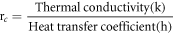Abstract
Efficient and secure operation of electric vehicles relies significantly on the cooling system for lithium iron phosphate (LiFePO4) batteries, a key component in electric vehicle technology. One of the critical challenges faced by electric vehicle is battery cooling to ensure optimal performance, extended battery life, and enhanced safety. The excessive heat generated during battery operation can lead to decrease in performance and potential safety hazards. Conventional cooling systems, such as air or liquid cooling, have limitations in terms of their cooling capacity, weight, and complexity. Therefore, there is a need to explore innovative cooling techniques that can effectively reduce the thermal issues associated with Electric Vehicles LiFePO4 batteries. Employing a thermal insulating coating and phase change material at the critical thickness emerges as an innovative approach to mitigate the surface temperature of battery cells. This is evident during the charging phase, where the bare cell, Teflon-insulated, and paraffin wax-coated cells reached respective peak temperatures of 69 °C, 57 °C, and 53.3 °C. Notably, the Teflon-coated cell exhibited a 17.39% reduction in peak temperature compared to the bare cell, while the paraffin wax-coated cell displayed a more substantial 23.18% reduction. A similar temperature reduction trend is observed during the discharging phase of the battery cell. Utilizing insulating materials or phase change materials with a critical thickness significantly lowers surface temperatures, enhancing the safety of the battery cell and ensuring prolonged life.
Export citation and abstract BibTeX RIS
1. Introduction
The lithium iron cell, commonly referred to as a Lithium Iron Phosphate cell, is a rechargeable battery that utilizes lithium iron phosphate (LiFePO4) as its cathode material. In the chemical composition, the cathode consists of lithium iron phosphate, the anode comprises a graphitic carbon electrode with a metallic backing, and the electrolyte is composed of an organic solvent containing lithium salt. LiFePO4 cells offer several advantages. Firstly, they boast a long lifespan, typically ranging from 2000 to 5000 cycles, surpassing other lithium-ion batteries. Additionally, they exhibit high safety levels, being less susceptible to thermal runaway due to the inherent stability of LiFePO4. Fast charging capability without causing damage to the battery, a wide operating temperature range suitable for diverse applications, and environmental friendliness by avoiding cobalt or nickel content is also notable advantages.
However, there are certain drawbacks associated with LiFePO4 cells. They have a lower energy density compared to other lithium-ion batteries, meaning they store less energy per unit volume. Moreover, the cost of LiFePO4 cells is generally higher than that of other lithium-ion batteries.
The applications of lithium iron cells are diverse and include electric vehicles hybrid electric vehicles, energy storage systems, marine applications, industrial applications, and medical devices. Despite their drawbacks, the unique combination of safety, longevity, and environmental considerations positions LiFePO4 cells as a compelling choice for specific use cases in the realm of rechargeable batteries.
Maintaining the efficient and safe functioning of electric vehicles relies heavily on the essential consideration of Electrical Vehicle battery cooling. Among the critical challenges challenging electric vehicles, effective battery cooling stands out as indispensable for achieving and sustaining optimal performance, prolonged battery life, and heightened safety standards. The substantial heat generated during battery operation poses a dual threat diminishing performance and introducing potential safety hazards. Traditional cooling systems, such as air or liquid cooling, encounter inherent limitations concerning cooling capacity, weight, and overall complexity. Consequently, a compelling imperative arises to delve into the realm of innovative cooling techniques capable of markedly mitigating the thermal challenges inherently associated with Electric Vehicle batteries. In doing so, advancements in cooling methodologies can pave the way for superior performance, increased safety, and extended longevity of Electric Vehicle battery systems.
Jindal et al [1] developed a customized Lithium Nickel Manganese Cobalt Oxide based battery pack, employing a Finite Element model and simulating it with a coolant containing 0.001 vol% and 0.005 vol% Graphene Nano platelets in a 50:50 mixture of Ethylene Glycol (EG) and water. The goal was to evaluate the efficacy of lowering the operating temperature within the battery pack. Three variations of the battery pack, configured as one tier, two tier, and three tier systems, were simulated to optimize the effectiveness and surface contact of the flowing coolant with the heated batteries. The simulations, conducted over a 15 s period of fluid flow with the pack's temperature set at 60 °C, revealed a 12% to 24% reduction in the operating temperature range for 0.001 vol% GNP/EG/water fluid and 24% to 29% for 0.005 vol% GNP/EG/water fluid compared to pure EG/water fluid. The enhanced cooling capacity was attributed to the high thermal conductivity and larger surface area of GNPs. The redesigned two and three-tier models facilitated a more uniform flow of the GNP/EG/water coolant, keeping the temperature within the recommended operational range for Lithium-Ion Batteries (LiFePO4) used in Electric Vehicles Zhao et al [2] addressed the limitation of low thermal conductivity in pure Phase Change Material (PCM) commonly used in Battery Thermal Management Systems. They enhanced the thermal conductivity of PCM by incorporating copper foam and active liquid cooling. The copper foam/paraffin composite PCM was studied using a three-dimensional numerical model and the impact of velocity of liquid inlet and material module on Battery Thermal Management Systems performance was examined. The study showed a significant improvement in thermal management using and liquid cooling, resulting in a 14 °C lower cell temperature. Shelkea et al [3] identified the Thermal Runaway (TR) onset temperature of a 21700 lithium-Ion Battery (LiFePO4) to be between 131 °C and 132 °C. The study observed similar heat generation patterns from the decompositions of Solid Electrolyte Interface and anode, while cathode heat generation increased sharply near the maximum cell surface temperature. Optimizing the cathode material was suggested to potentially delay TR onset temperature, and the time to maximum cell surface temperature decreased rapidly with an increase in heating power Sun et al [4] explored enhanced Lithium-Ion Batteries (LiFePO4) battery thermal management using phase change materials embedded in copper foam. The copper foam-PCM solution significantly improved heat dissipation and temperature uniformity, reducing the maximum temperature rise by 10.4% during intense operation. The cell-to-pack conversion efficiency with the copper foam-PCM cooling system was favourable, suggesting its effectiveness for Lithium-Ion Batteries (LiFePO4) batteries in electric vehicles and energy storage devices. Farulla et al [5] presented a systematic framework for designing passive and hybrid thermal management systems for Lithium-Ion Batteries (LiFePO4) batteries. Their study highlighted optimal passive and hybrid PCM cooling designs that reduced maximum cell temperature, emphasizing their potential for improving battery safety. Huang et al [6] focused on improving battery thermal management using phase change materials with core-shell microcapsules. The study synthesized composites with carbon nanotubes, exhibiting promising results for enhancing thermal conductivity and cooling efficiency in battery applications. Liu et al [7] presented a hybrid battery thermal management system combining Thermoelectric Coolers and PCM. The study emphasized the importance of PCM in achieving better temperature uniformity and identified optimal configurations for enhanced thermal performance. Verma et al [8] investigated geometric and thermo-fluidic parameters of a liquid coolant flowing through mini-channels in an electric vehicles battery. The study identified optimized parameters leading to a minimum temperature difference in the battery and reduced power consumption for reliable battery performance. Yang et al [9] introduced an innovative battery thermal management system featuring a Battery Thermal Cooling Module with Composite Phase Change Material and a carbon fiber thermally conductive gasket. The study demonstrated significant cooling benefits, offering effective temperature control for stable battery operation. Youssef et al [10] concentrated on novel and eco-friendly heat transfer coolant mediums for battery thermal management. The study reviewed traditional and novel coolant mediums, discussing their potential for enhancing thermal performance with higher safety and lower environmental impact. Wu et al [11] demonstrated the influence of heat generation performance on battery thermal management, highlighting the importance of technical parameters for a promising thermal management solution. Ali et al [12] Conducted a comprehensive review of BTMS, analysing design considerations, thermal stability, and optimization of various models. The study emphasized the critical role of BTMS optimization for the success of electric vehicles. Hosseinpour et al [13] numerically analyzed the melting process of PCM in a spiral coil, considering different fin configurations and percentages of hybrid nanoparticles. The study revealed the dominant effect of extra fins on melting time and the influence of inlet temperature on PCM melting. Moosavi et al [14] The key findings of the study include an evaluation of the effect of cell spacing on the maximum temperature within the cells. The results show a positive correlation between cell spacing ratios (transverse and longitudinal pitch ratios) and the maximum temperature within the cells. Additionally, the study explores the impact on temperature differences within the module and temperature uniformity, providing insights for balancing temperature rise and thermal gradients. The paper contributes valuable information on optimizing large-scale air-cooled battery systems by considering cell spacing as a crucial parameter. The combination of analytical and numerical models offers a comprehensive understanding of thermal behaviour, making it a relevant and insightful contribution to the field of energy storage and thermal management systems. Mahmud et al [15] this review delves into the crucial realm of thermal management for lithium-ion batteries (LiFePO4) in electric vehicles, emphasizing the role of phase change materials (PCMs). The authors thoroughly analyse various aspects of PCM-based thermal management systems, encompassing topics such as PCM selection, system configurations, and the effects of different parameters on thermal performance. The paper provides key insights by synthesizing information from diverse sources, shedding light on advancements, challenges, and potential applications of PCM-based solutions in enhancing the thermal performance of lithium-ion batteries (LiFePO4), particularly in the context of electric vehicles. Alqaed et al [16] the study likely explores the effectiveness of incorporating organic PCM in mitigating heat generation and regulating temperatures within the prismatic lithium battery pack. The paper is anticipated to discuss the experimental or numerical methods employed, the specific organic PCM utilized, and the observed impact on thermal performance. The paper contributes to the field of thermal management for lithium battery (LiFePO4) packs by examining the application of organic PCM. Ni et al [17] this study likely explores the use of PCM to mitigate thermal runaway incidents in batteries. The paper is expected to discuss experimental or numerical findings regarding how PCM can prevent or suppress thermal runaway, providing valuable insights into enhancing battery safety. The paper contributes to the understanding of thermal management strategies in batteries, specifically focusing on the role of phase change materials in preventing and suppressing thermal runaway incidents. The findings may have implications for improving the safety and reliability of battery systems. Wang et al [18] the research explores the application of a two-phase nanofluid flow with phase change materials to enhance the thermal performance of lithium batteries. Additionally, the paper incorporates machine learning techniques for optimizing horizontal and vertical distances between batteries. The paper contributes to the field of battery thermal management by investigating the effectiveness of a novel two-phase nanofluid approach with phase change materials. The integration of machine learning for optimizing battery spacing adds a unique dimension to the study, offering insights into potential advancements in battery system design and thermal control. Hossein Maleki et al [19] the study presents experimental thermal property data for the Sony US-18650 lithium-ion battery and its components, along with the techniques used for measuring thermal properties. The properties examined include specific heat capacity, thermal diffusivity and thermal conductivity both in the presence and absence of electrolyte The battery's heat capacity is reported at an open-circuit voltage of 2.75 V and 3.75 V, showcasing values of Cp. In the absence of electrolyte, the study observes an increase in thermal conductivity, with open-circuit voltage for both the negative electrode and the positive electrode (PE). Specifically, for the negative electrode, there is a 26% increase as the open-circuit voltage rises from 2.75 to 3.75 V, while for the positive electrode; the increase is in the range of 5 to 6%. The qualitative explanation for the dependence of specific heat and thermal conductivity on open circuit voltage is linked to the impact of lithiation and delithiation on the electron carrier density. This leads to n-type semi conduction in the graphitic negative electrode material and a shift from semiconducting to metallic character in the positive electrode material, collectively resulting in an increase in specific heat and thermal conductivity with open circuit voltage. Li, Yang, et al [20] explore battery thermal management through extensive simulations, evaluating different cooling strategies' effectiveness under high-rate discharging and external shorting conditions. Utilizing three-dimensional modelling, the research offers crucial insights into battery performance under extreme scenarios, facilitating the development of safer and more efficient battery systems. Additionally, the properties of a LiFePO4 battery cell are outlined, including a specific heat capacity of approximately 1200 J kgK−1, low thermal conductivity of 3 W mK−1, and a density of 2700 kg m−3.
Previous research has delved into the advantages of incorporating phase change materials (PCMs) to enhance the thermal management of battery cells. However, there exists a notable research gap pertaining to the durability and real-world implications of these materials within battery systems. Improving heat dissipation from the battery cell contributes to heightened durability and safety. The on-going experimental analysis is specifically concentrating on assessing the impact of utilizing critical thickness for insulating material and phase change material to address these concerns.
2. Experimental analysis in thermal management of LiFePO4 batteries
2.1. Material selection
The selection of Teflon and paraffin wax as coating materials for LiFePO4 battery cells is based on specific properties and performance considerations. The uniqueness of this choice lies in the fact that paraffin wax serves as a phase change material, while Teflon acts as an insulating material. While alternative materials could be considered, the decision to opt for these two is compelled by the following attributes they offer.
Teflon, known for its high non-reactivity and chemical inertness, remains unresponsive to the battery components and electrolyte. This characteristic ensures stability, preventing detrimental chemical reactions that might compromise the battery over time. With an impressive ability to withstand a broad temperature range without degradation, Teflon significantly contributes to the overall thermal stability of the battery. Its relatively high melting point, around 327 °C, adds to its stability at elevated temperatures. Moreover, Teflon exhibits low thermal conductivity and a minimal coefficient of thermal expansion. The material Teflon is sourced from 'Jayhind Polymers,' located in Sangli.
In contrast, Paraffin wax displays variable melting points, typically falling within the range of 37 to 70 °C, contingent on its specific type or blend. This material boasts low thermal conductivity paired with a notable heat storage capacity. Its effectiveness lies in the absorption and release of heat, facilitating efficient temperature management within the battery cell. The phase-change property of Paraffin wax, transitioning from solid to liquid at a specific temperature, is harnessed to absorb and store heat during operation, acting as a thermal buffer to prevent overheating. Additionally, Paraffin wax serves as a protective coating, sealing the battery cell and shielding internal components from external factors such as moisture or contaminants. Paraffin wax is procured from 'Mandira Products,' a candle store situated in Sangli.
Although PCM materials have garnered significant attention in battery thermal management literature, there is a scarcity of research exploring PCM materials in conjunction with critical insulation thickness. Teflon, despite being a noteworthy material, has received limited attention in research pursuits. This study seeks to combine PCM and Teflon in the thermal management of LiFePO4 battery cells. The selection of PCM and Teflon for experimental investigation is motivated by their cost-effectiveness, favourable thermo-physical properties, and ready availability, aiming to contribute novel insights to the existing body of knowledge.
The lithium iron cell, also recognized as a Lithium Iron Phosphate (LFP) cell, is a rechargeable battery that employs lithium iron phosphate (LiFePO4) as its cathode material. Its chemical composition includes a cathode composed of lithium iron phosphate, an anode featuring a graphitic carbon electrode with a metallic backing, and an electrolyte containing an organic solvent with lithium salt.
The thermo-physical properties [20] of a LiFePO4 battery cell include a specific heat capacity of around 1200 J kgK−1, low thermal conductivity 3 W mK−1, density of 2700 kg m−3, and these thermo physical properties are essential for designing effective thermal management systems and ensuring the safe and efficient operation of LiFePO4 battery cells, especially in applications such as electric vehicles and renewable energy storage.
2.2. Experimental methodology
- 1.The LiFePO4 battery cells, illustrated in figure 1, were acquired from the market. The 18650 rechargeable batteries, categorized as lithium-ion, are named based on its dimensions—18 mm in diameter and 65 mm in height.
- 2.The thermal conductivity, a critical property affecting heat conduction. The 'Scientific Indian' make apparatus is used (guarded hot plate method) for determining the thermal conductivity. The thermal conductivity was measured for paraffin wax and Teflon, resulting in values of 0.27 W mK−1 and 0.25 W mK−1, respectively. For natural convection heat transfer, a heat transfer coefficient of 19.28 W m2K−1 was considered.
- 3.The typical values of critical thickness of insulation are calculated by formula

- 4.The critical insulation thickness was calculated using the above equation, resulting in values of 14 mm for paraffin wax and 13 mm for Teflon.
Figure 1. LiFePO4 battery cells.
Download figure:
Standard image High-resolution imageTable 1 provides details on the specifications of LiFePO4 battery cells alongside the associated coating materials.
- 5.To prepare for experimentation, a mold was formed from copper sheet metal with dimensions of 28 mm inside diameter and 65 mm height. This mold, leaving a surrounding 14 mm thickness, accommodates the central placement of the battery cell. Paraffin wax was heated until it becomes semi-solid, and the resulting matrix was filled around the battery cell, coating it with the critical insulation thickness.
- 6.Similarly, Teflon tape was wound around LiFePO4 battery cells, ensuring a critical insulation thickness of 13 mm.
Table 1. Various parameters for LiFePO4 battery cells coating materials.
| Sr No | Description | Unit | Value | |
|---|---|---|---|---|
| 1 | PCM (Paraffin wax) | Thermal conductivity | W/m2K | 0.27 |
| Critical thickness of insulation | mm | 14 | ||
| 2 | Teflon | Thermal conductivity | W/m2K | 0.25 |
| Critical thickness of insulation | mm | 13 | ||
Therefore, the LiFePO4 battery cells, together with the applied insulation, were prepared for experimental testing.
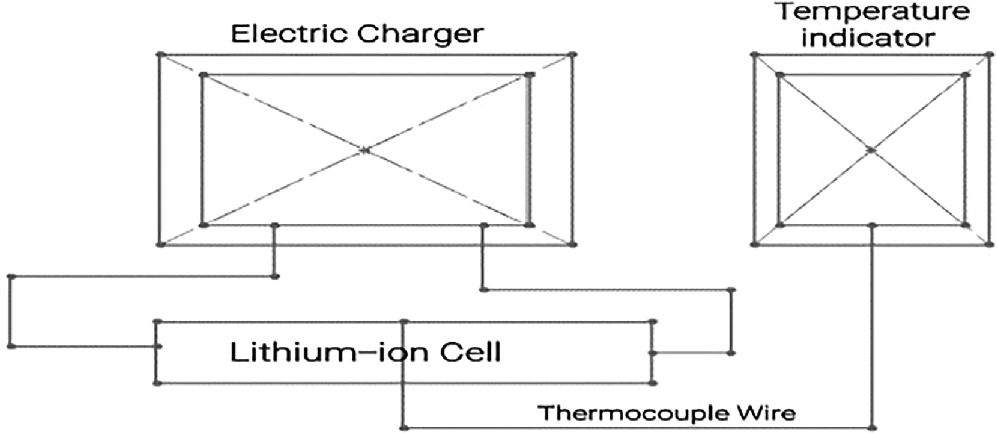
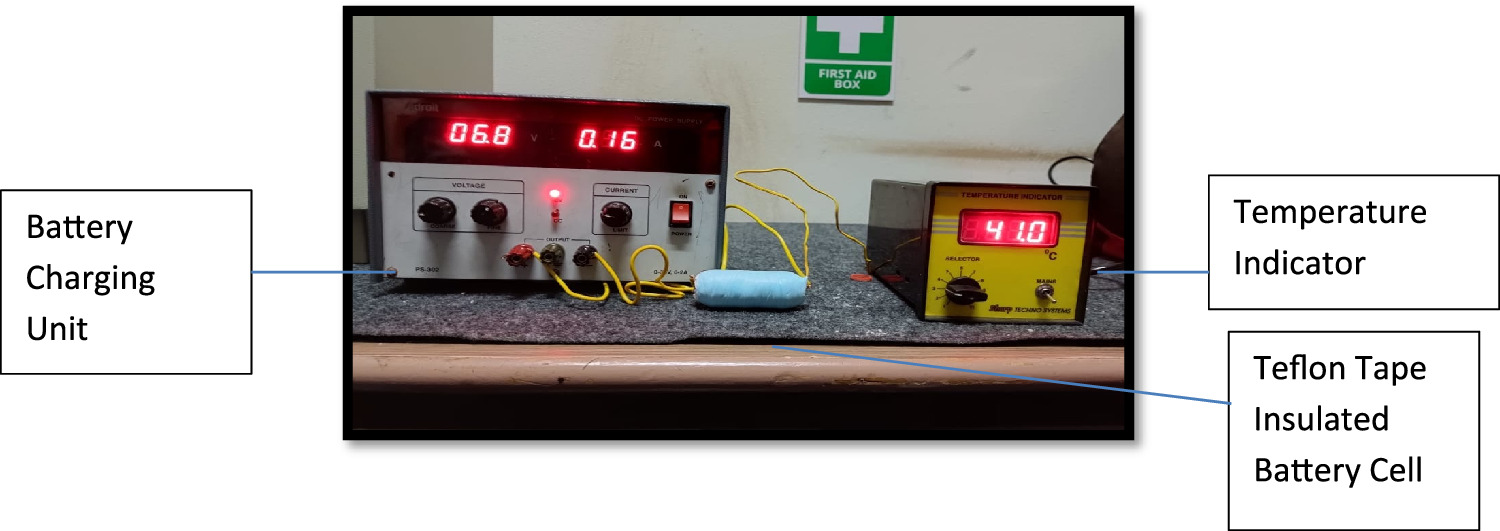
2.3. Experimental setup
The schematic representation of the experimental setup is depicted in figure 2. This setup includes a charging unit consist D. C. regulator power supply (0 to 30 V, 2Amp), Ammeter (0-5-10 Amp), LiFePO4 battery cells, and a temperature indicator with a thermocouple (K- Type 0–250 °C). The experimental procedure involves charging the cell by connecting it to the charging unit. A thermocouple wire is wound around the bare battery cell, and the surface temperature is monitored as the cell charges. The temperature increment is recorded per minute. Subsequently, the same battery cell is utilized, and the thermocouple wire is placed on the bare cell, which is then coated with paraffin wax to achieve the critical insulation thickness. Once again, during the battery charging process, the surface temperature increase is noted per minute. Following this, the paraffin wax coating on the battery cell is removed. The battery cell is then wrapped with Teflon tape, and similar temperature readings are recorded during the charging process.
Figure 2. Schematic of experimental setup for charging LiFePO4 battery cells.
Download figure:
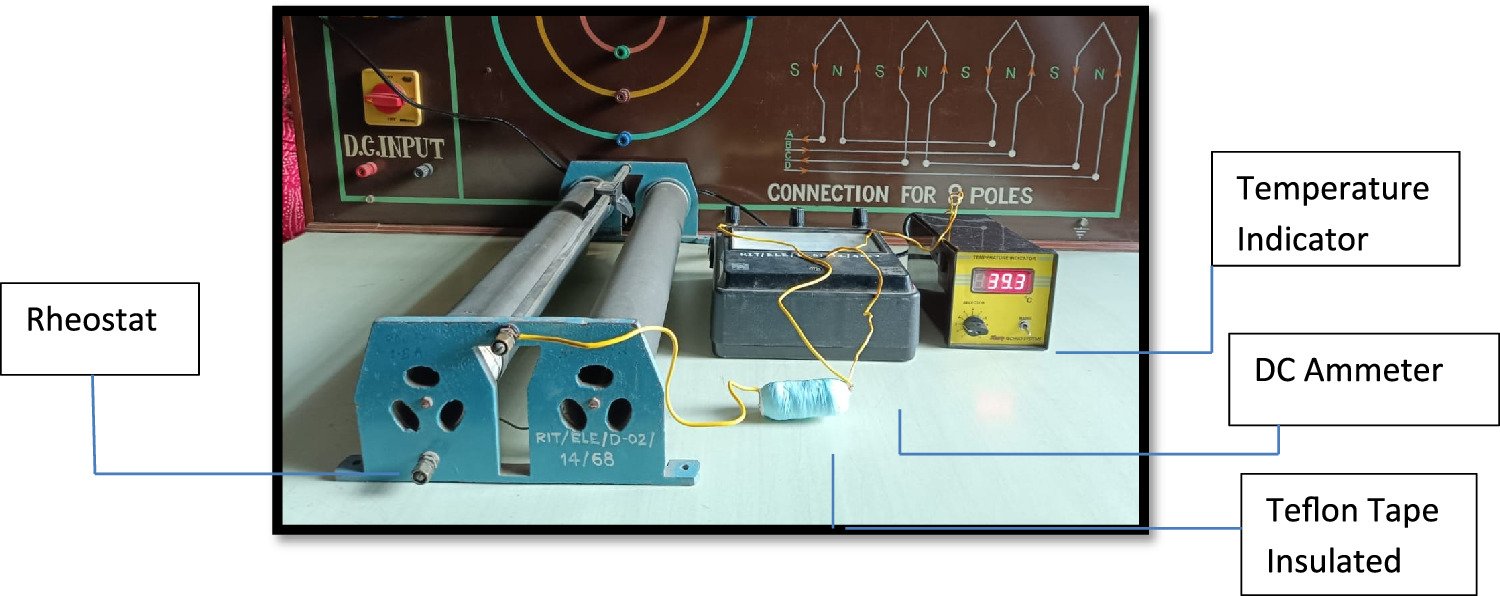
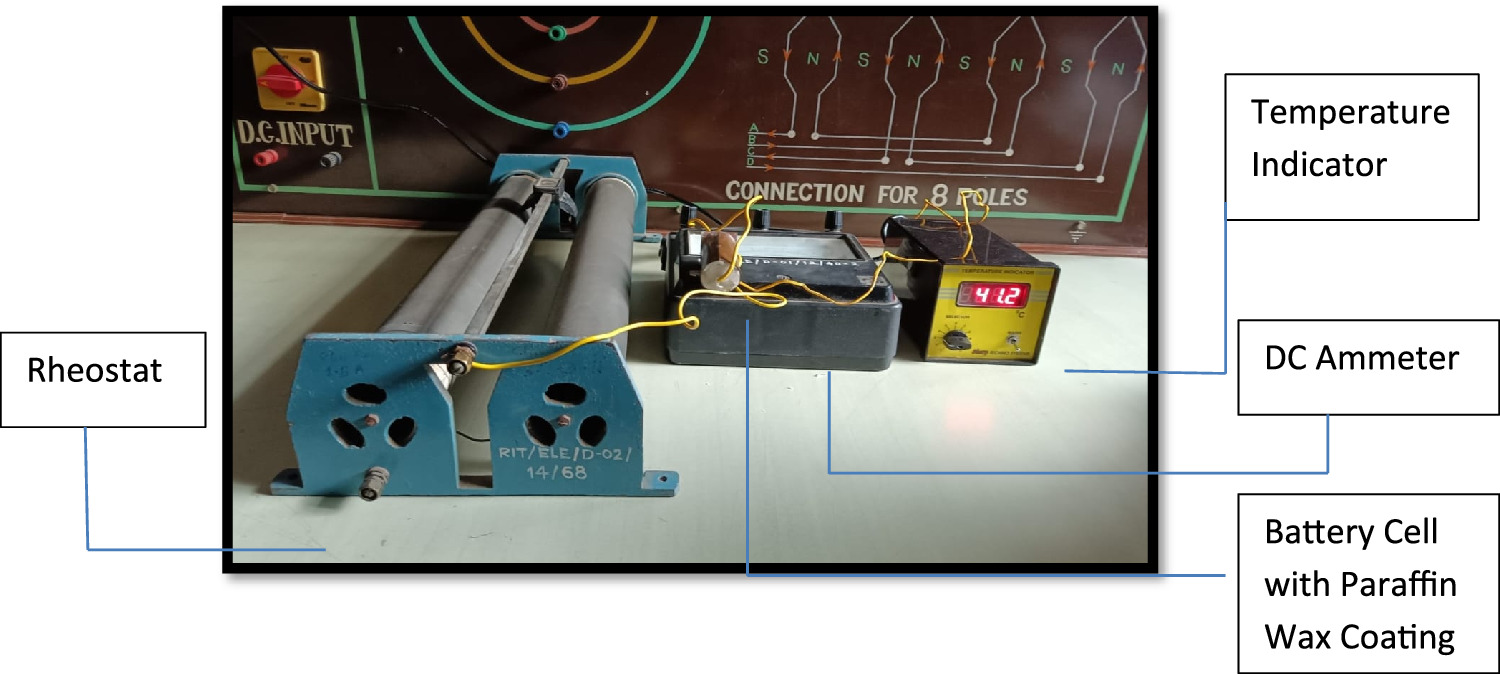
Standard image High-resolution imageFigure 3 illustrates a charging unit with a thermocouple sensor, an indicator, and a bare cell. Figure 4 displays a similar setup but with a Teflon-coated battery cell, while figure 5 shows a battery cell coated with phase change material. Heat is generated during the battery cell charging process, with a significant amount conducted radially outward and dissipated into the surroundings. The long-term performance and safety of the battery cell are depending on effective heat dissipation, necessitating substantial heat dispersion from the cell body to maintain lower temperatures.
Figure 3. Bare cell charging setup.
Download figure:
Standard image High-resolution imageFigure 4. Cell with Teflon insulation charging setup.
Download figure:
Standard image High-resolution imageFigure 5. Cell with paraffin wax insulation charging setup.
Download figure:
Standard image High-resolution imageThe battery cell features a cylindrical geometry, leading to most of the heat being conducted radially outward. Notably, a substantial amount of heat dissipation occurs when the insulation thickness matches the critical thickness of insulation. During experiments involving Teflon tape-insulated battery cells and Paraffin wax-coated battery cells, the insulation thickness is deliberately set to match the critical thickness. The term 'critical thickness' denotes the point at which heat flow increases up to a specific thickness and subsequently decreases. In the context of cylinders and spheres, this critical thickness is specifically known as the critical radius ( ).
).
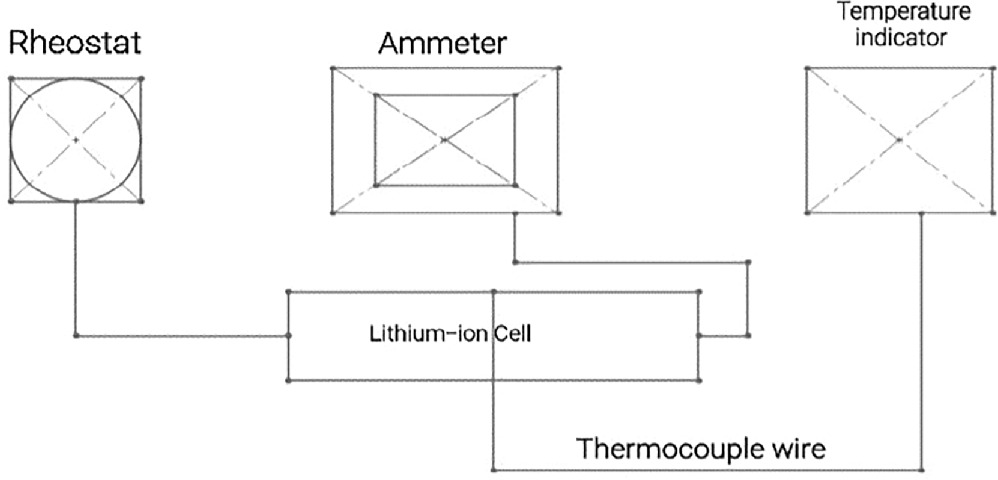
Figure 6 illustrates the schematic of the experimental setup designed for discharging a LiFePO4 battery cell. The setup comprises a Rheostat (370 Ω, 2.1 A), a DC ammeter (0-5-10 A), and a temperature indicator. The Rheostat serves to apply an electrical load, while the ammeter indicates the current flowing through the electrical circuit. Throughout the discharging process, the temperature of the battery cell is continually monitored and recorded on a per-minute basis. This experimental setup is utilized for evaluating the performance of bare cell, Paraffin Wax coating, and Teflon coating materials.
Figure 6. Schematic of experimental setup for discharging LiFePO4 battery cell.
Download figure:
Standard image High-resolution imageIn figure 7, a discharging unit is depicted, featuring a thermocouple sensor, a temperature indicator, and a Teflon-insulated battery cell, along with a multi meter and rheostat for applying electrical load. Meanwhile, figure 8 showcases a comparable discharging unit setup, comprising a thermocouple sensor, a temperature indicator, and a paraffin wax-insulated battery cell, alongside a multi meter and rheostat for the application of electrical load.
Figure 7. Cell with Teflon insulation discharging setup.
Download figure:
Standard image High-resolution imageFigure 8. Cell with paraffin wax insulation discharging setup.
Download figure:
Standard image High-resolution image3. Numerical analysis in thermal management of LiFePO4 batteries
3.1. Numerical analysis of bare LiFePO4 cell
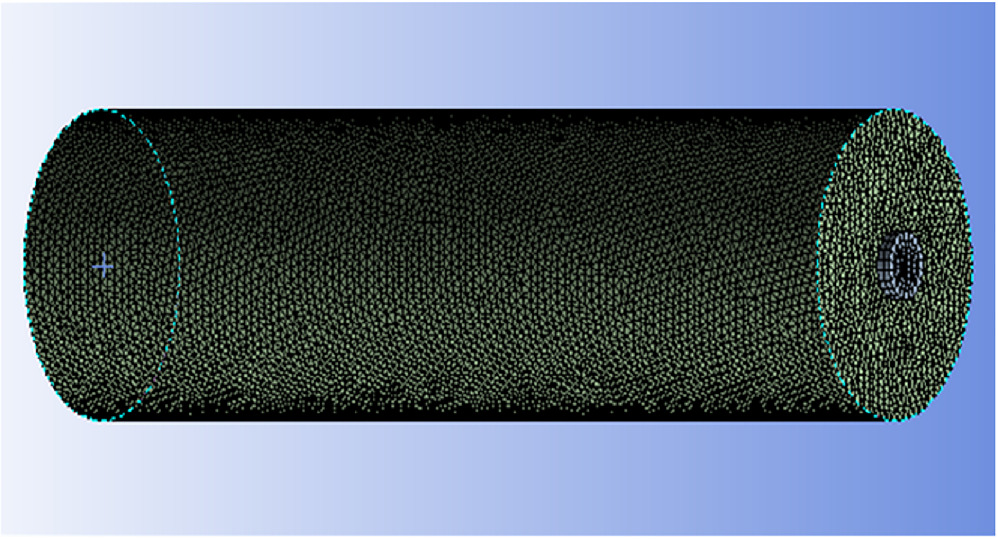
ANSYS 2022 R1 Educational software serves the purpose of modeling, meshing, and analysis. ANSYS Space Claim was employed to generate the geometry for the battery cell, and the subsequent meshing process utilized the ANSYS Meshing module. Figure 9 visually represents the numerical domain of the battery cell. The key geometric specifications include a diameter (D) of 18 mm and a length (L) of 65 mm. The geometry meshing was carried out using ANSYS Workbench.
Figure 9. Geometry of the battery cell LiFePO4 18650 battery cell.
Download figure:
Standard image High-resolution imageFigure 10 displays the 3D geometry of the battery cell with the mesh. The mesh quality parameters, specifically the Skewness of 0.22716 (with a prescribed limit of 0 to 1) and the aspect ratio of 1.8474, are within acceptable limits, close to 1. The element shape is tetrahedron, and the total number of meshed elements is 225,577.
Figure 10. Meshing of Bare battery cell.
Download figure:
Standard image High-resolution imageThe analysis incorporates a battery model, employing the MSMD solution method with the NTGK empirical model for the E Chemistry Model. The Joule heat passive zone and E-chem heat source have been activated for this model. Solution controls are configured with a current under-relaxation of 0.8 and a voltage under-relaxation of 1. Furthermore, the C rate is set at 3, with a minimum stop voltage of 3 V and a maximum stop voltage of 4.3 V. The thermo- physical properties of LiFePO4 already mentioned is used for analysis.
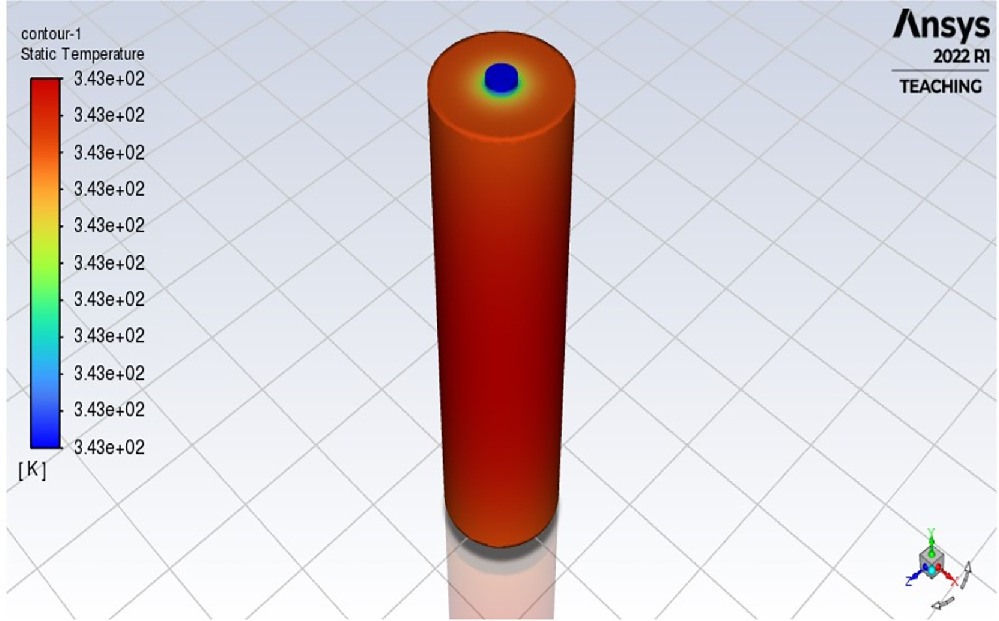
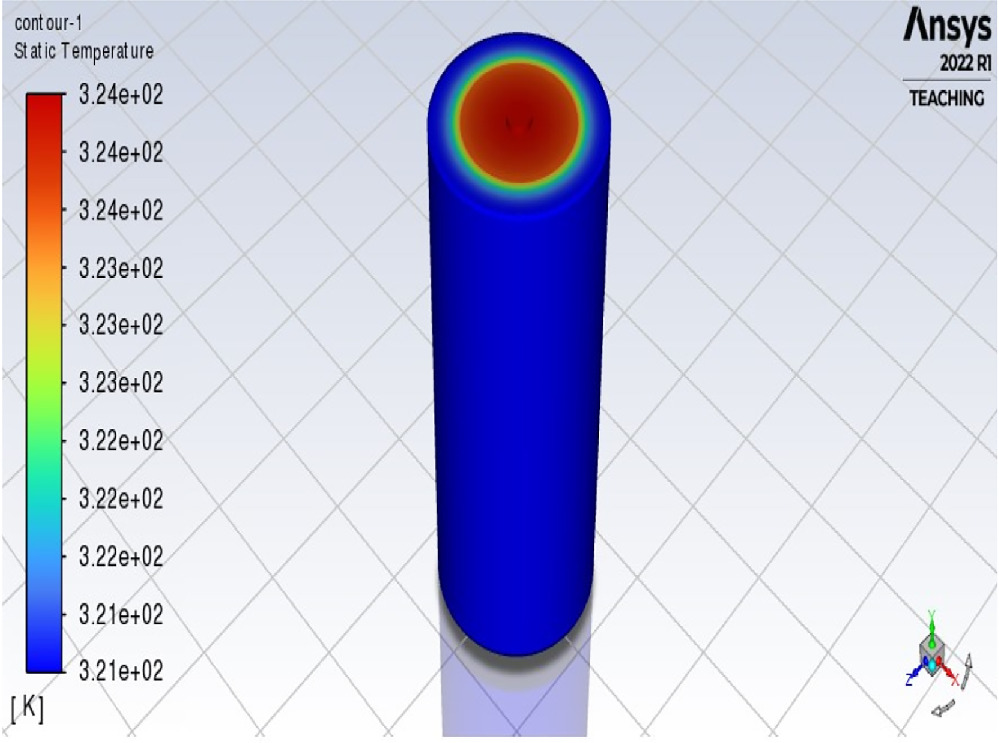
Figure 11 displays the temperature contour of the battery cell, indicating that the maximum temperature reached is 343 K, equivalent to 70 °C, within a timeframe of 1200 s.
Figure 11. Temperature contour of bare battery cell.
Download figure:
Standard image High-resolution image3.2. Numerical analysis of LiFePO4 cell coated with paraffin wax
The identical procedure employed earlier used to analyse the bare cell was followed, with the only distinction being that the bare cell is now coated with a 5 mm thickness of paraffin wax. Figure 12(a) presents a visual depiction of the numerical domain of the battery cell. The essential geometric parameters of the cell encompass a diameter (D) of 18 mm and a length (L) of 65 mm, with an additional 14 mm thickness of paraffin wax. The meshing of the geometry was conducted using ANSYS Workbench. Figure 12(b) illustrates the three-dimensional geometry of the battery cell coated with paraffin wax, including the mesh. The mesh exhibits a Skewness of 0.17472 and an aspect ratio of 1.6323, both falling within the prescribed limits. The total number of generated elements is 358,698.
Figure 12. (a) Geometry of the battery cell with PCM. (b) Meshing of battery cell with PCM.
Download figure:
Standard image High-resolution imageThe analysis integrates a battery model coated with paraffin wax, utilizing the MSMD solution method and the NTGK empirical model for the E Chemistry Model. The Joule heat passive zone and E-chem heat source have been enabled for this model. Solution controls are set with a current under-relaxation of 0.8 and a voltage under-relaxation of 1. Additionally, the C rate is specified at 3, with a minimum stop voltage of 3 V and a maximum stop voltage of 4.3 V.
The temperature contour of the battery cell is depicted in figure 13, revealing that the maximum temperature reached is 324 K, equivalent to 51 °C, within a timeframe of 1200 seconds.
Figure 13. Temperature contour of bare battery cell coated with paraffin wax.
Download figure:
Standard image High-resolution image4. Result and discussion
Experimental trials were undertaken during the charging and discharging of battery cells, exploring various insulation materials. The trials involved bare cells, Teflon tape as insulation, and paraffin wax serving as both insulation and a phase change material. In both charging and discharging trials on Teflon-coated and paraffin wax-coated cells, the insulation thickness was maintained at the critical thickness to optimize heat transfer.
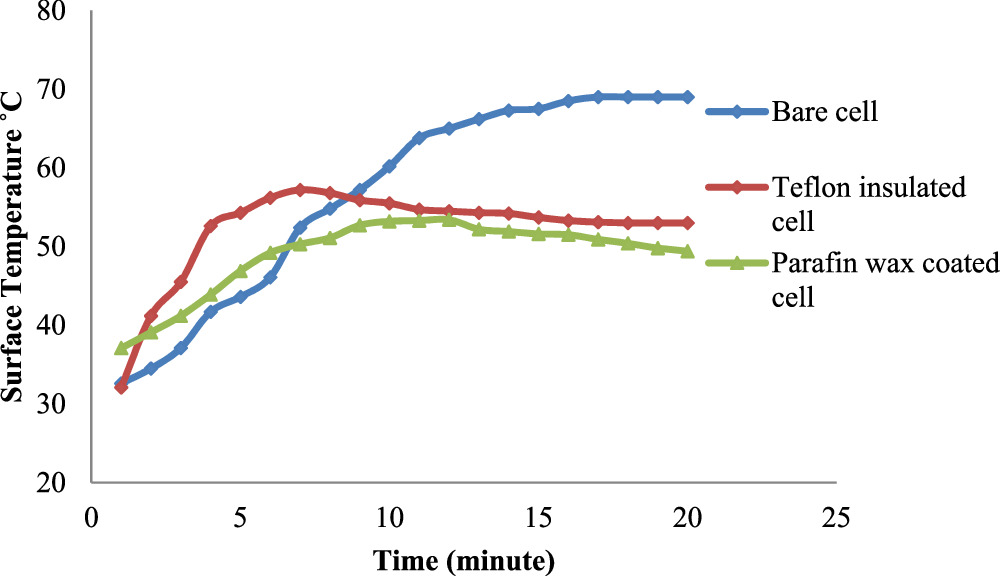
Following the trials, intriguing results were observed shown figure 14. In the charging phase, the surface temperature of the bare cell uniformly rose, reaching a maximum of 69 °C and maintaining that level for an extended period. The Teflon-insulated cell exhibited an initial temperature increase of 57 °C within the first 10 min, followed by a slight drop to 53.7 °C, which then remained constant. Meanwhile, the paraffin wax-coated cell showed a rapid initial temperature increase up to 53.3 °C within the first 10 min, followed by a slight drop to 49.8 °C and a stable temperature thereafter. Notably, the Teflon-coated cell demonstrated a 17.39% reduction in peak temperature compared to the bare cell, while the paraffin wax-coated cell showed a 23.18% reduction.
Figure 14. Surface temperature variations during battery cell charging.
Download figure:
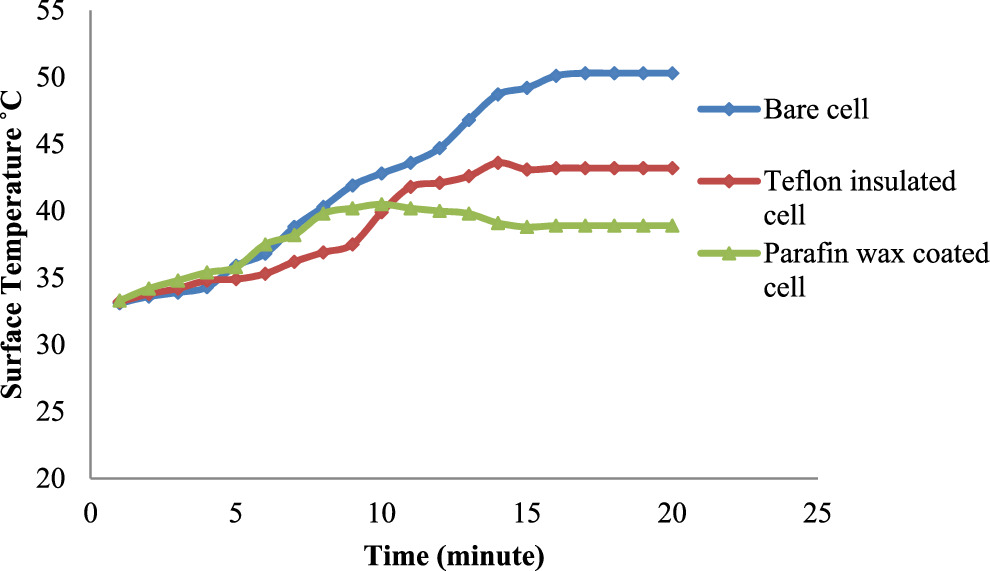
Standard image High-resolution imageDuring the discharging phase, similar trends were observed as shown in figure 15. The surface temperature of the bare cell reached a maximum of 50.1 °C and remained constant. The Teflon-insulated cell experienced an initial temperature increase of 43.6 °C within the first 15 min, followed by a drop to 43.2 °C, maintaining this level thereafter. The paraffin wax-coated cell exhibited a rapid initial temperature increase up to 40.5 °C within the first 10 min, followed by a slight drop to 38.9 °C and a stable temperature thereafter. Comparatively, the Teflon-coated cell showed a 14% reduction in peak temperature, while the paraffin wax-coated cell demonstrated a 19.88% reduction compared to the bare cell.
Figure 15. Surface temperature variations during battery cell discharging.
Download figure:
Standard image High-resolution imageThese findings suggest that the use of Teflon and paraffin wax as insulation materials is more beneficial, contributing to enhanced safety and prolonged battery cell life
This discrepancy arises from paraffin wax being a phase change material, capable of absorbing heat and distributing it throughout its bulk to transition from solid to liquid phase. Consequently, paraffin wax absorbs a greater amount of heat compared to Teflon, which serves as an insulating material. This characteristic leads to a reduction in the surface temperature of LiFePO4 battery cells coated with paraffin wax compared to those coated with Teflon.
The figure 16 presents a comparison between experimental and numerical results of the surface temperature of the battery cell. For the bare cell, the average deviation between experimental and numerical surface temperatures is approximately 5.9%. In the case of the paraffin wax-coated cell, the average deviation between experimental and numerical surface temperatures is 7.07%. These results indicate a favourable agreement between the experimental and numerical findings for the surface temperature of the battery cell.
Figure 16. Comparison between experimental and numerical results of surface temperature battery cell.
Download figure:
Standard image High-resolution image5. Conclusion
The implementation of a thermal insulating coating and phase change material at the critical thickness proves to be an innovative strategy for moderating the surface temperature of battery cells. This innovation is particularly evident during the charging phase, with the bare cell, Teflon-insulated, and paraffin wax-coated cells registering peak temperatures of 69 °C, 57 °C, and 53.3 °C, respectively. Noteworthy is the Teflon-coated cell's demonstrated 17.39% reduction in peak temperature compared to the bare cell, while the paraffin wax-coated cell exhibits a more significant 23.18% reduction. The experimental analysis presented is effectively complemented by numerical simulations. This temperature reduction trend carries over into the discharging phase. The strategic use of insulating materials or phase change materials with a critical thickness not only lowers surface temperatures but also fortifies the safety of the battery cell, ensuring its extended lifespan.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Financial statement
'We would like to acknowledge that this research received no financial support or funding from any organization or entity. All expenses related to this study were personally borne by the authors.'
Ethical statement
'At Engineering Research Express, we uphold the highest standards of ethical conduct in all aspects of our operations. We are committed to promoting integrity, honesty, and transparency in research and publication practices. Authors submitting manuscripts to our journal are expected to adhere to the principles of academic integrity, including proper citation and acknowledgment of sources, avoidance of plagiarism, and truthful reporting of research findings. We also prioritize the confidentiality of peer review and respect the intellectual contributions of reviewers and editors. Furthermore, we strive to ensure that all published research complies with ethical guidelines and does not involve misconduct or unethical behavior. We believe that upholding these ethical principles is essential for maintaining the trust of our readers, authors, reviewers, and the broader scientific community.'
This statement emphasizes the journal's commitment to ethical standards in research and publication, including integrity, honesty, transparency, proper citation practices, confidentiality, and adherence to ethical guidelines.
Supplementary data (0.7 MB DOCX)