Abstract
The objective of this study was to assess AISI 4340 and 300 M steels electropolishing performance in a concentrated perchloric/acetic acid electrolyte. The statistical analysis on a two-level fractional design (FFD) 24-1 was proposed to define an adequate tool to describe the dissolved thickness and the final surface via arithmetic roughness Ra. A compromise zone was defined for each steel by considering all the requirements for both responses of each steel: dissolved thickness between 15–17 μm and arithmetic roughness criteria less than 0.06 μm.
Export citation and abstract BibTeX RIS
1. Introduction
Electropolishing (EP) is an electrochemical surface cleaning-finishing process allowing metal to be electrolytically removed under specific conditions [1–19]. The first work dealing with this process was published in 1910 [1] and its development is due to Jacquet [2]. Nowadays, EP is traditionally used for esthetic applications resulting in an attractive mirror finish. This process can also be used for deburring, brightening and passivating—ferrous and nonferrous alloys. EP has become a common treatment for stainless steels [5, 13–18], copper [8, 10, 11], titanium [7, 12, 19] and niobium [20] in several high-technology applications such as cardiovascular and orthopedic body implants, pharmaceutical and semiconductor installations and so on.
The main objective of EP is to drastically minimize the surface micro roughness thus reducing the risk of dirt or product residues adherence and improving the clean ability of the surfaces [13–18, 21]. Another benefit of EP is that, contrary to classical cleaning processes (acid pickling, etc), this technique produces a surface free of hydrogen. Furthermore, this electrolytic process permits to obtain undisturbed and metallurgical clean surfaces [21, 22] contrary to the mechanical surface treatments which provide mechanical and thermal stresses.
Most of the published works related to the fundamental understanding of EP comprised the study of copper electropolishing in phosphoric acid [23–25] although some works on stainless steels and passive metals (Ti, Ni, Cr, Nb, Al) have also been reported [9, 14–20, 26, 27]. Readers can find numerous theories developed to understand the electrochemical fundamental mechanisms in recent reviews [14–17]. If little information is available on the mechanisms involved regarding ferrous alloys, the technological aspects of electropolishing, including electrolyte composition and operating conditions, have been described in literature [13, 28–30]. EP is carried out in electrolytes with high viscosity and/or low conductivity such as concentrated acids (e.g. sulfuric, phosphoric) or non-aqueous solutions (ethylene glycol, methanol-sulfuric acid). Tegart [4] and Shigolev [31] have reported overviews of typical formulas of EP electrolytes for different metals and alloys. It is well known that, for a given material, the surface property depends on numerous EP operating parameters such as applied current density, voltage, temperature, concentrations of the chemicals used, etc.
The aim of this paper is to determine a set of EP process experimental conditions for AISI 4340 and 300M steels in a perchloric/acetic solution allowing (i) the dissolution of a sufficient thickness to eliminate the layer affected by residual compressive stresses due to the mechanical polishing and (ii) a low roughness. Most of the previous works investigated the influence of each EP parameter one at a time while keeping the others constant. This conventional step by step approach for optimization purposes involves a large number of independent runs and does not take into account the possible interactions between factors. In order to overcome this problem, an experimental design is used. This approach has the dual advantage of taking into account the combined effects of several input variables and requiring only a moderate number of experiments [32–36]. In this respect, a two-level fractional factorial design (FFD), noted 24-1, is conducted. Based on the literature review, the following EP variables were investigated in the FFD study: (i) electrical charge amount, (ii) anodic current density, (iii) iron concentration of solution perchloric/acetic solution and (iv) temperature of EP solution. It is worth noting that in our knowledge any paper is devoted to study the AISI 4340 and 300 M steels electropolishing performance in a concentrated perchloric/acetic acid electrolyte.
2. Materials and methodology
2.1. Electropolishing apparatus and electrolyte
A typical electropolishing installation is used, comprising a direct current (DC) power source (Fontaine S30050 (300 V-50 A)), a cell fitted with a lead sheet (50 × 100 mm2) as cathode (−) and a steel part as anode (+) with an inter-electrode distance of 20 mm. The amount of metal removal depends on the electrolyte composition, the temperature, the current density and the metal being electropolished.
The electrolytes used are mixtures of concentrated analytic grade perchloric acid/acetic acid (5/95 Vol.%) [31]. To study the effect of the iron enrichment of EP electrolytes during electropolishing, an iron ion concentrate solution is preliminary prepared by dissolving iron sheet in a perchloric/acetic acid mixture. The working solution is obtained by diluting the iron -ion concentrate solution into adequate concentration. The temperature is maintained at a given level by thermostated water circulating through the jacketed electrochemical cell (250 ml).
2.2. Sample preparation and characterization
AISI 4340 and 300M steel sheets (electrode area 3 cm2) are used in this study. Their chemical compositions (wt.%) are given in table 1. 300 alloy is similar to 4340 with the addition of vanadium and higher silicon content. 300 M steel offers a combination of toughness and ductility at high strength levels without increasing carbon content.
Table 1. Composition (max) of AISI 4340 and 300 M steel sheets.
| Amount of the following elements (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Element | C | Mn | Si | P | S | Cr | Ni | Mo | Cu | V |
| 4340 | 0.43 | 0.85 | 0.35 | 0.015 | 0.008 | 0.90 | 2.00 | 0.30 | 0.35 | / |
| 300 M | 0.45 | 0.90 | 1.80 | 0.01 | 0.01 | 0.95 | 2.00 | 0.50 | 0.35 | 0.1 |
Before EP, the specimens are (i) degreased in an alkaline solution, (ii) mechanically polished with 600-grit SiC paper, (iii) rinsed with water then with alcohol, (iv) air-dried and then (v) weighted. After EP, the specimens are (i) rinsed with tap water, distilled water and alcohol, (ii) air-dried and (iii) weighted again. The dissolved thickness is computed using Faraday's law. Each test is repeated twice under the same conditions.
Surface morphology is observed by Contour GT-I 3D Optical Microscope and surface roughness measurements are made using a Mahr-Penthen perthometer S6R profilometer. Measurements are repeated three times for each specimen and the criteria roughness (Ra) is obtained thereby.
2.3. Methodology - experimental design [32–36]
As the literature indicates [1–15], the factors that affect the electropolishing process are numerous including pre-treatment of the metal surface, orientation from the workpiece in the electropolishing bath, choice of cathode material, electrode spacing, bath age, electropolishing time, temperature, composition of the bath, voltage or current density imposed, ...
As many factors are involved and must be optimized in an electropolishing process, there is no one-fit-all parameter set for all electropolishing setups. So, the following four variables are selected and investigated in the FFD study: U1: electrical charge amount Q (A min dm−2), U2: anodic current density (A dm−2), U3: Iron ions concentration of the EP solution, U4: temperature of the EP solution.
In addition to the current density and the electrical charge, the choice of factors is strongly linked to industrial practices. The electropolishing bath age i.e. the iron ions concentration in the EP bath is also a big concern during the process. Indeed, it is a standard practice in industry to reuse the electrolyte to keep a low profile of cost and minimize the detrimental effect to the environment [4]. Moreover, the temperature impacts the surface brightness which decreases with the decrease of the temperature. The reaction rate in the limiting current region becomes mass transport controlled as the temperature is increased [5, 14, 15].
A two-level fractional factorial design (FFD), noted 24-1, is implemented to identify the most influential variables affecting the studied responses and to carry out a low number of experiments, ensuring that the results are as precise as possible and to focus on the main effects and low-order interactions. A non-dimensional coded variable Xi is associated with each natural variable Ui. The limits of the experimental domain in terms of coded variables are identical for all variables and the extreme values are equal to ±1 (table 2). To check the validity of the empirical model, three central experiments need to be added to the experimental design.
Table 2. Experimental domain of the EP process.
| Factors | Unit | Associated variable | Lower level (−1) | Upper level (+1) |
|---|---|---|---|---|
| Electrical charge | A min dm−2 | X1 | 48 | 108 |
| Current density | A dm−2 | X2 | 12 | 18 |
| Iron ions concentration | mg l−1 | X3 | 0 | 500 |
| Temperature | °C | X4 | 14 | 26 |
The design is constructed by using the independent generator 1234. This type of design is classified as—'resolution IV' design [33, 34] i.e. all the main effects are confounded with three-factor interactions. From the principle of effect sparsity, a system is likely to be driven primarily by main factor and low-order interaction effects. So, the effects of the high-order interactions (three or greater) are assumed to be negligible, and therefore enabling all the main effects to be determined. Two-factor interactions are confounded with each other thus making it impossible to determine all of them for all responses. The eight selected effects and their aliases are listed in table 3. Eight experiments are replicated to determine the influence of the variables on the four responses noted:
- 1.Y1, dissolved thickness (μm) and Y2, mean roughness (arithmetic average of the absolute values (Ra)) for AISI 4340 steel,
- 2.Y3, dissolved thickness (μm) and Y4, mean roughness (arithmetic average of the absolute values (Ra)) for 300 M steel.
Table 3. Effects and aliases.
| l0 = b0 | l4 = b4 |
| l1 = b1 | l12 = b12 + b34 |
| l2 = b2 | l13 = b13 + b24 |
| l3 = b3 | l23 = b23 + b14 |
Nemrod-W software is used for regression, statistical analysis and graphical analysis of the obtained data [37].
3. Results and discussion
The requirements for each run and the measured response are summarised in table 4, considering the two levels defined for each of the four retained factors. The evaluation of the model quality of the four responses is done by means of the analysis of variance the results of which are reported in table 5. As can be seen, for each response the regression sum of squares is statistically significant at 99.9% confidence level (***). The main effect of a factor Xi (noted li) and the aliases of two-factor confounded variables interaction effects (noted lik) are estimated by least squares regression. The value and the significance of each coefficient, li or lik, determined by the p-values, are listed in table 6. It is important to mention that the p-value is the probability of getting the displayed value for the coefficient if its true value is zero. In other words, the 'null hypothesis' (H0 hypothesis) is tested for each li or lik. For a determined factor, if the H0 hypothesis is verified, this factor is said to be not influent. In practice, a confidence level of 95% is considered i.e. the alpha-level is set at 5%. The alpha level corresponds to the risk of rejecting the H0 hypothesis when this hypothesis is verified. The test of the H0 hypothesis is thus rejected, and the factor is considered as influent when p < 0.05. Accordingly, the smallest value of the p-value indicates the high significance of the corresponding coefficient.
Table 4. Experimental matrix in coded variables and measured responses obtained for AISI 4340 and 300 M steels.
| Run | X1 | X2 | X3 | X4 | Y1 | Y2 | Y3 | Y4 |
|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | 10.5 | 0.09 | 10.6 | 0.05 |
| 1bis | −1 | −1 | −1 | −1 | 10.7 | 0.10 | 10.2 | 0.07 |
| 2 | 1 | −1 | −1 | 1 | 16.4 | 0.06 | 15.8 | 0.07 |
| 2bis | 1 | −1 | −1 | 1 | 15.9 | 0.08 | 16.0 | 0.08 |
| 3 | −1 | 1 | −1 | 1 | 16.1 | 0.11 | 16.7 | 0.08 |
| 3bis | −1 | 1 | −1 | 1 | 15.8 | 0.09 | 16.9 | 0.09 |
| 4 | 1 | 1 | −1 | −1 | 24.0 | 0.22 | 24.4 | 0.19 |
| 4bis | 1 | 1 | −1 | −1 | 24.3 | 0.20 | 24.7 | 0.16 |
| 5 | −1 | −1 | 1 | 1 | 10.4 | 0.12 | 10.1 | 0.07 |
| 5bis | −1 | −1 | 1 | 1 | 10.2 | 0.09 | 10.4 | 0.11 |
| 6 | 1 | −1 | 1 | −1 | 15.4 | 0.07 | 16.1 | 0.08 |
| 6bis | 1 | −1 | 1 | −1 | 15.6 | 0.08 | 16.3 | 0.07 |
| 7 | −1 | 1 | 1 | −1 | 15.8 | 0.06 | 16.2 | 0.04 |
| 7bis | −1 | 1 | 1 | −1 | 15.6 | 0.08 | 16.0 | 0.05 |
| 8 | 1 | 1 | 1 | 1 | 24.0 | 0.11 | 24.2 | 0.10 |
| 8bis | 1 | 1 | 1 | 1 | 24.2 | 0.12 | 24.0 | 0.11 |
Table 5. ANOVA of the responses Y1 to Y4 for AISI 4340 and 300 M steels.
| Source of variation | Sum of square | Freedom degree | Mean square | F ratio | p-value | |
|---|---|---|---|---|---|---|
| Response Y1 | Regression | 383.1444 | 7 | 54.7349 | 1390.0930 | <0.01 *** |
| Experimental error | 0.3150 | 8 | 0.0394 | |||
| Total | 383.4594 | 15 | ||||
| Response Y2 | Regression | 0.0289 | 7 | 0.0041 | 17.7547 | 0.0277 *** |
| Experimental error | 0.0019 | 8 | 0.0002 | |||
| Total | 0.0307 | 15 | ||||
| Response Y3 | Regression | 397.7475 | 7 | 56.8211 | 1683.5873 | <0.01 *** |
| Experimental error | 0.2700 | 8 | 0.0337 | |||
| Total | 398.0175 | 15 | ||||
| Response Y4 | Regression | 0.0231 | 7 | 0.0033 | 15.1035 | 0.0497 *** |
| Experimental error | 0.0017 | 8 | 0.0002 | |||
| Total | 0.0248 | 15 |
The statistical significances of the model equations are evaluated by the F-test for analysis of variance (ANOVA), which show that the regression is statistically highly significant at a 99.9% (p < 0.001) confidence level (***).
Table 6. Values and statistical analysis of the effects for AISI 4230 and 300 M steels.
| Y1 response | Y2 response | Y3 response | Y4 response | |||||
|---|---|---|---|---|---|---|---|---|
| Effects | Estimates | p-value | Estimates | p-value | Estimates | p-value | Estimates | p-value |
| l0 | 16.56 | <0.01*** | 0.104 | <0.01*** | 16.79 | <0.01*** | 0.088 | <0.01 *** |
| l1 | 3.42 | <0.01*** | 0.012 | 1.14 * | 3.40 | <0.01 *** | 0.019 | 0.0763 *** |
| l2 | 3.42 | <0.01*** | 0.019 | 0.109** | 3.60 | <0.01 *** | 0.013 | 0.661 ** |
| l3 | −0.16 | 1.36 * | −0.013 | 0.855** | −0.12 | 2.62 * | −0.011 | 2.12 * |
| l4 | 0.07 | 20.3 | −0.007 | 8.7 | −0.02 | 60.1 | 0.001 | 90.9 |
| l12 | 0.73 | <0.01*** | 0.026 | 0.0132*** | 0.54 | <0.01 *** | 0.020 | 0.0675 *** |
| l13 | −0.02 | 71.5 | −0.009 | 5.5 | 0.09 | 9.3 | −0.008 | 5.7 |
| l23 | 0.08 | 14.0 | −0.018 | 0.173 ** | −0.16 | 0.764 ** | −0.018 | 0.138 ** |
*** Highly significant at the level 99.9%, ** Significant at the level 99%, *Significant at the level 95%.
3.1. FFD 2 4-1 for AISI 4340 steel
From an examination of the results in table 4, the dissolved thickness (Y1) and Ra (Y2) of AISI 4340 steel vary respectively from 10.2 to 24.3 μm and from 0.06 to 0.22 μm, indicating that certain factors and/or interactions should show significant effects on the measured responses (Y1 and Y2).
For the Y1 response, data from table 6 reveal that only X1, X2, X3 factors and two confounded interaction effects, 'l12' and 'l23' are significant at a confidence level greater than or equal to 95%. If we consider that interaction effects between the extra factors (X4) and the basic factors (X1, X2 and X3) are not taken into account in the theoretical analysis due to the hypothesis on the construction of the fractional design, we are able simplify the expression of the confounded effects. For the confounded interaction effect, l12 = b12 +b34, we can assume that the factor interaction effect b12 is the dominant term since electrical charge amount (X1) and current density (X2) have the greatest effects on the response, as emphasized in the literature. In this respect, we can conclude that the factor interaction effect b23 is the dominant term for the confounded interaction effect l23 = b23 + b14.
So, according to the empirical model obtained, the dissolved layer can be represented by the following equation:
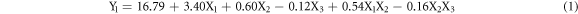
The equation (1) indicates that the positive coefficients of X1, X2 and X1X2 have a constructive contribution to the dissolved layer response. However, the negative coefficients of X3 and of X2X3 indicate antagonistic effects on the response.
From the analysis of ANOVA (table 5), the results shown in table 6 and following the same analysis as above, a fitted polynomial model (2) can be generated for the roughness criteria, Y2:
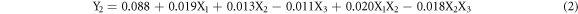
This model quantitatively elucidates the effects of the EP variables with statistical significance. The equation indicates the synergetic effects of X1, X2 and X1X2 and antagonistic effects of X3 and X2X3 on the Ra response. Note that for two models (1) and (2), the temperature (X4) shows no significant effect without any significant interactions.
All these results are confirmed by the high values of the multiple correlation coefficient squares (R2): 99%, 93% for Y1 and Y2 respectively. It should be noted that R2 values represent the percentage variation in the responses explained by the deliberate variation of the factors in the course of the experiments.
To validate the statistical models (1)–(2), three additional central experiments are conducted. The predicted values are in fair agreement with those measured suggesting that the first order models chosen are suitable and can be used as a prediction equation (table 7).
Table 7. Measured and calculated values for the confirmation experiments for AISI 4340 and 300 M steels.
| Runs | Experimental conditions | Measured responses | Calculated responses | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y4 | Y1 | Y2 | Y3 | Y4 | ||
| 17 | X1 = X2 = X3 = X4 = 0 i.e. | 16.82 | 0.084 | 16.67 | 0.097 | 16.79 | 0.088 | 16.56 | 0.104 |
| 18 | U1 = 78 A min dm−2, | 16.62 | 0.092 | 16.56 | 0.107 | ||||
| 19 | U2 = 15 A dm−2, | 16.80 | 0.085 | 16.58 | 0.112 | ||||
| U3 = 250 mg l−1, | |||||||||
| U4 = 20 °C | |||||||||
3.2. FFD 2 4-1 for 300 M steel
As previously mentioned, the fractional design allows the calculation of the estimates for factors and two confounded interaction effects for the dissolved thickness Y3 and the roughness criteria, Y4 of the 300M steel (table 6).
Following the procedure described above, the equations of the fitted models are:
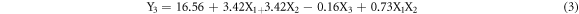
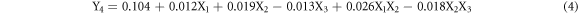
The equation (3) indicates that the positive coefficients of X1, X2 and X1X2 have a constructive contribution to the dissolved layer response. The negative coefficient of X3 indicates an antagonistic effect on the response whilst factor X4 has null effect. Accordingly, the dissolution is enhanced when factors X1 and X2 are respectively set as the highest level to obtain a synergistic effect and X3, as the lowest level. For Y4 response, X1, X2, X3 factors show significant effect with significant interactions (X1X2 and X2X3) whilst factor X4 has null effect.
The values of multiple correlation coefficients, R2, equal to 99% and 94% for Y3 and Y4 respectively show a good fit to the experiment data.
Three additional central experiments are conducted to validate the statistical models. As shown in table 7, the measured and the predicted values are in close-agreement. For overall results, we can conclude that the first order models chosen are considered suitable for this study and can be used as a prediction equation.
At this point, it is worth noting that the influence of the factors cannot be discussed separately due to the importance of their interactions [32–36]. Indeed, data from table 6 reveal that three factors, X1, X2 and X3, and their interactions are significant according the literature. However, the factor X4 is not significant which is at odds with literature [5, 14, 15]. Several authors have shown the importance of the temperature for electropolishing on the plateau current density and so on the diffusion coefficient of the rate limiting species in the electropolishing bath. The insignificance of the temperature in the FFD is likely due to the fact that the interval of variation is small.
3.3. Graphical exploitation of validated models—optimum' choice of the EP operating conditions for the two substrates
As indicated previously, the focus of this study is to satisfy one principal objective which is finding the EP operating conditions allowing (i) the dissolution of a sufficient thickness to eliminate the layer affected by residual compressive stresses due to the mechanical polishing and (ii) low roughness. For this purpose, we define an acceptable range for each response: (i) a dissolved thickness comprised between 15–17 μm and (ii) an arithmetic roughness criterium less than 0.06 μm. The evolution of the four considered responses Y1 to Y4 are plotted (figures 1–2). Note that, according to the expression of these responses, the X3 factor (iron ion- concentration) has been kept at its high level (+1). By mere inspection of these diagrams, it is easy to accurately choose each part of the domain that is acceptable according to the criteria above. A satisfactory zone is the part of the domain for which the value of each one of the calculated responses is acceptable (table 8). Taking into account all the requirements for the two responses of each steel, looking for a compromise where all the experimental responses fulfill the specifications imposed by the researchers to achieve the aims proposed is required (table 8).
Figure 1. Surface responses: (a) dissolved thickness, (b) Ra for AISI 4340 Steel- with X3 = +1.
Download figure:
Standard image High-resolution imageFigure 2. Surface responses: (a) dissolved thickness, (b) Ra for 300M Steel- with X3 = +1.
Download figure:
Standard image High-resolution imageTable 8. Compromise domain fulfilling the requirements for AISI 4340 and 300 M steels.
| AISI 4340 | 300 M | |||
|---|---|---|---|---|
| Requirements | Y1  [15; 17]/μm [15; 17]/μm |
Y2 ≤ 0.06/μm | Y3  [15; 17]/μm [15; 17]/μm |
Y4 ≤ 0.06/μm |
| Xi combinations fulfilling each Yi | X1  [−1.00; −0.60], [−1.00; −0.60], |
X1  [−1.00; −0.40], [−1.00; −0.40], |
X1  [−1.00; −0.65], [−1.00; −0.65], |
X1  [−1.00; −0.9], [−1.00; −0.9], |
| requirement (i = 1 to 4) | X2  [0.74; 1.00], [0.74; 1.00], |
X2  [0.60; 1.00], [0.60; 1.00], |
X2  [0.75; 1.00], [0.75; 1.00], |
X2  [0.84; 1.00], [0.84; 1.00], |
| X3 = 1.00 | X3 = 1.00 | X3 = 1.00 | X3 = 1.00 | |
| Compromise domain fulfilling | X1  [−1.00; −0.60], [−1.00; −0.60], |
X1  [−1.00; −0.9], [−1.00; −0.9], |
||
Yi  [15; 7] with i = 1;3 and [15; 7] with i = 1;3 and |
X2  [0.74; 1.00], [0.74; 1.00], |
X2  [0.84; 1.00], [0.84; 1.00], |
||
| Yj ≤ 0.06 with j = i+1 | X3 = 1.00 | X3 = 1.00 | ||
3.4. Surface morphology of steels
Figure 3 depicts typical 2 and 3-dimensional micrographies of 300M steel samples after electropolishing according to experimental design runs (runs 1, 4 and 8).
Figure 3. Typical 2D and 3D micrographies −300 M Steel.
Download figure:
Standard image High-resolution imageThe topography of the surface has been considerably modified and surface roughness is reduced or increased according to the operating conditions. As observed in figure 3(c), peaks and valleys are clearly observed. On the contrary, for figure 3(a) with a lower Ra value, the surface is flat due to a uniform dissolution. Similar profiles are observed for 4340 steel samples.
4. Conclusion
The electropolishing technique is a surface treatment that used to remove the metal surface rough irregularities without creation of internal stresses. The present study shows that AISI 4340 and 300M steel electropolishing can be achieved in a perchloric/acetic acid electrolyte using statistical methods. The effects of the operating conditions (current density, electrical charge, temperature) and electrolyte composition (Iron ions concentration) are studied using two-level fractional factorial design (FFD). Using the sequential experiment strategies (i.e., the fractional factorial design), the factors and interactions for the EP process with the dissolved thickness and the roughness criterium (Ra) varying respectively from ca. 10.5 to 24.3 μm and 0.06 to 0.22 μm for AISI 4340 and from ca. 10.1 to 24.7 μm and 0.04 to 0.19 μm for 300M steels are clearly demonstrated. The dissolved thickness and roughness criterium (arithmetic average of the absolute values (Ra)) are described using fitted models. Model validation using ANOVA analyses, check point-tests confirmed that the results are reliable and accurate. The predicted values obtained with models are in close agreement with the experimental data. As expected, these valuable results show that, dissolved thickness and Ra strongly depend on the polarization conditions (I and Q) effects for the two ferrous metals even if Iron ions concentration also affects the EP behaviors of steels. For all responses, the positive coefficients of X1 (Q), X2 (I), and X1X2 indicate a constructive contribution and the negative coefficient of X3 (Iron ions concentration), an antagonistic effect. Temperature (X4) has no effect in the domain studied.
The contour plots for the dependence of thickness and Ra on factors X1 (electrical charge) and X2 (current density) while keeping X3 (Iron ions concentration) constant were constructed by using the regression model for each substrate. This allowed us to define a compromise zone where all the experimental responses fulfill the specifications imposed by the researchers to achieve the aims proposed, i.e. a dissolved thickness comprised between 15–17 μm and arithmetic roughness criteria less than 0.06 μm.




