-
PDF
- Split View
-
Views
-
Cite
Cite
Itay Zelcer, Hagit Cohen, Gal Richter-Levin, Tom Lebiosn, Tomer Grossberger, Edi Barkai, A Cellular Correlate of Learning-induced Metaplasticity in the Hippocampus, Cerebral Cortex, Volume 16, Issue 4, April 2006, Pages 460–468, https://doi.org/10.1093/cercor/bhi125
Close - Share Icon Share
Abstract
Metaplasticity, the plasticity of synaptic plasticity, is thought to have a pivotal role in activity-dependent modulation of synaptic connectivity, which underlies learning and memory. Metaplasticity is usually attributed to modifications in glutamate receptor-mediated synaptic transmission. However, experimental evidence and theoretical considerations suggest that learning reduces the predisposition for further synaptic strengthening, while behavioral studies show that learning capability is enhanced by prior learning. Here we show that enhanced neuronal excitability in CA1 pyramidal neurons, but not enhanced synaptic transmission, occurs prior to rule learning of an olfactory discrimination task. This transient enhancement lasts for 1 day after rule learning, is apparent throughout the cell population and results from reduction in the medium and slow after-hyperpolarizations that control spike frequency adaptation. Such olfactory learning-induced increased excitability in hippocampal neurons enhances the rats' learning capability in another hippocampus-dependent task, the Morris water maze. Once olfactory discrimination rule learning is acquired, its maintenance is not dependent on the reduced post-burst AHP in hippocampal neurons. However, the enhanced spatial learning capability of olfactory-trained rats in the water maze is diminished once the post burst AHP in CA1 pyramidal cells resumes its initial value. We suggest that enhanced excitability of CA1 neurons may serve as a mechanism for generalized enhancement of hippocampus-dependent learning capability. In the presence of such enhanced neuronal excitability, the hippocampal network enters into a ‘learning mode’ in which a variety of hippocampus-dependent skills are acquired rapidly and efficiently.
Introduction
Predisposition for activity-induced strengthening and weakening of synaptic connections is highly dependent on the history of the synapse. It has been suggested that this phenomenon, termed metaplasticity, strongly affects long-term synaptic modifications that underlie learning and memory (Abraham and Bear, 1996). Although metaplasticity is usually attributed to activity-induced changes in glutamate receptors (Castellani et al., 2001; Sawtell et al., 2003), recent evidence raise the intriguing possibility that modifications in intrinsic neuronal properties, leading to enhanced neuronal excitability, are likely to underlie this phenomenon (for reviews, see Saar and Barkai 2003; Zhang and Linden, 2003). Enhanced neuronal excitability has been shown in hippocampal neurons following classical conditioning of the trace eye blink response or learning of the Morris water maze task (Moyer et al., 1996; Oh et al., 2003), and in piriform cortex neurons following olfactory discrimination learning (Saar and Barkai, 2003). This enhanced excitability is manifested in reduced spike frequency adaptation in response to prolonged depolarizing current applications (Moyer et al., 1996; Saar et al., 2001).
Neuronal adaptation is modulated by the medium and slow after-hyperpolarizations (AHPs), generated by potassium currents (Madison and Nicoll, 1984; Schwindt et al., 1988; Saar et al., 2001). Indeed, the AHP amplitude in hippocampal and cortical neurons is reduced after classical and operant conditioning (Moyer et al., 1996; Thompson et al., 1996; Saar et al., 1998, 2001; Oh et al., 2003). Learning-induced AHP reduction is transient. The AHP's amplitude returns to its initial value within days when training is suspended (Moyer et al., 1996; Saar et al., 1998). This recovery is not accompanied by memory loss (Moyer et al., 1996Saar et al., 1998). However, rule learning (manifested as the enhanced ability to acquire new memories rapidly and efficiently) is strongly correlated with reduced post-burst AHP; return of AHP to its initial value is accompanied by reduced learning capability. These data support the hypothesis that enhanced neuronal excitability underlies rule learning, but is not the biophysical trace of long-term memory for specific sensory inputs (Saar and Barkai, 2003).
It is still unclear whether such enhanced excitability occurs before the appearance of enhanced performance, and if such enhanced excitability is related only to acquisition of the rule or is crucial for its maintenance as well. Most importantly, the question whether enhanced neuronal excitability induced by one learning paradigm has any effect on learning of a different paradigm has to be addressed.
Rats demonstrate remarkable problem-solving capabilities based upon odor discriminations. These capabilities are greatly reduced by hippocampal damage (Staubli et al., 1986; Bunsey and Eichenbaum, 1996; Dusek and Eichenbaum, 1997), indicating that the hippocampus plays a major role in forming the representations of relations among odor memories (Eichenbaum, 1998).
Here we examine whether the induction and maintenance of olfactory discrimination (OD) rule learning is accompanied by enhanced excitability in hippocampal neurons, and whether such increased excitability may serve as a mechanism of metaplasticity, which is manifested by enhancing the animal's learning capabilities not only in the initial olfactory discrimination task but also in a novel hippocampus-dependent training paradigm.
Materials and Methods
Behavioral Methods
Olfactory Discrimination Training
Subjects.
Age-matched young adult Sprague–Dawly male rats were used. Prior to training they were maintained on a 23.5 h water deprivation schedule, with food available ad libitum.
Olfactory Discrimination.
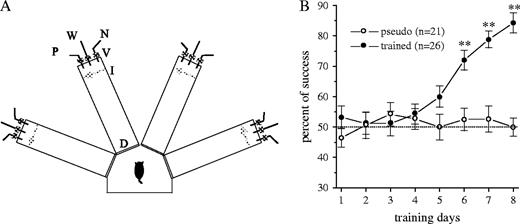
The olfactory discrimination training protocol was performed daily on each trained and pseudo-trained rat in a four-arm radial maze (Fig. 1A), with commercial odors that are regularly used in the cosmetics and food industries. Olfactory training consisted of 20 trials per day for each rat, as described in Figure 1A. In short, in each trial the rat had to choose between two odors (positive- and negative-cue) presented simultaneously. Rats designated to the trained group were rewarded upon choosing the positive cue. Rats in the pseudo-trained group were rewarded upon choosing any odor, in a random fashion. The criterion for completion of OD learning was at least 80% positive-cue choices in the last 10 trials of a training day, as was previously used (Saar et al., 1999, 2001). As we have previously reported (Saar et al., 1998, 1999), once the rats reach good performance with the first pair of odors, their capability to learn to discriminate between new pairs of odors increase dramatically; only 1 day of training is needed for reaching the criteria of 80% correct choices when a new pair of odors is presented. This enhanced learning capability suggests that when completing training with the first pairs of odors (after 7 or 8 day of training, as shown in Fig. 1B) rats acquire a rule which enables them to perform in the olfactory maze task rapidly and efficiently (Saar et al., 1998, 1999).
Training for odor discrimination. (A) Schematic representation of the olfactory maze. Protocols for ‘trained’ and ‘pseudo- trained’ rats were similar: an electronic ‘start’ command randomly opens two out of eight valves (V), releasing pressured airstreams with positive-cue odor (P) into one of the arms and negative-cue odor (N) into another. Eight seconds later, the two corresponding guillotine doors (D) are lifted to allow the rat to enter the selected arms. Upon reaching the far end of an arm (90 cm long), the rat's body interrupts an infrared beam (I, arrow) and a drop of drinking water is released from a water hose (W) into a small drinking well (for a ‘trained’ rat only if the arm contains the positive-cue odor; for ‘pseudo-trained’ rats randomly). A trial ends when the rat interrupts a beam, or in 10 s, if no beam is interrupted. A fan is operated for 15 s between trials, to remove odors. Each rat had 20 trials per day. (B) Learning curve for trained and pseudo-trained rats expressed as correct choices in the last 10 trials of the day. The learning curve shows a gradual improvement in performance in trained rats and no learning in pseudo-trained rats, which show no preference for any of the odors during the entire training period. Starting at day 6, there is a significant different between the performance of trained and pseudo-trained rats (**P < 0.01, independent Student's t-test). Values represent mean ± SE.
Rats in the naive group were water deprived, but not exposed to the maze. An important aspect of the OD task is that since the arm with the correct odor was determined randomly for each trial, learning the location of each arm relative to the other arms (e.g. spatial learning) did not give any information regarding the correct odor.
As the amount of drinking water awarded to each rat is modified by its performance in the odor-maze, trained rats get eventually somewhat more drinking water then rats in the control groups. However, this by itself is unlikely to have a significant effect, since all rats are given free access to drinking water for 30 min every day. Indeed, rats always finished drinking before the end of drinking time, implying that no more water was required at this time.
Water Maze Training
The procedure used was a modification of that described by Morris et al. (1986). Rats were trained in a circular pool 1.8 m in diameter and 0.6 m high, containing water at 26 ± 1°C. The pool occupied the center of a room and contained various salient cues. A 10 cm2 transparent platform was hidden in a constant position in the pool with its surface submerged 1 cm below the water level. The rats were given four consecutive training sessions per day, starting from random locations around the pool each time. Each session lasted up to 120 s. If the rat did not reach the platform within this time, it was guided to it. Each rat was allowed to remain on the platform for 30 s in order to learn its location. Each rat was trained for 4 days. The latency time to reach the platform was measured by using fixed overhead video recordings, analyzed off line.
Combined behavioral experiments, in which olfactory discrimination training was followed by water maze training one day after OD learning (Fig. 7A), were performed at two different locations, by two different teams of experimenters (training of 10 naive, 10 OD pseudo-trained and 10 OD trained rats was performed at the animal facility at BGU and the others at the animal facility at HU). All rats that were exposed to the water maze 3 days after OD training were trained at HU. The same person always trained the same rats in both the olfactory discrimination and the water maze tasks. As results of experiments in both locations were similar, data from the two training locations were combined.
Slice Preparation, Stimulation and Recording
Coronal brain slices were taken from 8 naive, 21 pseudo-trained and 28 trained rats at different times after the beginning of training; 400 μm coronal brain slices were cut from the dorsal hippocampus and kept in oxygenated (95% O2 + 5% CO2) Ringer's solution (in mM: NaCl 124, KCl 3, MgSO4 2, NaH2PO4 1.25, NaHCO3 26, CaCl2 2 and glucose 10). Intracellular recordings were performed at 36°C, with 4 M K-acetate-filled sharp glass microelectrodes. The identity of rats (naive, trained or pseudo-trained) from which the neurons were recorded was not known to the person conducting the experiments and measurements.
Intrinsic Neuronal Properties
Cell input resistance (Rin) was determined by linear regression fit to a voltage/current curve at the range from resting potential to −15 mV from resting. Spike width was measured at the spike threshold.
Post-burst AHP Measurements.
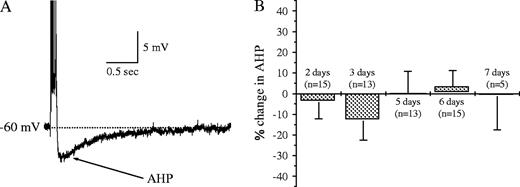
To standardize AHP recordings, cell membrane potential was depolarized to −60 mV with DC current application via the recording electrode, and AHP amplitude was measured following an additional 100 ms depolarizing current step that generated six action potentials (Fig. 2A), after which the DC depolarization was maintained. AHP amplitude was determined from an average of eight consecutive responses to stimuli applied once every 10 s. Under these conditions, a post-burst AHP that lasted up to several seconds appeared in all CA1 pyramidal neurons. The stimulus intensity applied to evoke a burst of six action potentials upon the 100-ms-long depolarizing pulse was adjusted to ensure that the last spike in the train would be evoked 10–15 ms before the end of the pulse. This procedure was undertaken to ensure that the firing frequency would be similar in neurons from all groups, so that the post burst AHP would not be affected by the firing frequency of the six action potentials train.
Post-burst AHP is not reduced after learning in neurons from pseudo-trained rats. (A) Post-burst AHP measurements in CA1 pyramidal neurons. Neurons were held at a membrane potential of −60 mV and an AHP was generated by a 100 ms depolarizing current step injection via the recording electrode, with intensity sufficient to generate a train of six action potentials. (B) AHP is not reduced in neurons from pseudo-trained rats. Amplitudes of AHPs recorded on different days after the beginning of training were compared with AHPs in naives. Presented are the averaged values for each day ± SE, each normalized to the averaged value of neurons from naive rats. The following number of rats was used for each of the days: three rats after 2 days of training, three rats after 3 days, five rats after 5 days, five rats after 6 days and two2 rats after 7 days. n, number of recorded cells.
Neuronal Adaptation Measurements.
At the neuron's resting potential, 1 s depolarizing current steps were injected into the cell body via the recording electrode in order to determine the current intensity needed to generate a single action potential (Ith). Adaptation was calculated from the response to a current step with intensity of Ith × 2. For each inter-spike interval (ISI), adaptation was defined as the ratio between the spike firing frequency at that interval, and the frequency of the first two spikes.
Post-synaptic Responses
Baseline Synaptic Responses.
For baseline synaptic potentials recordings, post-synaptic potentials (PSPs) were evoked in CA1 neurons by stimulating the Schaffer collaterals pathway. To standardize the PSP recordings, stimulus intensity was adjusted to evoke single EPSPs with amplitude of ∼10 mV (at Vm = −70 mV), and the responses in 10 consecutive trials were digitally averaged. PSP rise time was measured from 10 to 90% of the maximal voltage deflection, in a digital average of the 10 consecutive responses. PSP time constant of decay was measured by fitting a single exponential decay curve. PSP half-width is the time interval between points at the level of 0.5 of the PSP amplitude.
Paired-pulse Facilitation.
Paired-pulse facilitation (PPF) was evoked by pairs of stimuli applied at 50 Hz, with a 10 s interval between each pair. For measurements, the responses in 10 consecutive trials were digitally averaged. PPF was determined by calculating the ratio between the amplitudes of the second and first PSP (PSP2/PSP1) (Fig. 6E). As the second PSP sometimes overlapped the late part of the first PSP, its baseline was determined by digitally subtracting a trace of the average of 10 single PSPs, evoked in the same cell, from the averaged response to 10 double stimuli (Saar et al., 1999).
Statistical Analysis
An analysis of variance (ANOVA) was performed for all the separate days in each group. This procedure was followed by a between-group comparison of membrane properties, AHP amplitudes, instantaneous firing frequencies and performances in the water maze were done using a one-way ANOVA for the three learning groups, with post-hoc multiple t-tests for each pair of groups. Comparison of performances in the olfactory maze between trained and pseudo-trained rats was done by t-test. Values throughout the text are presented as mean ±SD.
Results
Olfactory Discrimination is Improved by the Sixth Day of Training
OD learning usually required 6–8 days of training for all rats to reach the criteria for rule learning. Figure 1B shows the time course of learning in 26 trained rats and the performance of 21 pseudo-trained rats. A significant difference in performance between the trained and the pseudo-trained group (which always performed at chance level) was apparent starting from day 6 onward. To examine the correlation between the time courses of olfactory discrimination learning and the appearance of intrinsic modifications in CA1 neurons, we studied biophysical properties of hippocampal neurons at different time intervals after the beginning of training.
Post-burst AHP is not Reduced in Neurons from Pseudo-trained Rats
As all rats exposed to the olfactory maze (trained and pseudo-trained groups) encountered a new environment when introduced to the training apparatus and the odors, hippocampal neurons could potentially have been modified in rats of both groups due to novel stimuli, regardless of the relation between the odors and the water reward. Therefore, we first examined whether the post–burst AHP in neurons from pseudo-trained rats is modified after olfactory learning by comparing neurons from these rats to neurons from naive rats. The averaged value of AHP in neurons from pseudo-trained rats, taken after 2–7 days of training, did not differ from that in neurons from naive rats (in mV, 3.73 ± 1.39, n = 61 in pseudo-trained compared with 3.74 ± 1.15, n = 17 in naives). We further examined the possibility that AHP amplitude is modified transiently in neurons from pseudo-trained rats at some point during training by examining the averaged amplitude of the AHP in neurons from pseudo-trained rats after each training day and comparing to the naive group. Averaged values of AHP amplitude for each training day in the pseudo-trained group were: 3.60 ± 1.17, n = 15 after two training days, 3.26 ± 1.25, n = 13 after three training days, 3.78 ± 1.42, n = 13 after five training days, 3.95 ± 1.23, n = 15 after six training days and 3.70 ± 1.34, n = 5 after seven training days. No significant difference was found between the pseudo-trained and the naive groups for any of the training days (Fig. 2B).
Post-burst AHP is Reduced in Neurons for Trained Rats Prior to Improvement in Performance
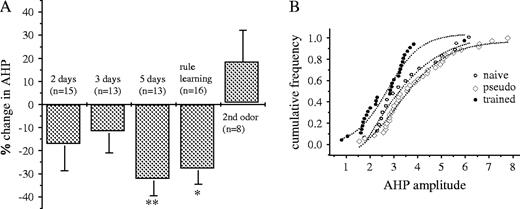
We next compared the AHP in neurons from trained rats with neurons from pseudo-trained and naive rats. Averaged values of AHP amplitude for each training day in the trained group were: 2.95 ± 1.47, n = 15 after two training days, 2.85 ± 1.00, n = 13 after three training days, 2.52 ± 0.86, n = 13 after five training days, 2.82 ± 1.20, n = 16 one day after OD learning (6 or 7 days of training for all rats in this time point). Comparison between neurons from trained and pseudo-trained rats was performed for each day; averaged values of AHP in neurons from trained rats one day after OD learning were compared with values in pseudo-trained rats after 7 days of training. During the first 3 days of learning, the averaged AHP amplitudes in neurons from trained rats did not differ from that of pseudo-trained and naives. However, starting from 5 days after the beginning of training and until rule learning (i.e. rats were trained for 5–7 days and AHPs were recorded on the next day), the AHP in neurons from trained rats was significantly smaller than from pseudo-trained ones (Fig. 3A). At time points when neurons from trained rats were significantly different from pseudo-trained ones, they also differed significantly from neurons in the naive group. Interestingly, 2 days after OD learning completion, the averaged AHP value in neurons from trained rats returned to the control levels (4.36 ± 1.42, n = 8) and did not differ from the averaged values of AHP in neurons from naive and pseudo-trained rats (Fig. 3A), even though training continued with a second pair of unfamiliar odors, which the rats learned within 1 or 2 days of training, as previously described (Saar et al., 1998).
Post-burst AHP in hippocampal neurons is reduced during olfactory discrimination learning. (A) Time window of reduction of AHP in neurons from trained rats. Amplitudes of AHPs recorded in neurons from trained rats on different days after the beginning of training were compared with AHPs in neurons from pseudo-trained rats. Presented are the averaged values for each day ± SE, each normalized to the averaged value of neurons from pseudo-trained rats for the same day. The following number of trained rats was used for each of the days: three rats after 2 days of training, five rats after 3 days, five rats after 5 days, four rats after 6 and 7 days (last day of training, rule learning), and four rats for 1 day after rule learning (second odor). n, number of recorded cells. *P < 0.05; **P < 0.01. (B) Cumulative frequency distribution of the AHP amplitudes in the three experimental groups. Each point represents the AHP amplitude in one cell. AHP amplitudes in neurons from trained rats, taken after 5–7 days of training, create a curve that is smoothly shifted to the left along the x-axis. Distributions of the amplitudes of neurons from pseudo-trained and naives are similar.
Reduced AHP after 5–7 days of training was apparent in most of the neurons recorded from trained rats (Fig. 3B), indicating that learning-induced AHP reduction is spread throughout the CA1 pyramidal neuron population.
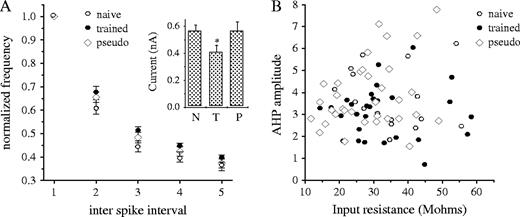
As the stimulus intensity of the 100 ms pulse was adjusted to ensure that the last spike in the six action potential train would be evoked 10–15 ms before the end of the pulse (see Materials and Methods), the averaged firing frequency was similar for the three groups throughout the train (Fig. 4A). Thus, the differences in post-burst AHP between neurons from trained rats and controls are not the consequence of different firing patterns. Notably, the averaged stimulus intensity needed to evoke the six action potentials train was significantly lower in neurons from trained rats (Fig. 4A, inset). This finding further suggests that the currents mediating the AHP are reduced after training.
AHP reduction is not a consequence of modified firing rate or input resistance. (A) Learning-induced AHP reduction is not the result of modified firing frequency during the 100 ms depolarizing pulse. Stimulus intensity was adjusted to evoke six action potentials, with the last spike occurring 10–15 ms before the end of the pulse. In these conditions, the averaged normalized frequency of all five ISIs was identical in all groups (firing frequency at each ISI along the train was normalized to the initial frequency at the train onset). However, the averaged stimulus intensity required to evoke the six action potentials train in was significantly lower in neurons form trained rats (inset). Values represent mean ± SE. Data were taken from 15 neurons recorded from naive rats 23 neurons recorded from trained rats (after 5–7 days of training) and 31 neurons from pseudo-trained rats that were exposed to the maze for the same period of time. Abbreviations: N, naive; T, trained; P, pseudo-trained. (B) The AHP amplitude is not directly related to the neuron's input resistance. For all three experimental groups no obvious relation exists between the AHP amplitude of each neuron and its input resistance.
Neuronal Adaptation is Reduced in Neurons from Trained Rats
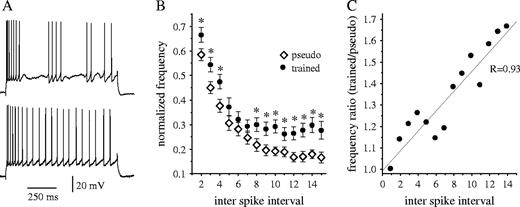
As the amplitude of the post-burst AHP reflects the magnitude of the potassium conductances that modulate the ability of neurons to generate repetitive spike firing, we examined whether learning-induced reduction in AHP is accompanied by reduced frequency adaptation. Neuronal adaptation was compared between 25 neurons recorded from trained rats (after 5–7 days of training) and 25 neurons from pseudo-trained rats that were exposed to the maze for the same period of time. When applying a 1 s intracellular pulse with stimulus intensity of twice the threshold current, responses varied from irregular patterns of repetitive firing interrupted by silent periods of various lengths (Fig. 5A, upper trace) to more continuous firing throughout the 1-s-long depolarizing pulse (Fig. 5A, bottom trace). Regardless of the firing pattern, the frequency of the first two spikes was always the highest. Neuronal adaptation differed considerably among neurons within each group. However, it was clearly reduced in neurons from trained rats. This reduction was apparent starting from the second inter-spike interval and was maintained for most of the response (Fig. 5B). Furthermore, the difference between neurons from trained and pseudo-trained rats increased monotonically with the prolongation of the response. In neurons from trained rats, the firing frequency remained almost stable starting from the seventh inter-spike interval, while neurons for pseudo-trained rats showed increased adaptation for as long as the spike firing persisted (Fig. 5B). Consequently, the ratio of neuronal adaptation between neurons from trained and pseudo-trained rats became more prominent as repetitive spike firing persisted (Fig. 5C), indicating that the differences in neuronal excitability increased when neurons were more active.
Neuronal excitability is enhanced in neurons from trained rats. (A) Two typical examples for neuronal firing patterns in layer CA1 pyramidal neurons taken from a pseudo-trained (upper trace) and a trained rat (lower trace). In response to th eapplication of prolonged depolarizing current steps via the recording electrode, with a stimulus intensity of Ith × 2, the cells fire trains of action potentials, with a spiking frequency that is highest at the onset of the pulse and decreases considerably thereafter. As shown for these two examples, neurons from trained rats had less spike frequency adaptation throughout the response. (B) A quantitative description of spike firing adaptation in pyramidal neurons from neurons of trained and pseudo-trained rats. With a stimulus intensity of Ith × 2, the firing frequency at each ISI along the train was normalized to the initial frequency at the train onset. Starting from the second interval, the averaged normalized frequency at most ISIs was significantly higher in neurons from trained rats (*P < 0.05). Values represent mean ± SE. Data was taken for 9 trained and 10 pseudo-trained rats. Twenty-five neurons were recorded for each group. (C) The ratio between the normalized frequencies of neurons of the trained and pseudo-trained rats increases linearly as a function of the inter-spike interval. Thus, as demonstrated with the two examples in (A), the difference in neuronal excitability between neurons from trained and control rats increases with prolongation of the stimulus.
Learning-induced AHP Reduction is not Due to Changes in Passive Membrane Properties
Post-burst AHP may have been reduced not only as a result of direct modifications in its underlying currents, but also as a consequence of modifications in the neurons' passive and active membrane properties, such as input resistance or action potential duration. Resting membrane potential (67.5 mV ± 2.8, n = 18 in naive, 67.7 ± 2.9, n = 31 in trained and 67.3 ± 2.5, n = 34 in pseudo-trained rats) and input resistance (34.9 ± 9.3 MΩ, n = 18 in naive, 32.5 ± 11.8, n = 31 in trained and 28.5 ± 10.2, n = 34 in pseudo-trained rats) did not differ among neurons from the three groups (neurons from trained and pseudo-trained rats taken 5–7 days after beginning of training). Moreover, for all three experimental groups no direct relationship was observed between the neurons' input resistance and its post-burst AHP amplitude (Fig. 4B).
However, for the same dates, the averaged action potential duration was significantly lower in neurons from trained rats (1.40 ms ± 0.16, n = 21 in naive, 1.26 ± 0.14, n = 47 in trained and 1.38 ± 0.24, n = 41 in pseudo-trained rats, P < 0.01). Notably, the reduced averaged width of single action potentials in neurons from trained rats was also observed after two days of training (1.15 ± 0.09 ms, n = 16). However, as the averaged AHP amplitude for the second training day did not differ among groups, this reduction in spike width was not sufficient by itself to cause a reduction in the averaged post-burst AHP in these neurons.
Enhanced Olfactory Discrimination Learning Capability is not the Result of Modifications in Synaptic Transmission in the CA1 Region
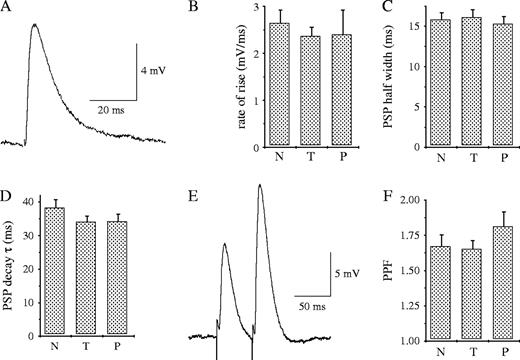
Previous work has shown that olfactory discrimination learning is accompanied not only by enhanced neuronal excitability of piriform cortex pyramidal neurons, but also by a series of later occurring physiological and morphological changes in synaptic transmission that enhance the connectivity between these neurons. These include enhanced synaptic release, as indicated by reduced PPF (Saar et al., 1999), reduced electrotonic distance along dendrites of pyramidal neurons, indicated by enhanced rate of rise of the PSPs, and increased spine density along dendrites of these neurons (Knafo et al., 2001). We have recently shown that spine density along apical dendrites of CA1 pyramidal neurons is increased 3 days, but not 1 day, after OD learning (Knafo et al., 2004). Here, we examined whether baseline synaptic transmission and PPF, evoked by stimulating the Schaffer collaterals, is modified during and after the first day of such learning. The properties of single, standardized (10 mV amplitude at Vm = −70 mV) postsynaptic potentials, evoked by stimulating the Schaffer collaterals pathway to these neurons, were similar in all groups (Fig. 6A–D). PPF was also not modified after 5–7 days of olfactory training (Fig. 6E,F). Thus, enhanced neuronal excitability is the only physiological modification observed in CA1 neurons during and one day after OD learning.
Baseline synaptic transmission and paired-pulse facilitation are not modified during and immediately after OD learning. (A) Example traces of standard PSPs evoked in a cell by stimulation of the Schaffer collaterals pathway (Vm = −70 mV). (B–D) Baseline synaptic transmission is not modified during days 5–7 of OD training. The averaged rate of rise, half width and time constant of decay of the single PSPs were similar for the three groups. (E) A typical response to pairs of stimuli applied at 0.1 Hz with an ISI of 50 ms. PPF was determined by calculating the ratio between the amplitudes of the second and first PSPs (PSP2/PSP1), in 10 consecutive responses, digitally averaged. (F) PPF is not modified during days 5–7 of OD training. Data were taken from five trained, five pseudo-trained and five naive rats. Thirteen neurons from naive rats, 19 neurons for trained rats and 15 neurons form pseudo-trained rats were recorded. Values represent mean ± SE.
Olfactory Discrimination Learning Enhances Learning in the Morris Water Maze
Learning-induced reduction in post- burst AHP in CA1 pyramidal neurons, similar to that described here, was recently reported to follow training in the Morris water maze (Oh et al., 2003). The intriguing finding that OD learning and water maze learning share similar enhancement in CA1 neuronal excitability raises the possibility that these two different learning paradigms may share a common mechanism. Thus, enhanced neuronal excitability may underlie a generalized enhancement of hippocampus-dependent learning capability. We examined this hypothesis by generating and testing the following prediction: if enhanced neuronal excitability of CA1 pyramidal neurons underlies a generalized increase in learning capabilities, then learning of one hippocampus-dependent task should enhance subsequent learning in a different hippocampus-dependent task. To test this hypothesis, we tested animals in the Morris water maze and compared the learning capabilities of olfactory discrimination-trained animals to the pseudo-trained and naive control groups.
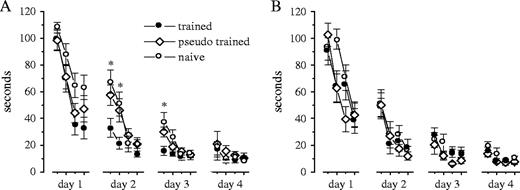
Training of the three groups in the water maze began on the same day in which the trained group demonstrated rule learning in the olfactory maze. Figure 7A shows that rats from the olfactory-trained group had a significant advantage over the rats from the two control groups, starting from the second day of training (i.e. 1 day after OD learning). In particular, rats from the two control groups showed a tendency towards decline in performance on the first trials of the second and the third days of training, compared with their performance on the last trial of the previous days. No such tendency was observed for rats of the trained group (Fig. 7A). On the last trail, rats of all groups were able to reach the platform in <10 s.
Behavioral consequences of post-burst AHP reduction. (A) OD learning in the olfactory maze enhances subsequent learning in the Morris water maze. Rats were exposed to the water maze on the same day that they acquired the rule in the olfactory maze. OD trained rats performed better then naive and pseudo-trained rats on the first two trials of day 2 and on the first trial of day 3 (*P < 0.05). Averaged values ± SE are shown for 22 naive, 21 trained and 19 pseudo-trained rats. (B) OD trained rats lose their advantage over control rats in water maze learning when the post-burst AHP resumes its control value. Training rats in the water maze after learning to discriminate between a second pair of odors (e.g. 3 days after rule learning, when the post-burst AHP resumes its initial value), results with performance similar to that of pseudo-trained and naive rats in the new task. Averaged values ± SE are shown for 17 naive, 17 trained and 17 pseudo-trained rats.
In a second experiment we tested whether the enhanced learning capability in the water maze disappears if water maze training begins only after rats are trained in the olfactory maze for a second pair of odors. Since by the time rats complete their training for the second odor pair the post-burst AHP in neurons from OD trained rats resumes its initial value (Fig. 3A), we expected that no difference would be evident in performance between the three groups. As shown in Figure 7B, in these conditions OD trained rats lose their advantage over the two control groups in learning the water maze task. In particular, they show the same tendency for decline in performance on the first trials of days 2 and 3 of training as the two control groups.
Discussion
In previous work, we showed that OD learning is accompanied by reduced post-burst AHP and enhanced neuronal excitability in piriform cortex pyramidal neurons (Saar et al., 2001; Saar and Barkai, 2003). Here we show that similar modifications occur also in CA1 hippocampal neurons just prior to and during rule learning of the same task. However, they are not required for maintenance of the rule. Furthermore, once enhanced neuronal excitability is induced in CA1 pyramidal neurons, rats perform better not only in the olfactory discrimination task in which they were trained, but also demonstrate enhanced learning capability in a novel hippocampus-dependent task — the Morris water maze. These data suggest that learning-induced modifications in intrinsic membrane properties, manifested as enhanced neuronal excitability, may serve as a cellular mechanism for metaplasticity, in the sense that they induce a general enhancement of hippocampus-dependent learning.
Effect of Learning on CA1 Neurons' Post-burst AHP and Firing Patterns
Enhanced firing frequency in neurons from trained rats was apparent immediately after the onset of the intracellular pulse (Fig. 5B), and the difference in neuronal excitability between neurons from trained and pseudo-trained rats became more pronounced as spike firing continued (Fig. 5C). These data indicate that the differences in excitability between neurons from trained rats and controls in hippocampal neurons are larger than those observed in the piriform cortex (Saar et al., 2001), and become more prominent with the prolongation of hippocampal activation.
Three types of AHP, fast, medium and slow, were described in CA1 neurons, based on their latencies to onset and duration (Sah and Faber, 2002). That the duration of a single action potential is shorter in neurons from trained rats suggests that the fast AHP is enhanced after olfactory learning. Notably, previous studies report that other forms of hippocampus-dependent learning are not accompanied by such a change in the action potential width (Moyer et al., 1996; Oh et al., 2003). The medium AHP has an onset latency of several milliseconds and peaks after 50 ms, and the slow AHP peaks at hundreds of milliseconds (Sah and Faber, 2002). Thus, a reduction in both the medium and slow AHP could account for the reduced spike frequency adaptation observed in neurons from trained rats.
In light of previous work showing that, following olfactory learning, acetylcholine loses its ability to modulate the post-burst AHP (Saar et al., 2001), one possible explanation for the learning-induced long-term reduction in the AHP is a long-lasting form of neuromodulation. However, studies performed with several learning models suggest that loss of cholinergic effect should be attributed to internalization of muscarinic receptors in hippocampal neurons (Van der Zee and Luiten, 1999). Moreover, acetylcholine was also shown to reduce synaptic release and thus enhance PPF in the Schaffer collaterals pathway (Hasselmo and Schnell 1994). A similar effect of noradrenalin was shown in piriform cortex neurons (Linster and Hasselmo, 2001). Here we show that PPF does not differ between slices from trained and control rats. This finding further suggests that cholinergic and adrenergic levels are not higher in slices from trained animals and that the reduction in post-burst AHP is not the consequence of enhanced neuromodulation.
Behavioral Relevance of Enhanced Neuronal Excitability
The prolonged training period (6–7 days) needed to complete rule learning in the olfactory discrimination task allows for a detailed comparison of the time courses in which the behavioral and cellular modifications appear and disappear. Such analysis is useful for revealing the particular phase of rule learning for which enhanced neuronal excitability is significant. AHP reduction and reduced neuronal adaptation occurred throughout the sampled CA1 neuronal population 1 day before rats showed any sign of enhanced learning capability in the olfactory maze. However, these intrinsic modifications were outlasted by enhanced learning capability. The averaged post-burst AHP in hippocampal neurons resumed its initial value within 2 days after OD learning, despite continuation of training with a new pair of odors. We have previously shown that rule learning is maintained for many weeks, as long as olfactory discrimination learning is continued (Saar et al., 1998). The differences in time courses and conditions by which the reduced AHP disappears in hippocampal and piriform cortex neurons following OD learning testify for the different roles of these two brain areas in acquiring and maintaining the enhanced OD learning capability; enhanced neuronal excitability in the hippocampus occurs prior to enhanced learning capability and disappears 1 day after the rule leaning, regardless of whether OD training is continued. In sharp contrast, enhanced neuronal excitability in the piriform cortex is maintained for as long as training with new pairs of odors is continued, and when training is suspended for 4 days or more the AHP returns to its pre-training value and the OD learning capability is reduced (Saar et al., 1998; Saar and Barkai, 2003). Hence, while enhanced neuronal excitability in piriform cortex neurons is essential for the maintenance of enhanced olfactory learning capability (Saar et al., 1998), similar modifications in CA1 neurons are crucial only for acquisition of the rule. Thus, our data support the notion that the hippocampus and the piriform cortex have different roles in OD learning; while the hippocampus fulfills the ‘higher’ role of acquiring the rule, once it is acquired it is preserved in the primary olfactory cortex for as long as its maintenance is essential (Saar et al., 1998).
Lack of Effect on Hippocampal Synaptic Transmission
Metaplasticity is usually attributed to activity-induced changes in glutamate receptors, triggered by calcium entry through the NMDA channels (Crump et al., 2001; Castellani et al., 2001; Gisabella et al., 2003; Mockett et al., 2002; Philpot et al., 2003; Sawtell et al., 2003; Tompa and Friedrich, 1998; Van Dam et al., 2004). Indeed, olfactory learning was shown to be accompanied by enhanced synaptic transmission between piriform cortex pyramidal neurons (Barkai and Saar, 2001). Enhanced synaptic release is indicated by reduced PPF (Saar et al., 1999), enhancement of post-synaptic potentials in pyramidal neurons is indicated by enhanced rate of rise of the PSPs (Saar et al., 2002) and enhanced connectivity is indicated by an increased number of spines along apical dendrites of these neurons (Knafo et al., 2001). However, these modifications only appear three days after OD learning (Knafo et al., 2001; Saar et al., 1999, 2002) and are subsequent to enhanced neuronal excitability in piriform cortex pyramidal neurons (Saar et al., 1998).
A similar sequence of events is apparent in the hippocampus: The generalized enhancement in hippocampus-dependent learning capability occurs one day after olfactory learning. At this time point, baseline synaptic activity, as well as paired pulse facilitation, is not enhanced, spine density along dendrites of pyramidal neurons is not yet increased (Knafo et al., 2004), and the subunit composition of the NMDA receptor is similar in the hippocampi of olfactory trained rats and their controls (Quinlan et al., 2004). Moreover, learning-induced potentiation of synaptic strength is accompanied by an increase in the threshold for further synaptic enhancement (Rioult-Pedotti et al., 2000; Lebel et al., 2001; Quinlan et al., 2004). Such increased threshold is at least partially mediated by a change in the subunit composition of the NMDA receptor that reduces the NMDA channel calcium conductance (Zinebi et al., 2003; Quinlan et al., 2004).
These results agree well with the sliding modification threshold theory, which suggests that LTP induction should be more difficult in more ‘experienced’ synapses (Bear, 1996). However, the results raise a central question: if hippocampus-mediated learning is accompanied by reduced predisposition for further activity-induced synaptic strengthening in this area, what is the mechanism underlying the learning-induced general enhancement in hippocampus-dependent learning capability? Taken together, these data and theoretical considerations indicate that enhanced neuronal excitability is a major widespread learning-induced modification that can enhance the computational power of the hippocampal network, although the possibility of the existence of currently unknown synaptic modifications can not be excluded.
Enhanced Neuronal Excitability and Metaplasticity
Unlike synaptic modifications, which can be restricted to specific synapses, a change in the neuron's firing patterns would affect many (if not all) of its synaptic connections with neighboring cells. Thus, widespread modifications in neuronal excitability can be expected to have a profound effect on the network composed from these neurons. Specifically, according to Hebb's rule (Hebb, 1949), when most of the excitatory neurons in the network fire more action potentials in response to afferent stimuli, activity-dependent synaptic modifications are more likely to occur. Namely, enhanced excitability would directly affect synaptic plasticity. The finding that enhanced neuronal excitability induced by one training paradigm enhances the learning capability of the animal in a second, seemingly unrelated paradigm strongly supports the idea that such modifications in intrinsic neuronal properties serve as a mechanism of metaplasticity. This notion is further supported by the finding that training rats in the water maze after the olfactory discrimination induced reduction in the post-burst AHP fades out, results with the loss of the advantage that olfactory discrimination trained rats have over the naive and pseudo-trained rats in learning the new task.
In conclusion, our data suggest that enhanced neuronal excitability in CA1 hippocampal neurons, which results from reduction in potassium currents that control neuronal adaptation, underlies the induction of rule learning. Moreover, although enhanced learning capability is mediated by the same biophysical modifications in the piriform cortex and the hippocampus, the behavioral consequences of these modifications are far more reaching when induced in the hippocampus. Enhanced neuronal excitability in hippocampal neurons enables a general enhancement of hippocampus-dependent learning by enhancing the creation of rule learning of novel tasks. In the presence of such enhanced neuronal excitability, the hippocampal network enters into a ‘learning mode’ in which a variety of hippocampus-dependent skills are acquired rapidly and efficiently.
Supported by grant from the Israel Science Foundation to E.B.
References
Abraham WC, Bear MF (
Barkai E, Saar D (
Bear MF (
Bunsey M, Eichenbaum H (
Castellani GC, Quinlan EM, Cooper LN, Shouval HZ (
Crump FT, Dillman KS, Craig AM (
Dusek JA, Eichenbaum H (
Gisabella B, Rowan MJ, Anwl R (
Hasselmo ME, Schnell E (
Knafo S, Grossman Y, Barkai E, Benshalom G (
Knafo S, Ariav G, Barkai E, Libersat F (
Lebel D, Grossman Y, Barkai E (
Linster C, Hasselmo ME (
Madison DV, Nicoll RA (
Mockett B, Coussens C, Abraham WC (
Morris RGM, Anderson E, Lynch GS, Baundry M (
Moyer JR, Thompson LT, Disterhoft JF (
Oh MM, Kuo AG, Wu WW, Sametsky EA, Disterhoft JF (
Philpot BD, Espinosa JS, Bear MF (
Quinlan E, Lebel D, Brosh I, Barkai E (
Rioult-Pedotti MS, Friedman D, Donoghue JP (
Saar D, Barkai E (
Saar D, Grossman Y, Barkai E (
Saar D, Grossman Y, Barkai E (
Saar D, Grossman Y, Barkai E (
Saar D, Grossman Y, Barkai E (
Sah P, Faber ES (
Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF (
Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE (
Staubli U, Fraser D, Kessler M, Lynch G (
Thompson LT, Moyer JR, Disterhoft JF (
Tompa P, Friedrich P (
Van Dam EJ, Kamal A, Artola A, de Graan PN, Gispen WH, Ramakers GM (
Van Der Zee EA, Luiten PGM (
Zhang W, Linden DJ (
Author notes
1Department of Physiology, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel, 2Mental Health Center, Faculty of Health Sciences and Zlotowski Center for Neuroscience, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel, 3Department of Psychology, University of Haifa, Haifa 39105, Israel and 4Department of Neurobiology and Ethology, Faculty of Sciences, University of Haifa, Haifa 31905, Israel