Abstract
Bone is a living composite material that has the capacity to adapt and respond to both internal and external stimuli. This capacity allows bone to adapt its structure to habitual loads and repair microdamage. Although human bone evolved to adapt to normal physiologic loading (for example from gravitational and muscle forces), these same biological pathways can potentially be activated through other types of external stimuli such as pulsed electromagnetic fields, mechanical vibration, and others. This review summarizes what is currently known about how human bone adapts to various types of external stimuli. We highlight how studies on sports-specific athletes and other exercise interventions have clarified the role of mechanical loading on bone structure. We also discuss clinical scenarios, such as spinal cord injury, where mechanical loading is drastically reduced, leading to rapid bone loss and permanent alterations to bone structure. Finally, we highlight areas of emerging research and unmet clinical need.
Export citation and abstract BibTeX RIS
1. Introduction
The skeleton plays an important physiologic and mechanical role in the body. It is a reservoir for calcium and contributes to several endocrine functions within the body. However, it clearly plays a critical mechanical function by facilitating movement and protecting internal organs. Because of this dual function, bone is constantly remodeling and adapting in response to its physiologic and mechanical loading environment. Thus, the interactions between external stimuli and bone lie along a continuum, with disuse osteoporosis on one end of the spectrum, and overuse injury on the other.
This review will summarize the current understanding of how external stimuli influence bone structure and mechanical behavior in humans. Bone fractures occur when applied loads exceed whole bone strength, and therefore maximizing bone strength is a key component of fracture prevention. Bone must be able to withstand high magnitude, high energy (impact), and repetitive loading at a minimal metabolic cost, requiring a material that is stiff, tough, and light. Therefore, we will focus on the mechanical function, which requires bone that well adapted to resist both habitual and occasional forces occurring during activities of daily living.
2. Bone mechanical function and physiology
2.1. Bone mechanical structure
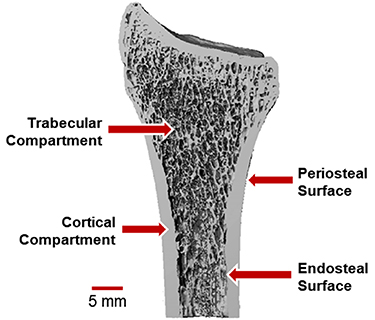
Bone tissue is a composite of hydroxyapatite mineral crystals bound to an organic matrix of collagen type I, proteoglycans, and other proteins [1]. The mineral provides stiffness and compressive strength, and the organic components provide flexibility and toughness. The basic building blocks of the organic matrix are triple helix tropocollagen molecules that polymerize to form aligned collagen fibrils approximately 100 nm in length [2]. Hydroxyapatite mineral crystals form between adjacent collagen molecules. At the microscale, aligned collagen fibers form lamellar sheets 3–7 µm thick [2], which are layered to form concentric osteons (200–300 µm diameter) in cortical bone and trabecular packets (50 µm thick) in trabecular bone [2]. Cortical bone forms the dense shaft and outer shell of long bones. Trabecular bone forms an interconnected lattice of individual struts, or trabeculae (Figure 1). Trabecular bone is found at the ends of long bones because its structure allows for absorption and transmission of joint loads away from the articular surface towards the cortex. The periosteal surface defines the outer boundary of the cortical shell, and the endocortical surface is the interface between the inner cortical boundary and trabecular compartment.
Figure 1. Definition of the trabecular and cortical bone compartments, shown for the distal radius bone in the forearm. The periosteal surface forms the outer bone surface, while the endosteal surface separates the cortical shell from the spongy trabecular compartment. Adapted from MacNeil (2008) Bone [3].
Download figure:
Standard image High-resolution image2.2. Bone cells and the dynamics of bone structure
Bone tissue contains cells that drive dynamic processes including growth, repair, and adaptation. There are four main cell types present in bone tissue: osteoclasts, osteoblasts, bone lining cells, and osteocytes. Osteoclasts are responsible for bone resorption. They are large (20–100 µm diameter), multinucleated cells derived from mononuclear/phagocytic cells in the bone marrow. Osteoclasts seal to the bone surface and release protons and proteolytic enzymes, forming a closed acidic microenvironment to dissolve mineral and degrade matrix proteins [4]. Osteoblasts are responsible for bone formation. They are cuboidal cells (∼10 µm in width) derived from mesenchymal stem cells that synthesize and secrete type I collagen and other proteins [5]. Bone lining cells are flat, quiescent osteoblasts that form a continuous monolayer covering periosteal, endosteal, and trabecular bone surfaces. While bone lining cells do not secrete new bone, they are thought to play a role in mechanosensation and the regulation of osteoblast and osteoclast activity [6] and may be converted to an osteoblast phenotype in the presence of parathyroid hormone [7]. Osteocytes, which make up 90% of bone cells, are mechanosensitive cells embedded within a complex network of bone pores. Osteocyte cell bodies reside within lacunar pores (0.5–1 µm), with cell processes extending into canalicular channels (50–100 nm) to form a communication network with nearby osteocytes and bone lining cells [1, 8]. Osteocytes in the lacunar-canalicular network are surrounded by fluid, which allows for transport of metabolic and biochemical signaling molecules and generates flow-based mechanical stimuli during skeletal loading.
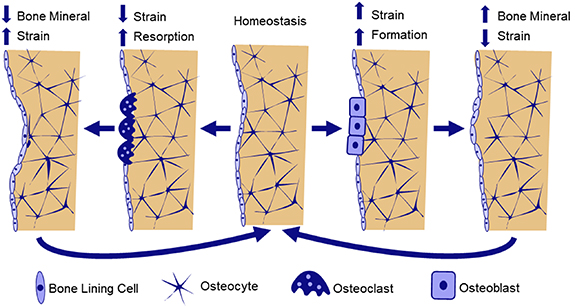
The collective activity of osteoblasts and osteoclasts result in bone remodeling and adaptation. In bone remodeling, osteoblast and osteoclast activity are coupled spatially and temporally, with cells working in 'basic multicellular units' (BMU) to resorb and replace small packets of tissue [5]. Remodeling is important for replacing older tissue with microdamage, and occurs throughout the lifespan. In healthy remodeling, bone formation and resorption are generally balanced, with no net changes in bone volume. However, there is a temporary increase in porosity and decrease in mechanical properties as formation lags behind resorption. Therefore, rapid increases in the initiation of new remodeling sites, such as during menopause, contribute to increased skeletal fragility and fracture risk. During bone adaptation, osteoblasts and osteoclasts are uncoupled, and add and remove tissue at distinct locations, resulting in net changes in bone size and shape. Bone adaptation is driven by mechanical stimuli, whereby osteocytes sense local tissue loading and recruit osteoblasts to build bone in areas of high loading and osteoclasts to areas of low loading (Figure 2). Adaptation can lead to net increases in bone mass, such as in response to increased physical activity, or bone loss during extended periods of bed rest, spinal cord injury (SCI), or space flight (Figure 3).
Figure 2. Hypothesized mechanism of strain-driven bone adaptation. Osteocyte cells embedded throughout bone tissue sense local mechanical strains. When strains are lower than the homeostatic setpoint, osteocytes release biochemical signals to recruit osteoclasts to nearby bone surfaces. Osteoclasts resorb bone, decreasing bone volume and stiffness, bringing strain back toward homeostasis. Alternatively, when bone strains are greater than the homeostatic setpoint, osteocytes release biochemical signals to upregulate osteoblast differentiation, leading to increased bone formation. As a result, bone volume and stiffness increase, and strains decrease toward homeostasis.
Download figure:
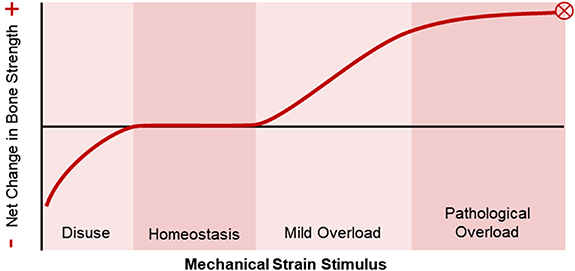
Standard image High-resolution imageFigure 3. The relationship between mechanical strain and bone strength as proposed by Harold Frost's mechanostat theory [20]. When mechanical stimuli are low, such as during bed rest, there is a net bone resorption response. The homeostasis window represents habitual mechanical loads to which bone is adapted and yield no net changes. In the mild overload window, such as during initiation of a new exercise intervention, there is a dose-dependent bone formation response. Ultimately, too much mechanical loading can be detrimental, with loads in the pathological overload window leading to fracture or bone stress injury. Adapted from Frost (2003) Anat. Rec. Part A [20].
Download figure:
Standard image High-resolution imageRecent advances in cell culture, microscopy, and molecular biology techniques have allowed researchers to identify several mechanisms by which osteocytes sense mechanical loading and regulate adaptation. Potential mechanosensors within osteocytes include integrin proteins, primary cilia, G protein-coupled receptors, and stretch-mediated ion channels [9]. Once stimulated, osteocytes undergo cellular changes such as increased intracellular calcium signaling [10] and release of adenosine triphosphate [11], nitric oxide, and prostaglandins, which upregulate bone formation [8]. Mechanical loading also decreases osteocyte expression of sclerostin [12], a protein that inhibits osteoblast differentiation by downregulating the canonical Wnt signaling pathway. Thus, loading increases Wnt signaling and promotes bone formation. During periods of disuse, lack of mechanical loading leads to osteocyte apoptosis, triggering the release of nuclear factor κβ ligand (RANKL), a critical biochemical stimulant of osteoclast differentiation and activity [13–15]. While there are likely additional pathways yet to be identified, these studies provide strong evidence of a biological basis for load-driven bone adaptation.
3. Theoretical and mathematical predictions of bone adaptation
3.1. Origins of the theory of load-driven bone adaptation
While the cellular mechanisms governing load-driven bone adaptation have been identified relatively recently, the relationship between bone form and (mechanical) function has been appreciated for centuries. In 1638, Galileo noted that larger vertebrates have stouter bones than smaller vertebrates, and suggested that there is an evolutionary relationship between body size and bone dimensions [16, 17]. In 1892, Julius Wolff published The Law of Bone Remodeling, which outlined his 'trajectorial hypothesis' that trabecular bone is 'designed' to follow stress trajectories within bone. This hypothesis was inspired by the work of anatomist Georg Hermann von Meyer and structural engineer Karl Culmann, who recognized the similarity between trabecular bone structure in the femur, as observed by von Meyer, and stress trajectories within a similarly shaped curved crane designed by Culmann [18]. While 'Wolff's Law' has largely dominated the bone adaptation narrative, it was in fact Roux who first, in 1881, hypothesized that bone is a self-regulating tissue in which cells align themselves and their matrix with principal stress trajectories [5]. This description of bone as an adaptive tissue was further developed by Harold Frost, who in 1987 published his 'mechanostat' theory [19]. Frost described bone adaptation as a homeostatic feedback mechanism, where non-customary loads act as a controlling stimulus driving activity of effector cells (osteoblasts and osteoclasts). He suggested that, like a thermostat, the bone mechanostat has threshold loading values; below a disuse threshold bone is removed, and above a minimum effective load threshold bone is added. Frost's theory served as the foundation for many subsequent theoretical descriptions of bone adaptation, and inspired decades of research to further characterize the mechanostat and determine its osteogenic thresholds.
3.2. Mathematical formulations of functional bone adaptation
In vivo loading animal models have been a valuable tool in characterizing the influence of specific loading parameters on adaptation. Starting in the 1960s, models in the rabbit tibia, sheep radius, and turkey ulna applied cyclic loads between transverse pins placed surgically through the cortical shaft. These models established that dynamic rather than static loads are required for adaptation [21–23]. They also demonstrated that strain rate [24], strain magnitude [25], and strain gradient [26] measured at the periosteal surface are related to changes in cortical bone area. Since the 1990s, most studies have used noninvasive models applying cyclic axial [27] or bending [28] loads to the limbs of rats or mice, which avoid a surgery-induced wound healing response and are feasible to apply in larger experiments with more animals. These models have provided further evidence of the importance of strain magnitude [29, 30], strain rate [31, 32], and the interaction of magnitude and rate [33] in generating a cortical response. They also demonstrated that only brief bouts of loading are needed to trigger an adaptive response, because bone becomes desensitized with continued loading cycles [34, 35]. In the trabecular compartment, artificial mechanical loading leads to increases in bone volume fraction via trabecular thickening in the rabbit femur [36], mouse tibia [37], and mouse vertebral [38, 39] loading models.
The value of the large empirical data sets generated by animal models is they provided a foundation for mathematical descriptions of bone loading dose related to their osteogenic potential. Several of these relationships have been proposed for use in humans, and the Daily Impact Stimulus was shown to be correlated to retrospective measures of bone mineral density (BMD) [40]. However, a major shortcoming of these relationships is that they lack prospective validation in humans. To address this limitation, we recently tested several of these relationships in a human upper extremity model and showed that a formulation of 'loading dose' including strain magnitude and rate was able to predict prospective changes to distal radius bone mineral content (BMC) [41]. Table 1 summarizes several of these proposed relationships.
Table 1. Proposed relationships between external mechanical stimuli and bone adaptation.
| Metric | Equation | Notes | Proposed by |
|---|---|---|---|
| Daily loading stimulus (strain-gauge based) |
![$S = {\left[ {\mathop \sum \limits_{j = 1}^k {N_j}\varepsilon _j^m} \right]^{1/m}}$](https://content.cld.iop.org/journals/2516-1091/4/1/012006/revision3/prgbac41bcieqn1.gif)
|
N is the number of cycles at strain magnitude,  , and m is a constant set to greater than 1 to account for the decay in response as number of cycles increases.
Supported by evidence from in vivo loading models. , and m is a constant set to greater than 1 to account for the decay in response as number of cycles increases.
Supported by evidence from in vivo loading models.
| [42] |
| Osteogenic index (strain-gauge/theoretical) |
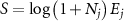
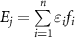
|
Ej
represents the intensity of the waveform, calculated as the product of the strain magnitude,  , and frequency, f, of a waveform with i frequency components. The logarithmic term accounts for 'diminishing returns' of increasingly high number of cycles due to desensitization.
Supported by evidence from small rodent in vivo loading models. , and frequency, f, of a waveform with i frequency components. The logarithmic term accounts for 'diminishing returns' of increasingly high number of cycles due to desensitization.
Supported by evidence from small rodent in vivo loading models.
| [34] |
| Osteogenic index (adapted for humans, GRF based; theoretical) |
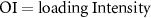
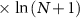
| Loading intensity is the product of peak ground reaction force (GRF) magnitude and loading rate. Supported by comparison of OI between activities known to be osteogenic, and retrospective data. | [43] |
| Daily impact score (accelerometer-based, for humans) |
![${\text{DI}}{{\text{S}}_{{\text{Exp}}}} = {\left[ {\sum\limits_{j = 1}^{32} {N_j}a_j^m} \right]^{1/m}}$](https://content.cld.iop.org/journals/2516-1091/4/1/012006/revision3/prgbac41bcieqn7.gif) Or Or
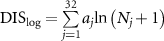
| Nj is the number of impacts at the jth acceleration level and m is an empirical constant set to 4. Both metrics significantly correlated to (human) hip bone mineral density (retrospective). | [40] |
| Enhanced daily load stimulus (EDLS) |
![${\text{EDLS}} = \left[ {\sum\limits_{j = 1}^k {N_j}{{\left( {G{z_j}} \right)}^m} + AL} \right]_{{\text{per day}}}^{1/2m}$](https://content.cld.iop.org/journals/2516-1091/4/1/012006/revision3/prgbac41bcieqn9.gif)
| N is the number of loading cycles, Gz is the vertical ground reaction force, AL is the 'accumulated load' from standing. Prescription of EDLS through exercise can reduce disuse-related bone loss at some sites (prospective, human) [44]. | [45] |
| Loading dose (adapted for humans, strain-based) | Loading dose = (mean strain) × (strain rate) × N | N is the number of loading bouts, with each bout consisting of 100 cycles. Strain is calculated from subject-specific finite element models that account for individual variations in bone structure. Significantly predicted changes in total and trabecular BMC (prospective, adult women). | [41] |
3.3. Summary
Collectively, in vivo animal loading models have demonstrated that mechanical signals related to bone strain magnitude, rate, and gradient are sensed by osteocytes. Based on a combination of mechanical and biochemical signals, osteocytes coordinate a cascade of biochemical responses that result in bone remodeling and adaptation. Several mathematical relationships have been proposed to explain or predict this response. However, very few of these have been prospectively tested in humans. This is primarily due to technical challenges with measuring both bone strain environment and bone adaptation with appropriate levels of precision in vivo in humans. For these reasons, the majority of the work on human bone adaptation has focused on observational studies comparing various specialized athlete groups, and clinical exercise interventions.
4. How do daily activities/habitual loads affect bone density and structure?
4.1. Retrospective studies
In general, the intensity of mechanical loading during exercise is positively correlated with the magnitude of bone adaptation in humans. Compared to non-athletes, female athletes in high impact (volleyball, track and field jumping) and odd impact sports (soccer and tennis), but not low impact activities (powerlifting and swimming), have greater tibia cortical area [45]. Female college athletes also have greater trabecular density at the distal tibia versus non-athletes, and moderate and high-impact sports (cross-country, volleyball, basketball) lead to increases in bending moment of inertia over the competitive season, while odd-impact sports (soccer) improve polar moment of inertia (i.e. torsional resistance) [46]. Longitudinal studies also suggest that long-term participation in high-intensity bone-loading sports leads to beneficial skeletal adaptation that is sustained into adulthood. A study in collegiate male and female swimmers showed greater increases in areal BMD (aBMD) at the hip and several tibia parameters in those who performed an additional weight-bearing sport versus those who only swam and non-athlete controls [47]. Faulkner et al showed that total body, lumbar spine, and hip aBMD were significantly greater for elite prepubertal female gymnasts versus age-matched controls [48], and a follow-up study showed that differences were maintained even when gymnasts had been retired for ten years on average [49]. Looking at the upper extremity, gymnastics is associated with increased forearm aBMD and BMC [50, 51]. Among adult female professional tennis players, BMC at the proximal humerus, humeral shaft, and distal radius has been shown to be 9%–16% higher in the playing versus non-playing arms, compared to 3%–5% side-to-side differences in age-matched controls [52]. These side-specific loading adaptations were generally maintained five years later, despite decreases in average training volume [53]. Similarly, a ten-year longitudinal follow-up of male sprinters found that those who maintained a high training volume were protected against age-related decreases in tibia trabecular bone density observed in those who decreased their training volume [54].
4.2. Abnormal/atypical loading
Habitual mechanical loads are important for the growth and development of normal bone and joint morphology. This relationship is most apparent in individuals with neuromotor control disorders such as cerebral palsy (CP), but may also play a role in the development of more subtle bone pathology such as femoral acetabular impingement syndrome. For example, in simulations based on data from children with CP, reduced posterior-oriented hip joint contact forces were shown to contribute to abnormalities in femoral growth [55]. Similar validated simulations suggest that large and habitual hip flexion loads are associated with alterations in the proximal femur growth plate orientation [56]. This may contribute to the development of cam morphology, a cause of femoral acetabular impingement. A recent 3 year longitudinal study reported that 14 year old male footballers had significantly greater increases in femoral head asphericity, a key feature of cam morphology, compared to controls [57]. This was attributed to excessive hip joint loading, though the direction of the loads was not quantified. In both children with CP and athletes who develop cam morphology, abnormal or altered loading during growth likely alters the orientation of the growth plate(s), resulting in potentially large morphologic changes.
4.3. Prospective interventions
Novel mechanical loading can also initiate bone adaptation in average individuals. A meta-analysis of nine controlled trials in premenopausal women showed that on average, impact loading interventions lead to significant increases in lumbar spine and femoral neck aBMD [58]. When impact interventions with and without resistive loading were compared, impact-only interventions (vertical jumping, skipping) showed a significant effect at the femoral neck only, while combined impact-resistive interventions (circuit training, group fitness) showed a significant effect at several sites [58]. A separate meta-analysis of six trials of brief (<30 min) high impact exercise protocols (vertical jumping) showed significant increases in bone density at the hip but not the spine [59]. This is in agreement with Zhao et al, who showed that jumping interventions increased bone density at the hip only [60]. Recent studies have examined the effect of exercise on bone microarchitecture. A 2019 study showed that among 90 female army recruits, eight weeks of basic combat training led to significant increases trabecular bone density driven by increases in trabecular thickness, and site-specific increases in cortical bone density, driven by increased cortical thickness, in regions of greatest loading [61]. Although many studies have reported equivocal or negative effects for exercise interventions in postmenopausal women, a recent systematic review [62] and meta-analysis [63] concluded that medium and high-intensity exercises—but not low intensity exercises—are effective at improving bone in the lumbar spine and hip in this population. These reviews specifically implicate resistance, potentially combined with impact training, as being most beneficial for bone.
Very few studies have systematically measured the influence of specific loading parameters on adaptation. Wang et al conducted a prospective trial of 24 healthy premenopausal women who performed a simple, controlled upper extremity 'dynamic impact loading' task over 24 weeks, and showed that reaction force magnitude was significantly and positively related to change in distal radius and total forearm aBMD [64]. A recent clinical trial in physically inactive young adult women comparing ten months of impact versus resistance training found that the effects were site-specific [65]. Impact training significantly improved volumetric BMD (vBMD) and bone strength index at the non-dominant distal radius (+8.5% and 15.4%) and tibia (+1.2% and 3.4%), while resistance training improved vBMD at the proximal femur (3.7%). These changes are clinically relevant, as reflected by a meta-analysis of therapeutic randomized controlled trials showing that a 2% increase in total hip aBMD corresponds to a 28% and 16% decrease in vertebral and hip fracture risk, respectively [66]. Several studies have calculated the osteogenic index [43] or other loading scores to characterize their loading interventions [67, 68], but data actually correlating loading scores to bone changes is limited and inconsistent [69–71]. Among adult women wearing accelerometers for 12 months, those with more large vertical peak accelerations had greater increases in femoral neck aBMD [72]. Data from this study were adapted to calculate daily impact scores (table 1), and was significantly correlated (R up to 0.550) to change in bone density at the hip [40]. These and similar accelerometer-based loading scores [73–76] may prove useful in monitoring bone loading, but lack of information about how accelerations generate strain distributions within bone tissue limit their application for characterizing bone adaptation.
We recently completed a randomized clinical trial to characterize the relationship between bone strain magnitude and rate and the resulting adaptive response in healthy adult women age 21–40 [41]. Our pilot data demonstrated that a simple distal radius loading task (leaning onto the palm of the hand to achieve a target force) was osteogenic [77] and that the resulting adaptation was somewhat site-specific, with higher strain regions having greater increases in local vBMD [78]. To eliminate variation stemming from individual anatomical differences, the trial used subject-specific finite element models as the basis for assigning each participant an individual target force to achieve a fixed strain magnitude. We found that bone 'loading dose' (table 1), a metric consisting of the total number of cycles, strain magnitude, and strain rate, explained 10%–15% of the variance in BMC gain [41]. Furthermore, we found that those participants who gained the most bone had significantly higher strain magnitudes, rates, and better compliance than those who did not.
4.4. Summary
Collectively, these retrospective and prospective studies demonstrate that mechanical signals related to strain magnitude and rate are osteogenic in both athletic and non-athletic populations. The responses are generally site specific, and different types of loading (e.g. high versus lower loading rates and sport-specific) may activate responses in cortical versus trabecular sites, and possibly peripheral versus central sites. In individuals with motor control and movement abnormalities, this can lead to alterations in bone and joint morphology. However, there is considerable individual variation in response, and it is unclear how additional factors (e.g. individual variations in hormonal environment, nutrition, and genetics) may modulate the relationship between external stimuli and bone adaptation. Despite clear evidence of a relationship, there are no evidence-based targets or thresholds for bone loading exercise, nor is it currently possible to predict osteogenic response of a given exercise intervention.
5. How does removal of daily/habitual loads affect bone structure?
Removal of daily/habitual loading to bone results in a net resorptive response, ultimately leading to disuse osteoporosis. In humans, disuse is not a normal physiologic state, although physical activity levels (and the resulting bone loads) typically decline with increasing age and decreasing health status. In small animal models, the effects of disuse have been studied using hindlimb unloading models [14, 79] and injection of botulinum toxin to induce paralysis [80, 81]. In humans, individuals with (SCI) and those subjected to microgravity offer insights into how removal of daily loading affects bone structure.
5.1. Bone loss following chronic SCI
SCI leads to an exponential loss of bone mass and subsequently, bone strength [82–84]. Persons with SCI lose 4.5%–20% BMD at the femur within 12 months post-injury [85, 86]. The weakened bone structure caused by resorption leaves the effected limbs vulnerable to fragility fracture during daily tasks of living and mobilization. Interventions that re-load the lower extremity skeleton through a combination of gravitational and muscle-induced loads have the potential to reduce or even reverse SCI-induced bone loss. However, the loss of trabecular structure and thinning of the cortex may not be fully reversible [87].
Evidence for bone reloading as a treatment for SCI-induced bone loss is currently weak due to small study sizes, inconsistent measurement sites, and (frequently) study durations that are too short to result in measurable changes [88]. (For a more detailed examination of this topic we refer the reader to the recent ISCD Official Position [89] and forthcoming clinical practice guidelines from the Paralyzed Veterans of America for monitoring and treating bone health in individuals with SCI). For example, functional electrical stimulation induced exercises, namely cycling and rowing, have been proposed to slow the progression of, and partially reverse, the early phases of bone loss [90–93]. Exoskeleton assisted walking has been shown to be an effective tool for improving some conditions associated with paralysis including body composition, spasticity, and bowel movement regularity [94, 95]. This and other applications that apply larger forces to the skeleton might be useful tools to supplement lower extremity bone loading in paralyzed individuals.
5.2. Bone loss due to microgravity
Loss of bone and muscle mass following prolonged microgravity exposure has been studied in humans and other animals [96–98]. In weightless environments, mechanical strain on the skeletal system is reduced and influences bone turnover. Bone resorption biomarkers increase as a result of load removal [99, 100]. Astronauts typically experience BMD deficits of 1%–1.6% per month at the spine and hip [96, 101]. Effects are mitigated by exercise [102], and individuals spending prolonged time in microgravity currently spend over 2 h per day exercising to maintain musculoskeletal health [103]. However, a 2021 study showed that the ability of in-flight exercise to prevent microstructural bone deterioration in the load-bearing tibia depends on pre-flight training volumes, and failure to maintain training volumes similar to pre-flight levels led to worse outcomes [104].
5.3. Summary
The removal or reduction of both gravitational and muscle-generated mechanical loads from the skeleton allows for accelerated bone resorption. Clinically, this is seen in individuals experiencing prolonged bed rest and those with partial or complete paralysis. It has also been observed in individuals exposed to microgravity, emphasizing the role that gravitational and muscle loads play in bone maintenance. Restoration of normal bone turnover is achievable through bone-loading exercises. However, not all changes to bone structure may be reversible, highlighting the need to prevent bone loss if possible.
6. Effect of other types of external stimuli on bone
6.1. Low magnitude, high frequency vibration therapy (VT)
The anabolic nature of bone is well-described for relatively low number of cycles at high strain magnitudes (2000–3000 microstrain), however some researchers have studied the possible anabolic response in bone due to many cycles of high-frequency (10–50 Hz), low magnitude (<1 g) stimuli. This is appealing for older and disabled adults, who may be unable to produce large strains in bone through exercise [105]. The exact mechanism(s) in which bone responds to low intensity, high frequency VT is largely unknown, but is speculated to be sensed directly by osteocytes, indirectly via muscle response, and/or indirectly through increased muscle strength and thus stronger postural muscle contractions [106]. In vitro studies suggest that osteocytes can respond directly to VT by reducing bone resorption through inhibition of RANKL, resulting in increased bone deposition [107]. Over the past two decades, several studies have reported promising results of VT in mice (14% increased trabecular bone volume after six weeks) [108], and sheep (34.2% greater trabecular density in the femurs of sheep after one year) [109].
Unfortunately, human studies have not shown the same success as animal models. A 2016 systematic review found that VT increased knee extensor strength, but had no significant effects on BMD in postmenopausal women with osteoporosis [110]. Similarly, a 2013 study recruited elderly men and women to undergo VT three times a day for 11 weeks but concluded that the therapy was not effective [111]. In contrast, a 2009 study found that VT produced an anabolic response in the tibias of disabled children (10 min per day, five days a week). Although compliance was low, (44%) the study group saw an increased trabecular BMD of 6.3% over the course of six months while the control group had an 11.9% decrease [112]. The lack of clear evidence of a benefit of VT may be related to lack of sensitive measurement tools. Rajapakse (2020) showed that one year of vibration daily therapy led to significant increases in tibial stiffness measured using high-resolution imaging, while DXA did not capture any significant treatment effect [113]. Overall, the literature has reported modest benefits for low-activity, high-risk populations [114], but not without issues with repeatability and participant compliance.
6.2. Pulsed electromagnetic field (PEMF) stimulation
PEMF stimulation for bone has been studied as a non-invasive option to bone healing and remodeling since 1957, when Eiichii Fukada and Iwao Yasuda published a paper on the piezoelectric properties of bone [115]. Today, PEMF bone stimulation is thought to inhibit osteoclast differentiation via the bone morphogenetic protein-2 (BMP-2) signaling pathway, increase rate of new bone formation by stimulating the release of growth factors, and promote the Wnt/β-catenin pathway to inhibit the NF-kB pathway, consequently preventing the release of inflammatory cytokines TNF-α and IL-1β [116]. Although promising, the effectiveness of PEMF for preventing or treating osteoporosis has not been well established and is not widely used [117]. PEMF is currently approved for clinical use in the United States and Europe as a treatment for enhancement of bone formation and facture nonunions, with some clinical evidence of PEMF decreasing time to union [116].
Although studies have seen inconclusive results on PEMF stimulation for nonunion fracture healing [118], a systematic review recently suggested that the therapy increases the rate of union in fractures with delayed union [119]. For example, 34 of 44 patients with delayed union who were treated with PEMF had healed fractures, with no relationships observed between union rate and medical factors such as age, fracture type, smoking, diabetes, time of treatment onset, nor initial treatment method [120].
6.3. Ultrasound and shockwave therapy
Low intensity pulsed ultrasound (LIPUS) is another method of external stimulation that is thought to work on similar pathways to VT and PEMF. It was first noted to be anabolic to bone in 1983 [121] and has since been approved in the United States for bone fracture healing. Mechanistic studies have shown that LIPUS primarily work to accelerate the speed of endochondral ossification through increased expression of vascular endothelial growth factor, BMP-2, and alkaline phosphatase [122]. A recent study found that LIPUS treatment of chronic nonunions led to a total healing rate of 86.2%, and observed that patient age was a significant factor in the failure to heal after LIPUS treatment [123]. A 2021 review reported conflicting results in the literature for the effect of LIPUS treatment on fracture healing; however, the researchers note that the differences may lie in that LIPUS is most effective in populations with elevated risk of fracture nonunion, such as diabetic and elderly patients [124].
Extracorporeal shock wave therapy (ESWT) is another non-invasive treatment studied for its success in healing nonunion and acute fractures, beginning with a 1991 study that reported fracture union 'within a reasonable time' in 70 out of 82 cases treated with ESWT [125]. Shock waves induce microtrauma that can stimulate increased vascularization and cell proliferation around the fracture site [126]. Almost 20 years later, a study including 204 patients with pseudoarthrosis and 16 patients with fresh tibia fractures treated by external fixation found that ESWT led to bone union of 85% of nonunions and 80% of fresh tibia fractures using both high (0.22 mJ mm−2) and low (1.10 mJ mm−2) energy flow densities [127]. Despite promoting fracture healing ESWT does not appear to have osteogenic effects on uninjured bone. A 2019 study found no significant differences in BMC and BMD in the healthy distal forearm of twelve non-osteoporotic post-menopausal women after six or twelve weeks of ESWT treatment [128]. The proposed mechanism of action focuses on the selective destruction of bony tissue to elicit an osteogenic response, so it is reasonable to speculate that ESWT yields the best results on fractures and nonunions rather than healthy bone.
6.4. Summary
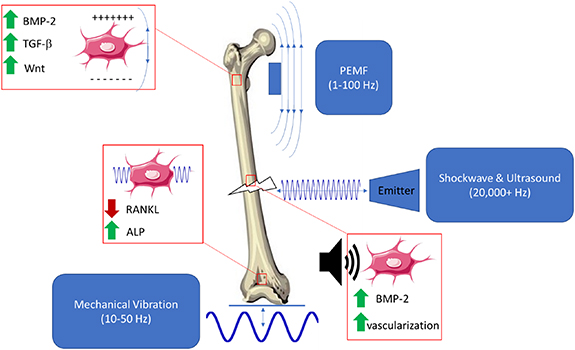
Several types of external stimuli may activate biological pathways within bone that are similar to or the same as those activated by mechanical stimuli (summarized in figure 4). These include VT, LIPUS, PEMFs, and ESWT. These technologies are appealing because of their potential to enhance bone strength without the need to exercise. However, while some animal studies have shown promising results, the clinical success of these methods have been limited primarily to fracture healing applications. VT has had some success in enhancing bone density in clinical populations such as growing children with mobility disorders, but not in older adults. PEMF, LIPUS and ESWT all appear to be effective in helping delayed and non-union fractures heal more quickly but have not been shown to enhance bone density or strength. Overall, these technologies have not been as well studied as exercise, although their potential for promoting bone healing or enhancing bone strength appears to be somewhat limited.
Figure 4. Pulsed electromagnetic fields (PEMFs), mechanical vibration therapy (VT), extracorporeal shockwave therapy (ESWT) and low intensity pulsed ultrasound (LIPUS) act in various ways on cells within a healing fracture.
Download figure:
Standard image High-resolution image7. Future areas of research
Clearly, there is a strong and direct relationship between external mechanical stimuli and bone structure and strength. However, much remains to be determined. For example, despite there being many theoretical quantitative relationships between mechanical stimulus and osteogenic response, very few of these have been prospectively validated in humans. The data that are available are widely scattered due to individual differences in underlying bone structure, diet, exercise, and other factors that modify physiological response to external stimuli. As a result of these and other challenges, there are no current guidelines on exercise or bone loading 'dose' to maximize peak bone mass or prevent or treat osteoporosis. How much bone stimulus is needed, at what magnitude and frequency, and how often, to elicit an osteogenic response? And, what are the factors that modify these parameters? Additional research is also needed to better understand the interactions between specific aspects of mechanical/external stimuli and the underlying bone physiology and adaptation. For example, individuals with chronic diseases such as type 2 diabetes mellitus, HIV, and rheumatoid arthritis all have increased risk of fractures, suggesting that these conditions may disrupt normal bone metabolism. Finally, the degree and manner in which bone adaptation is modified by various pharmaceutical treatments is not well understood. At worst, a drug may inhibit beneficial adaptation to external mechanical stimuli. At best, a drug may work synergistically along the same pathways to enhance overall response. This is an emerging and important area of research that requires good understanding of both bone physiology and mechanics.
Acknowledgements
Research reported in this publication was supported by NIAMS of the National Institutes of Health under award number R01AR063691. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This material is also based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1106756.
Data availability statement
No new data were created or analyzed in this study.
Conflict of interest
All authors declare that they have no conflicts to disclose.