Abstract
The production of functionalized clinoatacamite (Cu2(OH)3Cl) nanoparticles from Reactive Cavitation Erosion (RCE) of copper in 1 M aqueous guanidine hydrochloride (GHCl) solution was investigated for applications as potential quantum magnet materials. As-synthesized nanomaterial was characterized by Diffuse-Reflectance Infrared Fourier-Transform Spectroscopy (DRIFTS), XRD, and TEM. These analyses were compared to nanoparticles produced from RCE of Cu in KCl and RCE of Cu in GHCl in oxygen-depleted (Ar) and oxygen-rich (compressed air, CA) solutions to identify possible reaction pathways.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Most research on functionalized nanomaterials focuses on bottom-up synthetic routes. These methods often involve one or more of the following limitations: costly chemical precursors; high temperatures; toxic, highly corrosive, or pyrophoric chemicals; long reaction times; or multi-step processes. Top-down synthetic pathways have the possibility to overcome these limitations. Two common top-down nanostructured materials processing methods are mechanochemistry (e.g. Reactive High Energy Ball Milling (RHEBM) [1]) and sonomechanochemistry. Sonochemistry encompasses techniques which apply ultrasound to induce or accelerate chemical reactions. When solid surfaces are simultaneously generated (e.g. via cavitation erosion) and utilized as reactive species, it is called sonomechanochemistry [2]. Advantages of sonomechanochemistry include better control of contaminants and the availability of alternative chemical pathways.
Cavitation erosion is traditionally undesirable and most published studies focus on cavitation erosion resistance [3, 4]. Few studies examine the particulate created from cavitation erosion [5, 6]. The simultaneous or subsequent involvement of nanomaterial produced via cavitation erosion in surface and bulk reactions has not been studied. This newly emergent sonomechanochemical technique is known as Reactive Cavitation Erosion (RCE). To date, only one study has been published on RCE [7]. A greater understanding of the Reactive Cavitation Erosion process is needed in order to better utilize it as a nanomaterials processing technique.
Herein the production of functionalized nanomaterial via Reactive Cavitation Erosion was investigated for Cu in 1 M aqueous GHCl. Copper was chosen due to its high cumulative volume loss during cavitation erosion [8] and low reactivity with water. Guanidine hydrochloride was chosen due to evidence that GHCl inhibits corrosion of copper [9] and because the functionalized product—clinoatacamite—is of interest as a quantum magnet material [10].
2. Materials and methods
Cavitation erosion experiments were performed as described previously [8] using the apparatus shown diagrammatically in figure 1. Experimental specimens consisted of 12.5 mm discs of Cu 110 (Superior Washer and Gasket Corp.) with thicknesses of ∼1.54 mm and nominal grain size 50 nm. Reaction fluids were prepared with guanidine hydrochloride (CH5N3 · HCl, 98% Alfa Aesar), KCl (Fisher Scientific), and purified/deionized water. The 1 molar solution of GHCl was prepared with a molarity of 0.996 M and a pH of 4.93 ± 0.02. The 1 molar solution of KCl was prepared with a molarity of 0.991 M and a pH of 5.50 ± 0.02. The volume of fluid used in each experiment was 20 ml. Six experiments were performed per reaction fluid: 1 M GHCl; 1 M KCl; and H2O. Five additional GHCl experiments were performed in which a gas was bubbled through the solution via a 1/16' ID PTFE tube: three with argon (Ar) and two with compressed air (CA) to create oxygen-depleted and oxygen-rich environments, respectively. The gases were bubbled continuously through the solution, at a flow rate of ∼0.25 ml·min−1, from 10 min prior to the start of the experiment to the end. The specimens were sonicated at a sonotrode amplitude of 57 μm for 2 h. No fluids were added to the reactive medium during this time period. Following each RCE experiment, the particle-laden fluid was filtered sequentially through 10 μm, 1 μm, and 100 nm filters (Sterlitech). The filters with particles were dried overnight in a vacuum oven at 105 °C.
Figure 1. Schematic diagram of cavitation erosion apparatus.
Download figure:
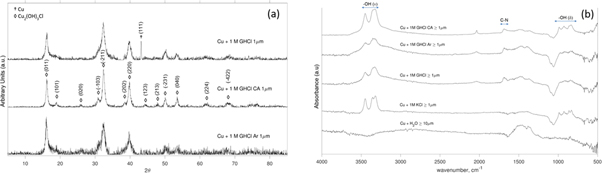
Standard image High-resolution imageFigure 2. (a) XRD of Cu + GHCl experiments and (b) DRIFTS spectra.
Download figure:
Standard image High-resolution imageParticulate samples were characterized by Diffuse-Reflectance Infrared Fourier-Transform Spectroscopy (DRIFTS) with a Nicolet 670 FTIR to provide chemical composition of the particulate surface. Particle morphology analysis was performed using a transmission electron microscope (TEM, FEI Tecnai G2 F30 Twin, 300 kV) equipped with a Bruker Energy Dispersive spectrometer (EDS). Electron microscopy samples were prepared by dispersing particles in ethanol and then a droplet of the suspension was deposited on a Ni TEM grid. X-ray diffraction (XRD) analysis was performed with a Scintag XDS 2000 (Cu K-α source, λ = 1.5406 Å) to identify the phases present in the bulk particulate material.
Figure 3. Nanoparticle TEM images (a) Cu + GHCl, (b) Cu + GHCl Ar, (c) Cu + GHCl CA, (d) Cu + KCl.
Download figure:
Standard image High-resolution image3. Results and discussion
3.1. Particle composition
The average mass losses of the copper discs and pH changes are shown in table 1. All experiments led to roughly equivalent mass losses. As previously reported, ionic solutions don't lead to significant differences in bubble dynamics, so any differences in particle production for KCl solutions can be attributed to chemical rather than mechanical effects [11].
Table 1. Average mass losses and changes in the pH of RCE experiments.
| Experiment | Average mass loss (g) ± std. dev. | Starting pH | Average ΔpH ± std. dev. |
|---|---|---|---|
| Cu + H2O | 0.117 ± 0.015 | Neutral | −0.22 ± 0.20 |
| Cu + KCl | 0.092 ± 0.027 | 5.50 ± 0.02 | 4.32 ± 0.15 |
| Cu + GHCl | 0.141 ± 0.010 | 4.93 ± 0.02 | 3.89 ± 0.16 |
| Cu + GHCl Ar | 0.164 ± 0.004 | 4.93 ± 0.02 | 3.77 ± 0.07 |
| Cu + GHCl CA | 0.150 ± 0.026 | 4.93 ± 0.02 | 5.03 ± 0.30 |
Distinct colors were observed for the particulates depending on the reactive medium: GHCl produced blue-green to deep green particles; KCl produced yellow-green particles; and H2O produced copper colored particles. XRD analysis of ≥1 μm particles from the different GHCl experiments revealed peaks attributed to clinoatacamite (Cu2(OH)3Cl), shown in figure 2(a). The XRD spectrum for Cu + GHCl also featured the Cu (111) peak. The Cu + KCl XRD spectrum (not shown) exhibited peaks for clinoatacamite, cuprite (Cu2O), and copper. The presence of cuprite caused the shift in color to yellow-green.
The DRIFTS spectra of the ≥1 μm particulates produced from the GHCl and KCl experiments, shown in figure 2(b), exhibited peaks characteristic of clinoatacamite from ∼3200 to ∼3500 cm−1 attributed to hydroxyl stretching vibrations ν(OH) and from ∼830 to ∼990 cm−1 attributed to hydroxyl deformation vibrations δ(OH). The NH2 stretching vibrations also appear between ∼3200 to ∼3500 cm−1. Therefore, surface functionalization with GHCl was confirmed by the peaks at 1685 cm−1, corresponding to a combination of C–N stretching vibrations and amine deformation vibration ν(CN3) + δ(NH2) [12], and ∼2035 cm−1, which is attributed to a combination of bonds from the guanidinium ion and clinoatacamite.
3.2. Particle morphology
Particle morphology provides information on the formation mechanism. The Cu + GHCl particles, (a) in figure 3, exhibited irregular morphologies with variable thickness, consistent with ablation. The Cu + GHCl Ar particles, (b) in figure 3, had similar irregular morphology but also exhibited large sheet-like regions. Some of which curl or fold to produce spike-like structures. The Cu + GHCl CA particles, (c) in figure 3, exhibited regular angular morphologies. Irregular structures occurred side-by-side with the angular structures in some particles, indicating that the particles likely originated from ablated material and that crystal growth had occurred. High levels of dissolved oxygen throughout the experiment led to alteration of the chemical environment such that crystal growth was possible. Particles produced from the Cu + KCl experiments, (d) in figure 3, exhibited highly faceted morphologies not observed in the GHCl experiments. No evidence of ablation was observed, though crystal growth could have obscured any evidence. The differences in the crystal morphologies between the Cu + GHCl CA and Cu + KCl experiments indicated that the guanidinium ion influenced the crystal growth process. There were no significant differences in composition of representative Cu + GHCl Ar particles (figure 4), where the presence of Ni is due to the support grid. EDS analyses of particles from the Cu + GHCl, Cu + GHCl CA, and Cu + KCl experiments were similar to that of the Cu + GHCl Ar particles in that they also exhibited peaks for C, O, Cl, Cu, and Ni with similar peak ratios.
Figure 4. Cu + GHCl Ar EDS spectra and associated nanoparticles. Scale bars in images are 50 nm.
Download figure:
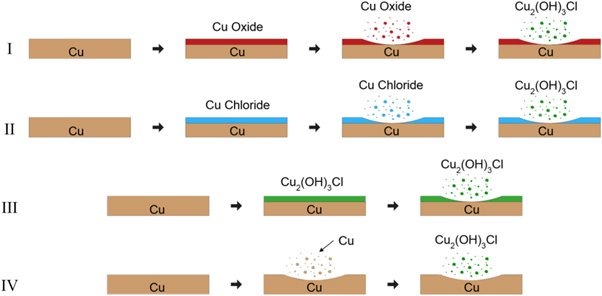
Standard image High-resolution imageFigure 5. Schematics of potential RCE mechanisms.
Download figure:
Standard image High-resolution image3.3. Reaction mechanism
Various reaction mechanisms have been proposed for Cu2(OH)3Cl synthesis from copper [13]. These mechanisms were based on experiments with copper substrates in quiescent chloride-containing media. The additional energy input from Reactive Cavitation Erosion means direct attribution to a known reaction pathway is difficult. This energy may allow for reactions that were not thermodynamically favorable at quiescent conditions to dominate. Due to limitations in analytic capability and the turbulence of RCE, in situ reaction analysis was not practical. Identification of possible reactions was performed via post-experimental analyses including XRD and FTIR. Potential clinoatacamite formation mechanisms via RCE include:
- I.Copper oxides form at the disc surface, ablate, and then reactions in solution lead to clinoatacamite;
- II.Copper chlorides form at the disc surface, ablate, and then reactions in solution lead to clinoatacamite;
- III.Clinoatacamite forms at the disc surface and subsequently ablates away;
- IV.Metallic copper is ablated from the disc surface and reactions in solution lead to clinoatacamite.
Schematics of these mechanisms are shown in figure 5.
Copper oxides were observed in the XRD spectra of the ≥10 μm particles from the Ar and CA GHCl experiments as well as the KCl experiments but were absent in the disc spectra. Additional experiments (results not shown) were performed on copper oxide powders to aid in reaction mechanism determination. No clinoatacamite or copper chlorides peaks were observed in the XRD of the copper oxide powder experiments. The lack of copper oxides at the disc surface and the results from the additional experiments rule out mechanism I.
Mechanism II is unlikely due to a lack of copper chloride peaks in any of the XRD spectra. The high water solubility of tolbachite CuCl2 (ca. 757 g·l−1 at 25 °C) [14] means that any CuCl2 formed during the experiments would likely have been dissolved rather than ablated and XRD detection would be complicated by removal during the water rinses following the experiments. The lack of nantokite (CuCl) peaks in the XRD spectra was a strong indication that CuCl was not produced since the low solubility of CuCl—ca. 0.05 g·l−1 at 20 °C [14]—meant that most unreacted CuCl formed would have remained after the water rinses.
No peaks attributable to copper hydroxychlorides were observed in the XRD spectra for the experimental discs. This information indicates that the dominant RCE mechanism did not proceed via formation of Cu2(OH)3Cl at the disc surface followed by ablation, thereby ruling out mechanism III.
The remaining mechanism involves metallic copper ablating from the disc surface, followed by conversion to clinoatacamite via reactions in solution (mechanism IV). This is also the simplest mechanism. The mechanistic steps for clinoatacamite formation from copper in 1 M GHCl under these conditions is the subject of future investigations.
4. Summary and conclusions
Reactive Cavitation Erosion of copper discs in 1 M GHCl produced surface-functionalized clinoatacamite nanoparticles. Bubbling argon and compressed air through the GHCl solution during the experiments affected nanoparticle morphology. The mechanism and reaction pathway for clinoatacamite nanoparticle formation were assessed based on XRD, DRIFTS, and electron microscopy. The dominant mechanism for nanoparticle formation was most likely ablation of metallic copper from the disc surface followed by reactions in solution. These findings provide insight into the newly emergent field of Reactive Cavitation Erosion.
Acknowledgments
Special thanks to Tanya Goehring for her aid with the XRD instrument.