Abstract
Integrating different functions in one material will decrease the volume, responding time, and price of device. Polyimides (PIs) are the optimal candidates because of excellent flexible mechanics, thermal stability, and transparent properties. In this work, a series of multifunctional flexible PIs were designed and synthesized from a novel functional triarylamine for electrochromic (EC) device. The PIs show promising application in EC with the efficiency of 205 cm2 C−1, memory devices with the ON/OFF ratio of 3.7 × 104, and photodetectors with a limit being of 0.07 V. Furthermore, the flexible EC device of PI-3 (short for PI 3 made of 4,4'-(hexafluoroisopropylidene) diphthalic anhydride) keeps excellent stability, and the optical contrast does not decrease even after 2000 cycles.
Export citation and abstract BibTeX RIS
1. Introduction
With the quick development of optic-electronic devices in numerous high-tech fields, such as calculation chip in computer, optical communication fiber, and chemical sensor, the minim device is crucial to decreasing responding time, improving performance and saving energy. It is an efficient way to integrate multifunction into one material, which is able to achieve excellent material utilization and maximally reduce the mass and volume of systems [1–4].
Recent years, in view of many advantages of multifunctional materials, scientists have used them in various fields, such as e-skin, wearable and implantable devices. [5–9]. Jianguo Mei designed and synthesized a stretchable e-skin with multiple functions, including stretchable, highly tunable resistive pressure sensor and electrochromism [10]. Pooi See Lee reported a facile synthesis of a 1D π–d conjugated coordination polymer (Ni-BTA), which exhibits superior multifunctional performances, such as electrochromism, energy storage, catalysis, sensing, and electronics [11]. Besides, Jerry Qi studied a multistimuli-responsive vitrimer with disulfide bonds, which can bear thermal- and photo-reversible as shape programmable and healable materials [12]. Furthermore, scientists have been still seeking kinds of electronic materials with more functions and novel features to expand their application fields [13–17].
Among electronic devices, the electrochromic (EC) device has been focused on for decades. Electrochromism is the optical property which can be reversibly changed by controlling a small voltage and can maintain a reversible cycle under applied voltage [18]. In addition, resistive memory device is a non-linear resistor with memory function, which have sudden change in resistance by the change of current, so as to achieve the information storage. The properties of memory device, which is sandwich structure of Al/Polymer/indium tin oxide (ITO), depend on the type and molecular structure of active layer materials. The active layer materials of organic resistive memory device usually are Triarylamine (TAA)-containing polymers [19, 20]. Meanwhile, photoelectric response is a kind of property which can detect the light by the change of the voltage of the polymer film after illumination [21].
TAA derivatives as typical hole transport materials can be oxidized easily and converted to the stable radical cations [22, 23], accompanied by obvious changes in color (from transparent/other colors to deep color). Inserting TAA group into the polymer backbone can endow the polymer with the excellent optic-electronic properties of TAA and remain the physical properties [24–26]. TAA-containing high performance polymers (HPs), such as polyamides, polyimides (PIs) and polyurethane, etc with excellent photo- and electro-active properties, fast response time and solubility, have been maturely applied to EC materials, electrofluorescent materials or memory devices [27–33]. Among the HPs, PIs with low cost, high performance and high durability have excellent mechanical property and thermal stability, which meets the requirements of practical applications. It can be made into tough, flexible, and free-standing film which caters to assemble flexible EC device (ECD) [34, 35]. In the last decades, Liou [36–38], Hsiao [39–41], Chao [42–44] and our [45–47] groups have synthesized many soluble PIs based on TAA, and studied the electronic properties. In addition, the PIs materials are also applied to memory device. Wang group report the synthesized PI possesses a volatile static random access memory characteristic with the threshold voltage of around 1.5 V and −1.8 V [48]. However, PIs is seldom reported as photoelectric response materials [49]. At present, in order to miniaturize the electronic device, it is the necessary to integrate multiple functions on PIs without reducing the performance of material.
Here, we report a series of PIs with TAA containing the isopropyl group. The multifunctional PIs retain excellent properties, namely, good flexibility and excellent thermal stability. Moreover, it can be used as the multifunctional materials in EC, memory device, photoelectric detectors, and wearable electronic fields. The flexible PIs ECDs could be bent by 20°, and the color changes from light orange to blue. Besides, the memory devices and photoelectric detector also show satisfactory performance. The PIs will promote the innovative development of multifunctional EC materials in optic-electronic field.
2. Experimental
2.1. Methods and chemicals
N-isopropyl-N-phenyl-1, 4-phenylenediamine (99%), bis-(3-phthalyl anhydride) ether (99%), 4,4'-(hexafluoroisopropylidene) diphthalic anhydride (99%), 5-(1,3-dioxo-2-benzofuran-5-carbonyl)-2-benzofuran-1,3-dione (99%), 3,3',4,4'-biphenyltetracarboxylic dianhydride (99%), 1,2,4,5-benzenetetracarboxylic anhydride (99%) were bought from Tokyo Chemical Industry Co., Ltd. (Shanghai) (TCI) and used as received. 4-fluoronitrobenzene (99%), palladium on charcoal (10%), hydrazine hydrate (80%), and triphenyl phosphite (99%) were bought from Aladdin. N, N-dimethylacetamide (DMAc), dimethyl sulfoxide (DMSO), pyridine and N-methyl-2-pyrrolidone (NMP), tetrahydrofuran (THF), ethanol, methanol and N, N-dimethylformamide (DMF) were bought from Fuyu Fine Chemical Co., Ltdj. Sodium chloride (NaCl), calcium hydridesodium (CaH2), hydride (NaH), calcium chloride (CaCl2) were bought from Tianjin Guangfu Technology Development Co. Ltd And DMAc, DMF, and NMP were dried with CaH2 and were distilled under reduced pressure before use.
The structures of PIs were characterized by the PerkinElmer Spectrum 100 Model FT-IR spectrometer. The Bruker AC-400 MHz and AC-100 MHz spectroscopies were used to obtain the NMR spectra of M1, M2, and PIs. Mass spectrometer (MS) spectrum was performed on a Finnigan LC-MS/MS system (Thermo Electron, San Jose, CA, USA). Matrix assisted laser desorption ionization-time of flight (MALDI-TOF) spectrum of monomer was obtained on the AXIMA Performance (Shimadzu, Japan) spectroscopy. Gel permeation chromatography (GPC) analysis was carried out on a Malvern instrument connected with the refractive index detector to measure the Mn and Mw using DMF as the elution solvent with the flow rate of 0.8 ml min−1, at the constant temperature of 55 °C. Ten milligram powder solid was used to obtain thermogravimetric analysis (TGA) curve by a PerkinElmer Pyris 6 TGA at a heating rate of 10 °C min−1 and a flowing nitrogen rate of 20 cm3 min−1. Scanning electron microscopy (SEM, Zeiss, Gemini SEM 300, Germany) was applied to observe the surface morphology of films. The thickness of PIs films is determined by 6JA interference microscope (Shanghai Guangxue Co.). Ultraviolet–visible spectroscopy (UV–Vis) absorption spectra were measured by the Shimadzu UV-3600 spectrophotometer (Kyoto, Japan) in solution of 10−5 mol l−1. The cyclic voltammetry (CV) curves were measured by the CH Instruments (660E electrochemical workstation) with the three-electrode electrochemical cell at a scan rate of 50 mV s−1 in 0.2 M lithium perchlorate LiClO4/CH3CN solution at room temperature. The three-electrode electrochemical cell consists of the ITO coated with polymers as the working electrode, a Pt wire as the counter electrode, and an Ag/AgCl electrode as the reference electrode, respectively. The photoelectric response of PIs films was measured using the same structure of ECD in the LiClO4/CH3CN under a 500 W Xe arc lamp (Changtuo Co. light changes every 20 s). Density functional theory (DFT) were calculated by using the Gaussian program based on B3LYP/6-311 G (d, p) basis [50]. Keithley 2636B system sourcemeter was used to test the memory performance of sandwich memory device with Al and ITO/glass as top and bottom electrodes, respectively.
2.2. Synthesis of monomers and PIs
Synthesis of N-isopropyl-N, N'-bis(4-nitrophenyl) -N'-phenylbenzene- 1,4-diamine (M1), N, N'-bis(4-aminophenyl) -N-isopropyl- N'-phenylbenzene- 1,4-diamine (M2) was carried out according to the previous work and described in ESI [51, 52]. The schematic of synthesis process is shown in scheme S1 (available online at stacks.iop.org/MFM/4/034002/mmedia).
2.2.1. Synthesis of PIs
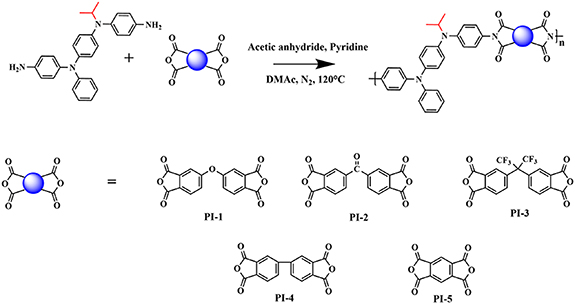
The synthesis of TAA-containing PIs is described in scheme 1 as following. Taking PI-3 as an example, the whole process was carried out under nitrogen atmosphere. In the 50 ml three-neck round-bottom flask which equipped with a magnetic rotor, M2 (0.204 g, 0.5 mmol) and 4,4'-(hexafluoroisopropylidene) diphthalic anhydride (0.222 g, 0.5 mmol) were dissolved in 4 ml of dried DMAc. The reactor was stirred at room temperature for 12 h to give poly (amic acid) solution. Then, 1.0 ml of pyridine and 2.0 ml of acetic anhydride were added to the three-neck round-bottom flask, and the mixture was heated at 120 °C for 5 h. After the reaction was finished, the mixture solution was poured slowly into 300 ml methanol to give a fiber-like precipitate, then it was collected by reduced pressure filtration. After the product was dried under vacuum at 60 °C for 48 h, the collection was purified by Soxhlet extractor with methanol for 72 h. PI-2, PI-3, PI-4 and PI-5 were synthesized in the similar way.
Scheme 1. Synthetic route of PIs.
Download figure:
Standard image High-resolution imagePI-1: Light yellow powder solid. FTIR (KBr, cm−1): 1780 and 1726 (imide ring C=O stretch); 1379 (C–N); 744 (imide ring deformation). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 0.94–0.96 (−CH3), 7.03–7.96 (aromatic ring of benzene). Yield: 88%.
PI-2: Red-brown powder solid. FTIR (KBr, cm−1): 1780 and 1724 (imide ring C=O stretch); 1380 (C-N); 721 (imide ring deformation). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 0.95–0.97 (−CH3), 7.04–8.16 (aromatic ring of benzene). Yield: 86%.
PI-3: Light red-brown solid. FTIR (KBr, cm−1): 1786 and 1725 (imide ring C=O stretch); 1376 (C–N); 723 (imide ring deformation). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 0.97–0.98 (−CH3), 7.06–7.63 (aromatic ring of benzene). Yield: 81%.
PI-4: Orange-red powder solid. FTIR (KBr, cm−1): 1774 and 1723 (imide ring C=O stretch); 1375 (C−N); 739 (imide ring deformation). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 0.97–0.98 (−CH3), 7.07–8.37 (aromatic ring of benzene). Yield: 83%.
PI-5: Light brown powder solid. FTIR (KBr, cm−1): 1778 and 1728 (imide ring C=O stretch); 1377 (C–N); 727 (imide ring deformation). 1H NMR (400 MHz, DMSO-d6, δ, ppm): 0.96–0.98 (–CH3), 7.05–8.24 (aromatic ring of benzene). Yield: 79%.
2.3. Preparations of PIs films and devices
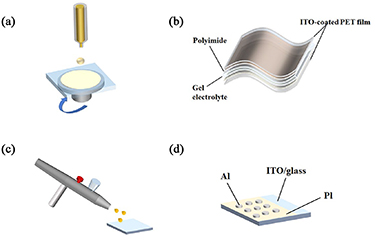
The PIs films were coated on ITO/glass by spin coating (figure 1(a)). The concentration of the solution sample was 10 mg ml−1. The films were taken shape on the spin coater, at a spinning rate of 500 r min−1, then dried at 60 °C under vacuum for 12 h.
Figure 1. The schematics of spin coating (a), flexible ECD (b), spray process (c), and memory devices (d).
Download figure:
Standard image High-resolution imageFlexible ECD was assembled step-by-step (figure 1(b)). The PIs films were prepared on ITO/PET by spray process (figure 1(c)). The concentration of the solution sample was about 20 mg ml−1. A gel electrolyte based on polymethyl methacrylate (PMMA) (1.25 g) and LiClO4 (0.15 g) were mixed with propylene carbonate (2.75 g) to form a highly transparent and conductive gel. The device was sealed by hot melt adhesive finally.
Sandwich memory devices were assembled with the PI as an interlayer, Al electrode as the top electrode, and ITO/glass as the bottom electrode (figure 1(d)), respectively. Al electrode was deposited by vacuum evaporation (1250 °C; 1 × 10−4 Pa).
3. Result and discussion
3.1. Synthesis and characterization
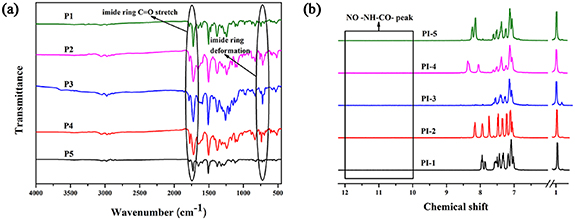
In this article, five new TAA-based PIs (Scheme 1) were designed and prepared. The 1H NMR spectrum and the FTIR spectrum of M1 are collected in figures S1(a) and (b). The absorption peaks at 1296, 1582 cm−1 are attributed to the −NO2 of M1, which indicate the M1 has been synthesized successfully. The C–H HSQC spectrum and HBMC spectrum of M2 in CDCl3 are collected in figures S3(a) and (b) and all peaks have been assigned and the structure of that is also fully confirmed. NMR spectra combined with FTIR spectra (figure S4) indicate that M2 was successfully synthesized, namely, –NH2 absorption peak is shown in the 1H NMR spectrum at 3.58 (s, 4H, –NH2) and the FTIR spectrum at 3330, 3024 cm−1. The structures of these PIs were characterized by FTIR (figure 2(a)) and characteristic bands of PIs have been marked, such as imide ring C=O stretch and imide ring deformation. The 1H NMR spectra of PIs are shown in figures 2(b)–(f). There are no –NH–CO– peaks at 10–12 in 1H NMR spectra which proves the poly(amic acid)s have reacted completely to form PIs [53].
Figure 2. FTIR spectra of PIs (a), 1H-NMR of PI-1 (b).
Download figure:
Standard image High-resolution image3.2. Thermal properties, solubility and films morphology
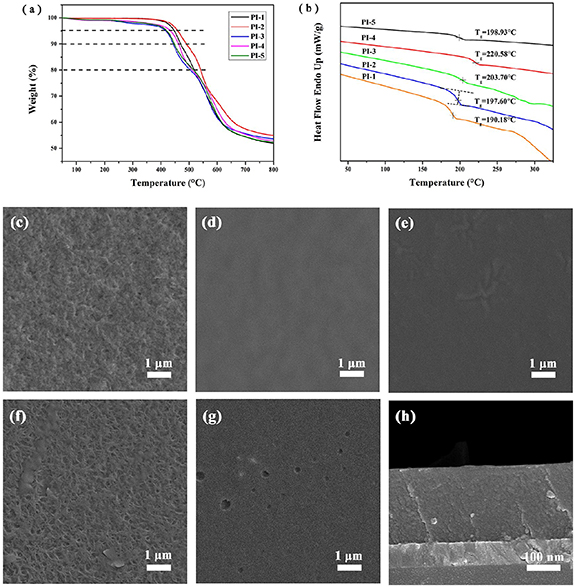
TGA curves show that PIs start losing weight when the temperature reaches about 400 °C under the nitrogen atmosphere (figure 3(a)). Besides, in table 1, the decomposition temperatures (Td) at 5%, 10%, 20% weight loss are 416 °C–460 °C, 441 °C–489 °C, and 502 °C–540 °C, respectively. When PIs were heated to 800 °C, the char yields of PIs were 52%–55%. In summary, PIs have excellent thermal stability according to TGA analysis, due to the aromatic rings and imide linkage. Compared with those of the other four PIs, the thermal stability of PI-3 is the best, which is attributed to trifluoromethyl in the structure. The glass transition temperature (Tg) curves of PIs (figure 3(b)) and Tgs (table 1) reveal that Tgs of PI-1, PI-2, PI-3, PI-4, and PI-5 are 190, 198, 204, 221, and 199 °C, respectively. The H bonds and benzene ring aggregation contribute high Tg together. Mn, Mw, and polydispersity index (PDI) of PIs are shown in table 2. Average degrees of polymerization of PIs are calculated from Mn, to be 33, 28, 29, 26, and 32, respectively. Besides, the PDIs of PIs are 1.5–1.8, which means the ratio of the two monomers to react is close to 1:1 and the PIs have narrow molecular weight distribution.
Figure 3. Td curves (a) and Tg curves (b) of the PIs, the top-view SEM images of PI-1 (c), PI-2 (d), PI-3 (e), PI-4 (f), PI-5 (g), and lateral-view SEM images of PI-3 (h).
Download figure:
Standard image High-resolution imageTable 1. Thermal properties and average molecular weights of the PIs.
| TGA and DSC techniquea | GPCb | |||||||
|---|---|---|---|---|---|---|---|---|
| Polymer code | Td5% | Td10% | Td20% | Char yield (%) | Tg (°C) | Mn (Da) | Mw (Da) | PDI |
| PI-1 | 448 | 470 | 518 | 52 | 190 | 2.3 × 104 | 3.8 × 104 | 1.6 |
| PI-2 | 460 | 447 | 514 | 52 | 198 | 2.0 × 104 | 3.6 × 104 | 1.8 |
| PI-3 | 416 | 489 | 540 | 55 | 204 | 2.4 × 103 | 4.3 × 104 | 1.8 |
| PI-4 | 434 | 463 | 507 | 53 | 221 | 1.7 × 104 | 3.0 × 104 | 1.7 |
| PI-5 | 421 | 441 | 502 | 54 | 199 | 1.9 × 104 | 2.9 × 104 | 1.5 |
aThe decomposition temperatures (Td) at 5%, 10% and 20% weight losses in nitrogen. Char Yield: measured at 800 °C. bPDI: Mw/ Mn.
Table 2. Solubility of the PIs.
| Solventa | |||||||
|---|---|---|---|---|---|---|---|
| Polymer code | DMSO | DMAc | DMF | NMP | THF | CHCl3 | CH2Cl2 |
| PI-1 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| PI-2 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| PI-3 | ++ | ++ | ++ | ++ | +− | +− | ++ |
| PI-4 | ++ | ++ | ++ | ++ | +− | ++ | ++ |
| PI-5 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
aSolubility: ++, soluble at room temperature; +−, partially soluble at room temperature; −, partially soluble on heating (80 °C).
The solubility of PIs is shown in table 2, with a 10 mg sample in 1 ml of seven kinds of solvents. PIs can be completely dissolved in DMSO, DMAc, DMF, NMP, and CH2Cl2 at room temperature, which is resulted from the isopropyl group on diamine monomer. Good solubility is a crucial performance for obtaining flat surface film by simple, cheap techniques, such as roll to roll, spray, or printing.
In figures 3(c)–(g), the top-view SEM images of PIs, the surfaces of PIs films are without broken or insoluble particles which show the excellent film-forming ability of PIs. However, the surface of PI-1 and PI-4 films is fibrous, which is different from the flat surface of PI-2, PI-3 and PI-5 films. It indicates that there will be some ordered molecular assembly, which will make ions intercalation and deintercalation easier, and increase the conductivity of films. Meanwhile, the contact area between the film and ions in the solution will increase, which is conducive to charge transport. Although the charge transfer efficiencies of PI-1 and PI-4 films surpass the other three, they are not to the extent of excellent, which may be caused by poor ion transmission. The thickness of PIs films is in the range of 212–233 nm (determined by 6JA interference microscope). The lateral-view SEM image of PI-3 film (show in figure 3(h)) indicates the film thickness is 227 nm which is the same as the result tested by 6JA interference microscope, and the inside of the PI film is integrated and dense without voids.
3.3. Optical and electrochemical properties
The optical properties of PIs were studied by the UV-visible absorption in CH2Cl2 (concentration: 1 × 10−5 M) (figure 4(a)). All PIs have an intense absorption peak at 305–312 nm (table 3 and figure S5). The strong absorption peaks locate at UV region due to π–π* transition of the C=C bond of benzene ring in TAA of PIs [25]. The absorption spectra of the PIs films are red-shifted compared to that in the CH2Cl2 solution. This may be caused by J (head to tail) type π–π stacking of aromatic rings, when the PIs were coated on the ITO/glass [54].
Figure 4. UV-visible absorption spectra of PIs in CH2Cl2 (a) and the CV curves of PIs films on the ITO/glass substrate in 0.2 M LiClO4/CH3CN solution at a scan rate of 50 mV s−1 (b).
Download figure:
Standard image High-resolution imageTable 3. Optical properties of the PIs.
| In solutiona | As films | |||
|---|---|---|---|---|
| Polymer code | λmax | λcutoff | λmax | λcutoff |
| PI-1 | 312 | 359 | 299 | 402 |
| PI-2 | 307 | 369 | 324 | 417 |
| PI-3 | 305 | 363 | 307 | 403 |
| PI-4 | 310 | 367 | 327 | 406 |
| PI-5 | 309 | 405 | 321 | 420 |
aThe concentration of polymer in CH2Cl2 is about 1 × 10−5 M.
The electrochemical properties of PIs were investigated by CV curves during the scan between 0.0 V and 1.6 V in figure 4(b), and the oxidation potentials are summarized in table 4. All the CV curves of PIs have two reversible redox peaks which are attributed to two N atoms of TAA. Taking the PI-3 as an example, the first oxidation peak and the second oxidation peak are at 1.11 V and 1.54 V, respectively. We can observe the color of PI-3 film on ITO/glass changes from transparent to light orange, then to blue. In table 4, the E1/2 of PIs are similar, the reason for which the TAA unit plays a leading role in the redox process of positive potential. When negative voltage was applied on the PI-3 film between 0.0 V and −1.6 V, there is a pair of redox peaks in CV curves, which is one-electron reduction induced by the aromatic-carbonyl π-system of the imide functional group could be observed from the imide ring of PIs [55]. The isopropyl group adjusts the E1/2s of the PIs. Figure 4 shows that the E1/2s of PIs at negative voltage are −1.21 V, −1.35 V, −1.29 V, −1.13 V and −1.15 V, respectively. In addition, the E1/2s of PI-4 and PI-5 are obviously lower than the other three dues to that the diimides structures of PI-4 and PI-5 have the stronger electron withdrawing character compared to that of PI-1, PI-2 and PI-3 [56].
Table 4. Electrochemical properties of the PIs.
| Polymer code |
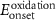 a
a
|
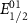 a
a
|
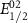 a
a
|
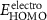 b
b
|
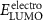 c
c
|
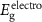 c
c
|
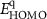 d
d
|
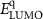 d
d
|
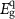 d
d
|
|---|---|---|---|---|---|---|---|---|---|
| PI-1 | 0.84 | 1.01 | 1.48 | −5.19 | −1.56 | 3.08 | −4.52 | −2.86 | 2.02 |
| PI-2 | 0.75 | 0.96 | 1.44 | −5.10 | −1.35 | 2.97 | −4.93 | −3.13 | 1.70 |
| PI-3 | 0.79 | 1.00 | 1.50 | −5.14 | −1.46 | 3.07 | −5.10 | −3.14 | 1.96 |
| PI-4 | 0.80 | 0.99 | 1.45 | −5.15 | −1.44 | 3.05 | −5.07 | −3.23 | 1.84 |
| PI-5 | 0.77 | 0.93 | 1.45 | −5.12 | −1.33 | 2.95 | −5.01 | −3.33 | 1.68 |
a
 : onset potential of PIs.
: onset potential of PIs.  : average potential of redox couple peaks. b
: average potential of redox couple peaks. b
 = −(
= −( vs. Ag/AgCl + 4.80 − E1/2, ferrocene) eV. Ferrocene is used as an external reference for calibration (E1/2, ferrocene = +0.45 V vs. Ag/AgCl) in LiClO4/CH3CN. c
vs. Ag/AgCl + 4.80 − E1/2, ferrocene) eV. Ferrocene is used as an external reference for calibration (E1/2, ferrocene = +0.45 V vs. Ag/AgCl) in LiClO4/CH3CN. c
 = −(
= −( vs. Ag/AgCl + 4.80 − E1/2, ferrocene). d
vs. Ag/AgCl + 4.80 − E1/2, ferrocene). d
 = 1240/λcutoff.
= 1240/λcutoff.
3.4. Quantum chemistry calculation
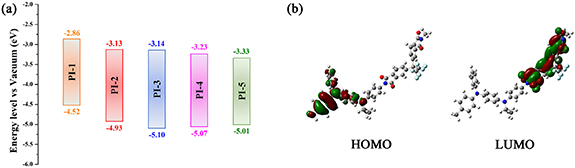
The electronic properties of structure for PIs were investigated with DFT. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of PIs are shown in figure 5(a). Taking PI-3 as an example, the electron density of the dimers is shown in figure 5(b). The HOMO locates at the central nitrogen atom which links three phenyl groups on the TAA part, and the LUMO level locates at the (difluorophenylmethyl) benzene ring. It indicates electrons can transfer from TAA moiety to benzene part of polymer. Theoretical data of  s,
s,  s and
s and  s and experimental data of
s and experimental data of  are summarized in table 4. The order of PIs
are summarized in table 4. The order of PIs  s is PI-5 < PI-2 < PI-4 < PI-3 < PI-1, which is the same as that of
s is PI-5 < PI-2 < PI-4 < PI-3 < PI-1, which is the same as that of  . However, the experimental data is smaller than the theoretical data due to some reasons, such as the polarity of the solvent, the temperature, the kind of electrolyte, and collisions between polymer and solvent molecules [57].
. However, the experimental data is smaller than the theoretical data due to some reasons, such as the polarity of the solvent, the temperature, the kind of electrolyte, and collisions between polymer and solvent molecules [57].
Figure 5. The frontier molecular orbitals computed for the PIs (a) and the electron density in the frontier molecular orbitals of the PI-3 dimers (b).
Download figure:
Standard image High-resolution image3.5. EC characteristics
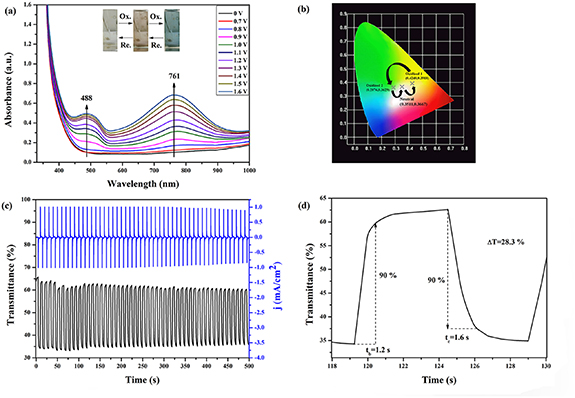
The UV–vis absorption spectra combined with CV techniques are used to study EC characteristics of the PIs. The applied potential is increased linearly from 0.0 V to 1.6 V. During the process of applying potential, the new absorption peaks of PIs at 484–491 nm to 752–778 nm (figures S6–S9) become intense.When the operating potential reaches 1.6 V, the color of film changes to blue completely. The color coordinates corresponding to the coloration phenomenon of PI-4 are demonstrated in figure 6(b). This can be attributed to the formed bistriphenylamine cation during oxidation [58].
Figure 6. The absorption spectra (a), color coordinate (b) of PI-4 film in 0.2 M LiClO4/CH3CN solution as the supporting electrolyte, and dynamic changes of the transmittance and current (c), optical switching (d) of PI-4 film at λ = 761 nm as the applied voltage was stepped between 0.0 V and 1.6 V of PI-4 film.
Download figure:
Standard image High-resolution imageDynamic changes of the transmittance combined current (figure 6(c)) are used to study the EC reversibility of PI-4. Multi-step cycle transmittance of PI-4 keeps stable reversible changes in 500 s with a cycle time of 10 s, and the optical contrast (ΔT) of PI-4 is 28.3%. Besides, the response time of PI-4 film is 1.6 s (colored time (tc)) and 1.2 s (bleached time (tb)), respectively. The fast response time is an advantage of ECDs. As for the other four PIs, the ΔTs of PIs are 23.1%, 8.5%, 29.0%, and 12.6%, respectively (figures S10–S13). The EC coloration efficiencies (CEs) of PIs are summarized in table 5, and CEs of PIs are 159 cm2 C−1, 110 cm2 C−1, 205 cm2 C−1, 186 cm2 C−1, and 98 cm2 C−1, respectively. The CE of PI-3 is bigger than that of others which indicates the higher optical modulation companies with smaller intercalation charge density. These data indicate that the new type of PIs have great application potential in EC materials.
Table 5. EC properties of the PIs.
| Response time b | |||||||
|---|---|---|---|---|---|---|---|
| Polymer code | λa (nm) | ΔT (%) | tc (s) | tb (s) | ΔOD c | Qd d (mC cm−2) | CEe (cm2 C−1) |
| PI-1 | 760 | 23.1 | 2.8 | 2.3 | 0.238 | 1.5 | 159 |
| PI-2 | 762 | 8.5 | 3.4 | 3.9 | 0.055 | 0.5 | 110 |
| PI-3 | 778 | 29.0 | 2.2 | 1.5 | 0.246 | 1.2 | 205 |
| PI-4 | 761 | 28.3 | 1.6 | 1.2 | 0.261 | 1.4 | 186 |
| PI-5 | 752 | 12.6 | 4.9 | 3.0 | 0.078 | 0.8 | 98 |
aWavelength of absorption maximum. bTime for 90% of the full-transmittance change. cOptical density (ΔOD) = log (Tb/Tc), where Tb and Tc denote the transmittances of the film before and after colorations, respectively. d Qd is ejected charge. e Coloration efficiency: CE = ΔOD/Qd.
3.6. Flexible ECD
Owing to the excellent film-forming and mechanical properties of PIs, the PIs films can form a free-standing film. The PI-3 film has the best flexible behavior in the five PIs, which can be bent at a certain degree without brittle fracture (figure 7(a)). Besides, PI-3 can adhere on the ITO/PET tightly (figure 7(b)). Whether PI-3 is in an independent free-standing film state or in a film state coated on ITO/PET, it maintains good integrity during the bending process without little damage which is attributed to the trifluoromethyl group. Therefore, we prepared flexible ECDs based on PI-3. Figures 7(c) and (d) show the flexible ECD in the normal state. When the applied potential is increased linearly from 0.0 V to 3.0 V, the color of ECD can be switched from faint yellow at 0.0 V to blue upon 3.0 V. The flexible ECD can be bent by 20° and keep the color state (shown in figures 7(e) and (f)).
Figure 7. The free-standing flexible film (10 mm ×20 mm) (a), and the flexible PI-3/ITO/PET film (30 mm ×50 mm) (b). Color change of the flexible EC device (PI-3) before (c) and after (d) coloring; color change of the flexible EC device in bending state before (e) and after (f) coloring.
Download figure:
Standard image High-resolution imageDynamic changes of the transmittance for flexible ECD prove that the flexible ECD has excellent EC cycle stability (figure S14). Multi-step cycle transmittance of flexible ECD was measured in 20 000 s with a cycle time of 10 s (2000 cycles). When the flexible ECD works after 1000 s, the optical contrast keeps stable and does not decrease even after 20 000 s. In summary, this flexible kind of ECD shows excellent EC performance in bending state.
3.7. Photoelectric properties
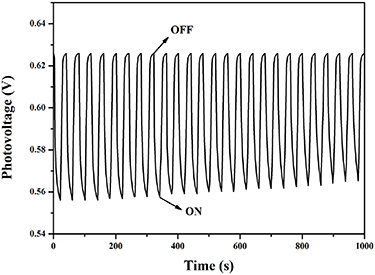
The photoelectric response of the PIs films for photodetector or photovoltage cell is studied. Take PI-3 film as an example (figure 8). A reversible photovoltage is observed when the light switches many times. The photovoltage quickly increases as soon as the PI-3 film is irradiated with the light ('on' state). On the contrary, when the light is turned off ('off' state), photovoltage drops dramatically to the primary value. The photovoltage under light illumination is 0.07 V higher than that in dark, revealing charge separation and electron transfer between the electrolyte and the PIs film when PI-3 film is illuminated [46]. The excited photovoltage of PI-3 film decreases little after many on/off cycles, which indicates that PI-3 has a stable response to light. After the PIs absorb light energy, the photo-generated electrons transfer from the LUMO to the conduction band of the ITO surface, and then move to the external circuit. The open-circuit photovoltage is engendered by the hopping of electrons. Compared with PI-3, the other four PIs show similar behavior (shown in figures S15–S18). The limit photovoltage of photodetectors based on PI-1, PI-2, PI-4 and PI-5 are 0.07 V, 0.08 V, 0.06 V and 0.05 V. It indicates their photoelectric response sensitivity are similar, but comparing their cyclic stability, it was found that PI-3 and PI-5 have more stable cyclic reversibility, which is attributed to the excellent film-forming ability of PI-3 and PI-5. Accordingly, these PIs can be utilized as photoelectric conversion materials or in photodetectors.
Figure 8. Photovoltaic responses of the PI-3 film on ITO glass upon exposure to light with switching at room temperature.
Download figure:
Standard image High-resolution image3.8. Memory performance
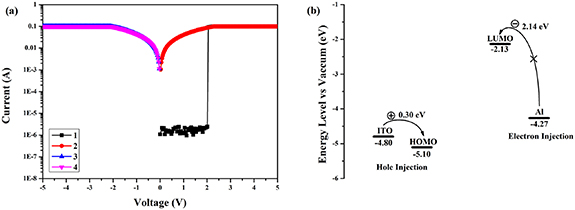
CV characteristics were investigated by ITO/PIs/Al sandwich memory device. Taking the ITO/PI-2/Al as an example (figure 9), the device displays typical electrical switching and non-volatile write-once read-many-times (WORM) storage characteristics. The other four PIs have similar storage characteristics (figures S19–S22). In '1' scan, the applied voltage (Va) of memory device is increased progressively from 0.0 V to 2.0 V (figure 9(a)), the current keeps about 2.4 × 10−6 A. When Va is raised after 2.0 V, the current suddenly increases to 9 × 10−2 A, which indicating the resistance is decreased by about 105 times. After that, the Va of WORM memory device switches from the 'off' state to the 'on' state, and it represents the conversion of the 'write' process in the digital storage device. In particular, the current switching ratio of the memory device is 3.7 × 104, which is sufficient to allow the memory device to have excellent data storage performance without misreading. In the subsequent multiple scans (2, 3, 4), even if a negative voltage is applied to the device, the device still remains in the 'on' state and keeps WORM memory characteristic.
Figure 9. I–V curves (a) and electron energy level and transport diagram (b) of the ITO/PI-2/Al memory devices.
Download figure:
Standard image High-resolution imageThe energy levels of WORM memory devices of PI-2 are displayed in figure 9(b). As for PI-2 film working as the active layer, the hole injection energy barrier (0.30 eV) is calculated by work functions of ITO (−4.80 eV) and HOMO energy level (−5.10 eV). Meanwhile, the electron injection energy barrier (2.14 eV) is calculated by work functions of Al (−4.27 eV) and LUMO energy level (−2.13 eV). By comparing the electron injecting energy barrier with the hole injection barrier, it indicates that the conduction process of WORM memory device mainly depends on hole transport, due to hole is more easier to transfer. The memory devices based on PIs have the excellent threshold voltage (Vthreshold) and WORM characteristic, which are attributed to the TAA group containing the isopropyl group. However, the switching Vthreshold of WORM memory devices based on PIs are 3.6 V, 2.0 V, 2.8 V, 3.8 V and 2.1 V, respectively. Vthreshold of PI-2, PI-3 and PI-5 are lower than the other two, which is attributed to the low HOMOs of them make hole to transfer easier. In summary, it indicates that these kind of PIs are excellent multifunctional materials which can be applied to polymer memory devices.
4. Conclusions
In summary, a series of multifunctional PIs containing asymmetry TAA have been designed and synthesized. The novel PIs materials exhibit excellent solubility, thermal stability and electrochemical properties. The flexible ECD can maintain properties when it is operated in bending state. Thanks to TAA, the ITO/PIs/Al memory device exhibits typical WORM memory performance and the ON/OFF switch current ratio of device of PI-2 is 3.7 × 104 at Vthreshold of 2.1 V. Meanwhile, PIs show a reversible and stable photovoltaic response with the switch of light. In summary, the new multifunctional EC PIs have broad potential applications in the fields of flexible ECDs, photoelectric detector and memory storage devices.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (Grant Nos. 51773053, 51373049); Heilongjiang Province Foundation for Returners from Oversea (LC2018024).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare no conflict of interest.