induced by air flow in the nostrils during a sniff and odorants per se, each induce activation in both primary olfactory regions within the ventral temporal lobe (Sobel et al., 1998a), and in the cerebellum (Sobel et al., 1998b). This activation may in part represent information regarding the air flow-rate being made available to the olfactory cortex for computation of overall stimulus concentration, and to the cerebellum for participation in the planning and execution of sniffs.
The finding that birhinal sniff duration is not long enough to satisfy the LFR nostril optimal duration suggests that during each sniff, each nostril conveys to the brain a different image of the olfactory world. At threshold levels, one nostril (the HFR) may have reached detection threshold, whereas the other nostril (the LFR) may have not. But are these different images superior (more concentrated) and inferior (less concentrated) images of the same olfactory content, or does each nostril convey different olfactory content? The following is a working hypothesis for the latter: for an odorant to act on the olfactory epithelium, it first must cross the olfactory mucosa. Different odorants have different mucosal sorption rates (Mozell and Jagodowicz, 1973). The effects of flow-rate on the magnitude of the olfactory response in the bullfrog can range from negative to positive, depending on how strongly the odorant in question sorbs to the mucosa (Mozell et al., 1991). A high-sorption-rate odorant will induce a greater response when flowing at a greater flow-rate relative to a lower flow-rate. This is because a high flow-rate enables the odor molecules to spread across a larger mucosal/epithelial surface area before they are all sorbed. At a slower flow-rate, the odorant would all be sorbed before it spread and a smaller portion of the receptor surface would be involved in the response (Mozell et al., 1991). In contrast, a low-sorption-rate odorant will induce a greater response when flowing at a lower flow-rate relative to a higher flow-rate. This is because at higher flow-rates, such an odorant would clear the nasal passage before maximal sorption could occur. Only at a low flow-rate would the odorant spend sufficient time within the nasal passage to maximally sorb (Mozell et al., 1991). This type of airflow-imposed mucosal activity pattern has been demonstrated in the rat as well [although within physiological limits, increases in flow-rate decreased the effect (Kent et al., 1996) and the mucosal odorant-specific activity patterns in the rat may be related in part to an odorant-inherent rather than to an airflow-imposed response pattern (Kent et al., 1995)]. Considering that in the human, during the same sniff, each nostril has a different flow-rate, the above findings suggest that perhaps the HFR nostril is more highly tuned towards high-sorption-rate odorants and the LFR nostril towards low-sorption-rate odorants. It remains to be shown how the olfactory system combines two competing olfactory images (different in either concentration or content or both) into a single unified olfactory percept.
Paired comparisons of data obtained from the high and low flow-rate nostrils. The HFR nostril is plotted on the horizontal axes and the LFR on the vertical axes. Thus, for measures that are greater in the HFR nostril, data points will accumulate below the dotted unit slope line, and for measures that are greater in the LFR nostril, data points will accumulate above the unit slope line. For measures that are equivalent across nostrils, data points will disperse around the unit slope line. Filled elements represent two subjects with identical scores. (a) Air flow-rate at baseline was significantly greater in the HFR in comparison to LFR nostril (by definition). (b) Detection thresholds did not significantly differ for either odorant between the HFR and LFR nostrils (circles = vanillin, squares = propionic acid). (c) Air flow-rate remained greater in the HFR nostril during the task. (d) Detection accuracy remained equal for both nostrils during the task. (e) Sniff duration was significantly longer in the LFR nostril in comparison to the HFR nostril during the task. (f) When duration was kept equal, i.e. the LFR nostril was prevented from sniffing for longer duration than the HFR nostril, LFR detection accuracy dropped significantly below detection accuracy in the HFR nostril.
The difference in flow-rate between the left and right nostrils in relation to the difference in sniff duration between the left and right nostrils for all subjects. The gray cross-lines represent the axes of symmetry, i.e. equal flow and duration in both nostrils. Percentage difference was computed by: [(left—right)/left] × 100 A larger difference in flow-rate predicted a larger difference in duration [F(1) = 8, R2 = 0.35, P = 0.01].
Paired comparisons of sniffs of a high concentration and sniffs of a low concentration odorant within the same nostril. Sniffs of the high concentration odor are plotted on the horizontal axes and sniffs of the low concentration odor on the vertical axes. Thus, for measures that are greater in the high concentration condition, data points will accumulate below the dotted unit slope line, and for measures that are greater for the low concentration condition, data points will accumulate above the unit slope line. For measures that are equivalent across odor concentrations, data points will disperse around the unit slope line. (a, b) Flow-rate remains equal in both nostrils for both concentrations. (c, d) Sniff duration is always greater for the low concentration in comparison to the high concentration. (e, f) Sniff volume is always greater for the low concentration in comparison to the high concentration.
This work was supported through NIH grant AA10723. R.M.K. wishes to thank the Samoan government for their support. We thank Ilana Hairston and Amnon Saltman for their thoughts and comments. We also thank Lubert Stryer for his comments, help and excellent advice. Finally, we thank Arak Elite.
References
Amoore, J.E. (
Bellas, D.N., Novelly, R.A., Eskenazi, B. and Wasserstein, J. (
Betchen, S.A. and Doty, R.L. (
Cain, W.S., Gent, J.F., Goodspeed, R.B. and Leonard, G. (
Eccles, R., Jawad, M.S. and Morris, S. (
Eskenazi, B., Cain, W.S., Lipsitt, E.D. and Novelly, R.A. (
Frye, R.E. (1995) Nasal airway dynamics and olfactory function. In Doty, R.L. (ed.), Handbook of Olfaction and Gustation. Marcel Dekker, New York, pp. 471–491.
Gilbert, A.N. (
Gilbert, A.N. and Rosenwasser, A.M. (
Hornung, D.E., Chin, C., Kurtz, D.B., Kent, P.F. and Mozell, M.M. (
Kent, P.F., Youngentob, S.L. and Sheehe, P.R. (
Kent, P.F., Mozell, M.M., Murphy, S.J. and Hornung, D.E. (
Keyhani, K., Scherer, P.W. and Mozell, M.M. (
Keyhani, K., Scherer, P.W. and Mozell, M.M. (
Laing, D.G. (
LeMagnen, J. (1945) Etude des Facteurs Dynamiques de L’Excitation Olfactive. L’annee Psychol., 44–45, 77–89.
Mirza, N., Kroger, H. and Doty, R.L. (
Mozell, M.M. and Jagodowicz, M. (
Mozell, M.M., Sheehe, P.R., Swieck, S.W., Kurtz, D.B. and Hornung, D.E. (
Mozell, M.M., Kent, P.F. and Murphy, S.J. (
Principato, J.J. and Ozenberger, J.M. (
Rehn, T. (
Schneider, R.A. and Wolf, S. (
Shimomura, J. and Motokizawa, F. (
Sobel, N., Prabhakaran, V., Desmond, J.E., Glover, G.H., Goode, R.L., Sullivan, E.V. and Gabrieli, J.D. (
Sobel, N., Prabhakaran, V., Hartley, CA., Desmond, J.E., Zhao, Z., Glover, G.H., Gabrieli, J.D. and Sullivan, E.V. (
Tatchell, R.H., Lerman, J.W. and Watt, J. (
Teghtsoonian, R. and Teghtsoonian, M. (
Teghtsoonian, R. and Teghtsoonian, M. (
Teghtsoonian, R., Teghtsoonian, M., Berglund, B. and Berglund, U. (
Toulouse, E. and Vaschide, N. (
Tucker, D. (
Youngentob, S.L., Kurtz, D.B., Leopold, D.A., Mozell, M.M. and Hornung, D.E. (
Youngentob, S.L., Stern, N.M., Mozell, M.M., Leopold, D.A. and Hornung, D.E. (
Youngentob, S.L., Mozell, M.M., Sheehe, P.R. and Hornung, D.E. (


![The difference in flow-rate between the left and right nostrils in relation to the difference in sniff duration between the left and right nostrils for all subjects. The gray cross-lines represent the axes of symmetry, i.e. equal flow and duration in both nostrils. Percentage difference was computed by: [(left—right)/left] × 100 A larger difference in flow-rate predicted a larger difference in duration [F(1) = 8, R2 = 0.35, P = 0.01].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/25/1/10.1093/chemse/25.1.1/2/m_7-99f02.jpeg?Expires=1716410717&Signature=Ym~GdL24QhbF~p-CNyjnwsj~IyphIaO-r~949tYICj1wpd3-cmF~3DX3Y8ooB8awALS6xQOgyiHy8Cz6-dbS1aKXMc0k4DP6lSkJzL4OMRtTwuOfQByUJclGWGdKvb5oEw4RcUr4PpR9C3YT~bExRognH1~x~Y0K3sDVbMXx0EqMZYcDczgAOnl8glDzUEvtDx0thySfuEbq8GZrlg7l1N~pjNRHz44OX7fvuIkM0myphHTbrIh8ZPsNLlcOoLCPabsiYvxOjoDF0cJWEXRGWw~aqxs8GQKzbuM-J9J8iBMhTv2i~bISiPK37TfpTLgChV-C1ZerDaW~lA9ryu0LsA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)