Abstract
Defect engineering of metal-organic frameworks has attracted increasing attention in recent years for potential applications in gas storage and catalysis. In this study, defective UiO-66 is obtained by Ar and H2 plasma treatments. Compared with the pristine UiO-66, a new aperture with a size of ∼4 nm appears for a sample with the plasma modification, indicating the formation of mesopores within UiO-66 framework. Characterization results demonstrate that the pore volume, surface area and the number of Lewis and Brönsted acid sites can be easily tuned by varying the discharge parameters. The adsorption performance of UiO-66 is evaluated for the adsorption of methyl blue. In comparison to the pristine UiO-66 and the sample with H2 plasma treatment, the Ar plasma modified sample shows excellent adsorption activity due to the suitable pore size and volume. Equilibrium adsorption capacity as high as 40.6 mg·g−1 is achieved for the UiO-66 (Ar) sample.
Export citation and abstract BibTeX RIS
1. Introduction
In recent years, metal-organic frameworks (MOFs) have attracted great attention due to their unique structure. As a new class of porous materials, MOFs consist of metal ions or clusters (nodes) and organic ligands (linkers) through coordination bonds. This construction supplies MOFs with ultra-high surface areas and large porosities, enabling them to be used in broad applications in gas storage, liquid or gas separation, catalysis, and chemical sensing [1–4]. Among various MOFs, the UiO family (UiO-66, UiO-67 and UiO-68) has been extensively studied, due to the feasible production conditions, versatile connectivity and ease of reproducibility [5, 6]. Generally, UiOs are composed of Zr4+ and dicarboxylic acid and have the same network topology [7]. As the prototype of the UiO family, UiO-66 is the first zirconium cluster-based material with the composition of Zr6O4(OH)4(BDC)6, where 6 Zr4+ are situated at the vertices of an octahedron with OH and O alternately located at the center of each of the eight faces. A strong affinity and bonding between Zr4+ and carboxylate O atom provides UiO-66 with exceptional physicochemical and thermal stability, which makes UiO-66 suitable for various applications, such as catalysis, small molecule adsorption and separation, toxic chemical sensing and drug delivery [8].
In an ideal UiO-66, each Zr center connects to 12 BDC linkers, forming a face-centered cubic crystal structure. However, such a stoichiometrically perfect framework does not exist, and all UiO-66 materials contain a large number of defects, including missing cluster/Zr6 node defects and missing-linker defects. In fact, some predetermined properties of UiO-66 MOFs closely relate to the controlled numbers of particular defects. Wu et al reported that the tunable missing-linker defects were able to improve the pore volume and the surface area of UiO-66 (up to 150% and 60%, respectively), and found that the excess CO2 adsorption isotherm for the UiO-66 sample with the most defects was ∼50% higher than the sample with the least defects when the gas pressure was 3.5 MPa [9]. Liu et al investigated the catalytic performance of different kinds of defects contained in UiO-66 for the conversion of glucose to fructose [10]. The missing cluster defects showed superior actively catalytical performance in comparison to the missing linker.
The most common methods to synthesize and quantitatively control the defects within UiO-66 are de novo synthesis and post-synthetic treatment approaches [11–13]. The de novo synthesis method includes incorporation of modulators, linker modification and metal cation substitution, which are related to adjusting the synthesis conditions during UiO-66 production. The post-synthetic treatment involves the treatment of the synthesized parent UiO-66 with harsh activation procedures, such as thermal activation/dehydration, acid/base or high-temperature treatments.
Recently, the plasma technique has been used to produce the missing-linker defects of UiO-66 [14]. It is known that plasma consists of numerous high-energy electrons, ions, active radicals, excited atoms and molecules, while the bulk gas temperature remains close to room temperature [15, 16]. Based on these merits of high reactivity and reduced thermal effect, plasma has been widely used for the materials fabrication [17–19] and surface modification [20]. With regard to the post-synthetic treatment of UiO-66 by plasma, the defective framework was fabricated by active species reaction or energetic ion bombardment with the parent UiO-66 [14]. Compared to other approaches, the plasma technique is a time-saving, efficient and green method for the preparation of certain quantitatively defective UiO-66 MOFs.
This study aims to resolve the defect formation within UiO-66 via the Ar and H2 rapid plasma treatment (no more than 5 s). We use either Ar or H2 gas as the discharge gas to modify UiO-66 materials. The effects of plasma treatments on UiO-66 adsorption performance for methylene blue (MB) have been carefully investigated.
2. Experiment
All chemicals and solvents were purchased from Aladdin Industrial Inc. (Shanghai, China) and used as received without further purification. UiO-66 MOFs were prepared using a solvothermal method in accordance with [21, 22]. In a typical synthesis, 0.1463 g of ZrOCl2·8H2O and 0.1463 g of terephthalic acid (HBDC) with a Zr/HBDC ratio (fZr/HBDC) of 1:2 were dissolved in 50 ml dimethylformamide (DMF). The solution was then transferred to a 100 ml Teflon-lined autoclave, sealed and placed in a preheated oven at 120 °C for 24 h, after which it naturally cooled to room temperature. The resulting product was collected by centrifugation and washed three times with DMF to remove the unreacted terephthalic acid. The white powder was further washed with CH3OH to remove the DMF adsorbed in the pores of the product and dried at room temperature. Finally, the product was calcined at 373 K in a vacuum oven for 10 h.
The plasma treatment of as-synthesized UiO-66 was performed in a homemade horizontal dielectric barrier discharge (DBD) reactor. The detailed structure of the reactor can be found in previous reports [15, 23], and only the main features are outlined here. The reactor mainly consisted of a 80 cm long fused silica tube (inner diameter 40 mm) and quartz boat placed along the axis of the outer tube as the UiO-66 container. Two copper coils were wrapped on the outside upstream of the fused silica tube to serve as the high-voltage and ground electrodes with a distance of 100 mm separating the two electrodes . The high-voltage electrode was connected to a 13.56 MHz radio-frequency power source with a matching network for producing plasma. High-purity (99.999%) Ar or H2 gas was employed as the discharge gas, which continuously flowed into the reactor at a flow rate of 50 standard cubic centimeters per minute, and the corresponding working pressure was 50 Pa. LabVIEW software was used to control the plasma treatment time of the UiO-66 framework with an input power of 60 W. UiO-66 (Ar) and UiO-66 (H2) denote the UiO-66 samples modified by Ar and H2 plasma in this paper, respectively.
The x-ray diffraction (XRD) patterns of the UiO-66 MOFs were inspected by x-ray diffractometer (Rigaku, MiniFlex600) with Cu Kα radiation performed at 40 kV and 15 mA. The chemical composition and impurity of UiO-66 were determined by x-ray photoelectron spectroscopy (XPS) (Thermo Escalab 250Xi). The spectrometer was equipped with a monochromatic Al Kα source (1486.6 eV). The amount of Zr in the UiO-66 MOFs was measured by inductively coupled plasma optical emission spectrometry (ICP-OES) (EXPEC 6000), while the quantities of C, O and H were evaluated by an elemental analyzer (Elementar, Vario Micro Cube). The acidity of the UiO-66 samples was determined by pyridine-Fourier transform infrared spectroscopy (Py-FTIR), and the measurements were performed with an FTIR spectrometer (Thermo Nicolet 6700).
The specific surface area of the UiO-66 before and after the plasma treatment was analyzed by the Brunauer–Emmett–Teller (BET) (Micromeritics, ASAP 2020 Plus 3155) method from the N2 adsorption isotherms at 77.3 K. The total pore volume was estimated based on the amount of N2 adsorbed at P/P0 = 0.99, and pore size distributions were determined by means of the Barrett–Joyner–Halenda method. Thermogravimetric analysis (TGA) of the UiO-66 sample was examined using a thermogravimetric analyzer (NETZSCH, TG 209C), and the measurement was carried out in a N2-ambient glovebox with a heating rate of 5 °C·min−1 from 25 °C to 700 °C.
The adsorption of methyl blue was carried out at room temperature in a 100 ml beaker containing 50 ml 1 × 10−5 MB aqueous solution and 10 mg UiO-66 with continuous stirring in a dark place. About 1 ml solution was taken from the beaker at certain time intervals and filtered by a 0.45 μm membrane filter. The methyl blue concentration in the solution was determined by a UV–vis spectrophotometer (Agilent, Cary 4000). The amount of MB absorbed on UiO-66, q (mg·g−1), was determined from,
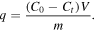
In this expression, C0 and Ct are, respectively, the liquid-phase concentrations of MB solution at the initial time and at any other time. m is the mass of UiO-66 powder and V is the volume of MB solution.
3. Results and discussion
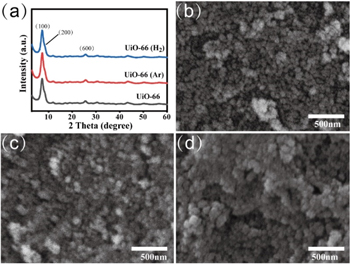
The crystal structure of the synthesized samples and the corresponding morphology are shown in figure 1. As shown in figure 1(a), the XRD patterns for the UiO-66 sample demonstrate crystalline diffraction peaks centered at 7.4°, 8.5° and 25.6°, which can be attributed to the (100), (200) and (600) planes of the face-centered cubic structure [24]. After Ar or H2 plasma treatment for 5 s, both the UiO-66 (Ar) and UiO-66 (H2) samples maintain highly crystalline structure. These results indicate that the main structure does not collapse via rapid plasma treatment. The SEM images shown in figure 1(b) show that UiO-66 is aggregated particles. As Yao et al pointed out, UiO-66 synthesized employing ZrOCl2·8H2O as Zr precursor consists of agglomerated particles [25]. Moreover, numerous defects exist due to the partial displacement of hydroxyl ion groups and the coordinated water on Zr ions or nodes. When Ar or H2 plasma is used, unobvious changes in the morphologies of the UiO-66 samples can be found (figures 1(c) and (d)), implying again that the main framework structure of UiO-66 remains stable.
Figure 1. (a) XRD results for the UiO-66 samples and corresponding SEM images, (b) UiO-66, (c) UiO-66 (Ar) and (d) UiO-66 (H2).
Download figure:
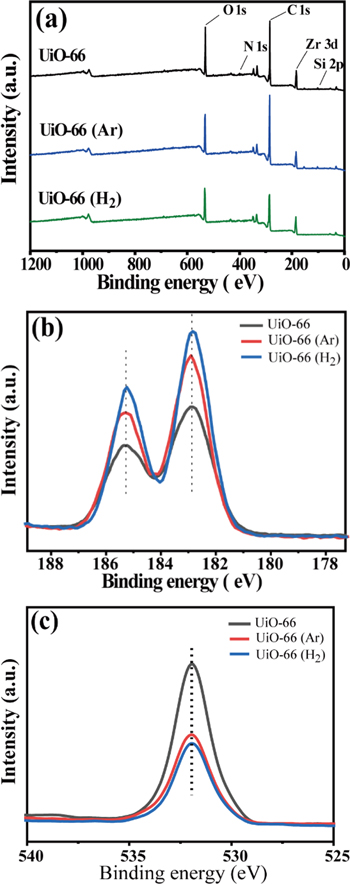
Standard image High-resolution imageThe chemical compositions of the pristine UiO-66, UiO-66 (Ar) and UiO-66 (H2) samples were examined using XPS. The survey spectra shown in figure 2 exhibit the peaks from Zr, C, O and N photoelectron emission. The high-resolution spectra are used to analyze Zr 3d, as shown in figure 2(b). Two main peaks are found, which can be attributed to the spin–orbit splitting peaks of Zr 3d. For the pristine UiO-66, the binding energies of Zr 3d3/2 and Zr 3d5/2 are 185.23 and 182.88 eV, respectively. After Ar plasma treatment for 5 s at a working pressure of 50 Pa, the corresponding binding energy shifts to higher values (185.31 and 182.91 eV). In contrast, H2 plasma treatment makes the binding energy move to lower values (185.24 and 182.84 eV). These results imply that, in comparison with the pristine UiO-66, plasma treatment may cause a change in the coordination bonds of Zr ions or clusters. Figure 2(c) shows the high-resolution spectra of O 1s core-level emission. The binding energies of O 1s for the pristine UiO-66, UiO-66 (Ar) and UiO-66 (H2) samples are all found at 531.94 eV.
Figure 2. XPS spectra for UiO-66, UiO-66 (Ar) and UiO-66 (H2). (a) Survey spectra, (b) high-resolution spectra of Zr and (c) O.
Download figure:
Standard image High-resolution imageTGA was used to further analyze the relationship between the plasma treatment and the missing-linker defects within the UiO-66 samples. Figure 3 shows the TGA curves of the pristine UiO-66, UiO-66 (Ar) and UiO-66 (H2), which can be divided into three main weight loss regions. The first weight loss region corresponding to the temperature range of 30 °C–150 °C can be ascribed to the desorption of physisorbed DMF solvent and H2O. The second weight loss that occurred in the temperature range 150 °C–400 °C is a direct consequence of the dehydroxylation of Zr6 clusters. The last weight loss in the temperature range from 400 °C–600 °C is attributed to the decomposition of BDC linker coordinated to Zr6 cluster [26]. All three TGA curves show that the weight losses of pristine and plasma-treated UiO-66 samples correspond to the same temperature region, implying that Ar or H2 plasma treatment does not reduce the thermal stability of the UiO-66 samples. When the temperature is higher than 600 °C, the structure of the UiO-66 framework collapses and ZrO2 is left. The residue weight of UiO-66 (Ar) is a little bit higher compared with that of the pristine UiO-66, indicating that Ar plasma treatment leads to the removal of BDC linker. In the case of H2 plasma treatment, the amount of the residue weight increases further to ∼51%. A possible reason is that H2 plasma contains more reactive H species, and results in more BDC missing-linker defect formation in comparison with Ar plasma treatment. These results are consistent with those presented in the following ICP measurements.
Figure 3. TGA profiles of UiO-66, UiO-66 (Ar) and UiO-66 (H2).
Download figure:
Standard image High-resolution imageThe quantities of C, O, H and Zr within the UiO-66 samples with and without plasma treatment were determined by an elemental analyzer and ICP-OES. As shown in table 1, Zr and O weight percentages for the pristine UiO-66 are 20.2% and 22.7%, which are lower than their theoretical values of 32.9% and 30.8%. In contrast, the actual C and H contents (39.0% and 4.0%) are higher than the theoretical quantities (34.6% and 1.7%). Moreover, 4.4% N is observed in the pristine UiO-66. These results suggest that the removal process of DMF solvent during UiO-66 fabrication is incomplete, and a small portion of DMF remains in the synthesized UiO-66. In the case of UiO-66 treated by Ar plasma for 5 s, the Zr amount increases to 27.2%, while the contents of C, H, O and N decline to 38.2%, 3.7%, 22.2% and 3.2%, respectively. A possible reason for the increase in Zr content and the decrease in other elemental quantities is the energetic Ar ion collisions with UiO-66. On one hand, the ion impact will remove partial DMF molecules adsorbed on the UiO-66. On the other hand, the ion bombardment will result in the elimination of coordination bonds between the metal ions/Zr6 clusters and organic ligands (linker). As a result, the missing-linker defects within the UiO-66 are generated. In the case of post-synthetic treatment of UiO-66 by H2 plasma, the Zr amount will further increase to 36.8%, and the C, H, O and N contents decrease continuously to 38.0%, 3.6%, 21.8% and 3.0%. In comparison to Ar plasma, H2 plasma contains a large number of active H and H* 2 species. These active species are capable of reacting with UiO-66, leading to the removal of BDC linker. Consequently, the number of non-metal elements further decreases and the Zr weight percentage increases after H2 plasma modification.
Table 1. Elemental composition of UiO-66, UiO-66 (Ar) and UiO-66 (H2).
| Sample | C (at%) | H (at%) | O (at%) | N (at%) | Zr (at%) |
|---|---|---|---|---|---|
| UiO-66 | 39.0 | 4.0 | 22.7 | 4.4 | 20.2 |
| UiO-66 (Ar) | 38.2 | 3.7 | 22.2 | 3.2 | 27.2 |
| UiO-66 (H2) | 38.0 | 3.6 | 21.8 | 3.0 | 36.8 |
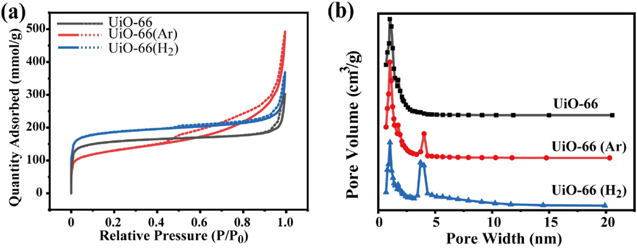
Figure 4 exhibits N2 adsorption-desorption isotherms and pore size distributions of the pristine UiO-66, UiO-66 (Ar) and UiO-66 (H2) samples. As shown in figure 4(a), the N2 adsorption isotherm for the pristine UiO-66 exhibits type I isotherm with no hysteresis. In contrast, N2 adsorption isotherms for UiO-66 (Ar) and UiO-66 (H2) display typical IV model isotherms, suggesting the existence of mesopores in these two samples. The H4 type hysteresis loops very likely come from the capillary effects in mesopores. Capillary evaporation and capillary condensation do not occur at the same pressure, resulting in the appearance of hysteresis loops. Figure 4(b) shows that the aperture size of UiO-66 is mainly concentrated on ∼1.1 nm. After H2 or Ar plasma treatment, a new aperture with a size of ∼4 nm appears, indicating the formation of mesoporous UiO-66. This change again confirms that the plasma plays an important role in the expansion of partial UiO-66 micropores.
Figure 4. (a) Nitrogen sorption isotherms at 77 K for UiO-66, UiO-66 (Ar) and UiO-66 (H2), (b) pore size distribution as functions of pore width.
Download figure:
Standard image High-resolution imageBased on N2 adsorption-desorption isotherms, mesopore parameters related to the specific area (SBET), total pore volume (V) and pore size (D) are calculated and shown in table 2. The values of SBET, V and D for the pristine UiO-66 are 606.3 m2·g−1, 0.41 cm3·g−1 and 2.72 nm, respectively. After Ar plasma modification, SBET slightly decreases to 604.9 m2·g−1, whereas V and D increase to 0.48 cm3·g−1 and 3.21 nm, respectively. With H2 plasma treatment, SBET dramatically declines to 422.0 m2·g−1, and the corresponding V and D sharply rise to 0.67 cm3·g−1 and 5.80 nm, respectively. In response to the plasma treatments, coordination bonds between the Zr ions and BDC linkers break and the defects within UiO-66 are generated. In particular, more BDC linker is removed in the case of H2 plasma treatment due to the reaction of hydrogen species with BDC, resulting in the minimum value of SBET, and maximum values for V and D. These results are in agreement with ICP-OES and elemental analysis results.
Table 2. Surface area, pore volume and pore size of UiO-66, UiO-66 (Ar) and UiO-66 (H2).
| Sample | BET surface area (m2·g−1) | Pore volume (cm3·g−1) | Pore size (nm) |
|---|---|---|---|
| UiO-66 | 606.3 | 0.41 | 2.72 |
| UiO-66 (Ar) | 604.9 | 0.48 | 3.21 |
| UiO-66 (H2) | 422.0 | 0.67 | 5.80 |
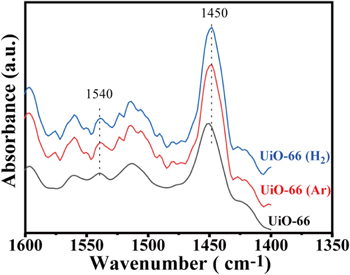
The formation of defects definitely affected the acidity of UiO-66, thus changing its adsorption performance for MB. Pyridine infrared spectrum is used to determine the variation of the acidity of the UiO-66 samples after the defects are generated. For the defective UiO-66, Lewis acid sites are related to the exposed Zr4+, and Brönsted acid sites can stem from Zr-OH, Zr-OH2 and μ3-OH [27, 28]. Figure 5 shows Py-FTIR spectra of UiO-66, UiO-66 (Ar) and UiO-66 (H2). The peak centered at 1447 cm−1 is associated with Lewis acidity due to the unsaturated Zr ion. The Brönsted acid site is associated with the peak at 1538 cm−1, corresponding to pyridinium [29]. Based on the area of the peaks shown in figure 5, the number of Lewis and Brönsted acid sites are calculated and presented in table 3. For the pristine UiO-66, the quantities for the Lewis and Brönsted acid sites are 79.51 and 2.86 μmol·g−1, respectively. When Ar plasma treatment is used, these two values increase to 300.85 and 10.57 μmol·g−1, respectively. The increase in Lewis acid sites can be ascribed to the contribution of exposed Zr4+ initiated energetic Ar+ collision with UiO-66. Meanwhile, the partially unsaturated Zr atoms will be compensated for the negative charge by –OH/H2O pairs or the terminal –OH groups, leading to an increase in Brönsted acid sites. In comparison to Ar plasma treatment, both Lewis and Brönsted acid sites decrease after H2 plasma modification. A possible reason is derived from the fact that the reductive H2 plasma is able to reduce a small amount of highly positive charged Zr ions to lower values or Zr atoms, which can be seen in the XPS results. The decline in Zr ion amount leads to a decrease in Lewis and Brönsted acid sites.
Figure 5. Py-FTIR spectra of UiO-66, UiO-66 (Ar) and UiO-66 (H2).
Download figure:
Standard image High-resolution imageTable 3. Amounts of Lewis and Brönsted acid sites of UiO-66, UiO-66 (Ar) and UiO-66 (H2).
| Sample | CB (μmol·g−1) | CL (μmol·g−1) | B/L |
|---|---|---|---|
| UiO-66 | 2.86 | 79.51 | 0.036 |
| UiO-66 (Ar) | 10.57 | 300.85 | 0.035 |
| UiO-66 (H2) | 1.76 | 171.65 | 0.010 |
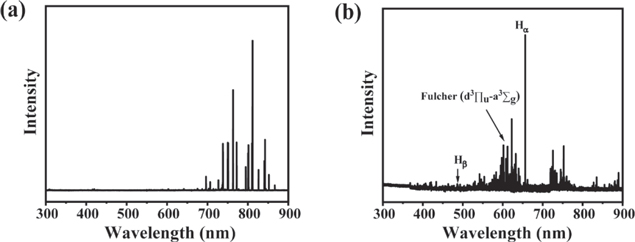
OES was further used to investigate the plasma systems since the active species in plasma have their own characteristic optical emission. The optical emission spectrum of Ar DBD plasma scanned from 300 to 900 nm mainly contains excited Ar lines, as shown in figure 6(a). The lines centered at 476.48 and 487.98 nm corresponding to Ar+ are too weak to be observed, which can be attributed to the low concentration of Ar+. The emission spectrum of H2 plasma (figure 6(b)) mainly consists of atomic H* (e.g. Hα at 656.3 nm and Hβ at 486.1 nm) and molecular H* 2 (e.g. Fulcher-α d3Πu → a3Σg + bands at 580–650 nm).
Figure 6. Typical optical emission spectra of Ar plasma (a) and H2 plasma (b).
Download figure:
Standard image High-resolution imageFigure 7 shows the schematic illustration and the crystallographic model of defective UiO-66 formed by Ar and H2 plasma treatments. In the case of Ar DBD plasma, the formation of defective UiO-66 may occur via physical modification of MOFs due to the bombardment with energetic Ar+. The effect of excited Ar atoms on the modification can be neglected due to their inert properties. For H2 DBD plasma, the active H* atoms and H* 2 molecules tend to react with UiO-66, and more BDC linker is removed, thus making the original UiO-66 highly defective.
Figure 7. (a) Schematic illustration of defective UiO-66 synthesized by Ar and H2 plasma treatments, (b) crystallographic model of the perfect and defective UiO-66 structure.
Download figure:
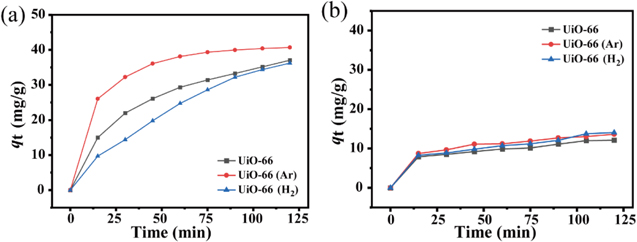
Standard image High-resolution imageIn the following section, the adsorption activities of the UiO-66, UiO-66 (Ar) and UiO-66 (H2) samples are evaluated for the adsorption of MB. Figure 8(a) shows the adsorption of MB as functions of contact time for samples with Zr/HBDC ratio (fZr/HBDC) of 1:2. All three adsorption curves show that the adsorption capacities rise sharply during the first 50 min, and then reach almost stable values. As Pelekani and Snoeyink pointed out, the size of monomer of MB molecule was 1.43 nm in width, 0.61 nm in depth and 0.4 nm in thickness [30]. For the microporous UiO-66, the average aperture size is 2.72 nm (table 2), which is comparable to the size of MB molecules. Thus, the MB can penetrate the micropores and be adsorbed by active sites in addition to the adsorption on the UiO-66 surface. Several adsorption functions exist between the MB and UiO-66, such as the π-π stacking effect, electrostatic interaction, hydrogen bonding formation and covalent bond formation [24]. The π-π stacking effect can be ascribed to the interaction between the aromatic structure of BDC ligands and the aromatic ring on the surface of MB. The electrostatic interaction is related to the attractive force of the negatively charged carboxyl group in BDC linker and the positively charged nitrogen in MB. The covalent bond formation involves the interaction of the electron acceptor (the aromatic ring of MB) and the electron donor (the carbonyl oxygen of BDC linker). Among these four adsorption factors, the π-π stacking effect is the main adsorption force. Compared with the pristine UiO-66, UiO-66 (Ar) shows an improved adsorption activity for MB due to the existence of mesopores within the UiO-66 frame structure. On the one hand, the presence of mesopores allows MB molecules to have easy access to the active sites within UiO-66, resulting in a faster absorption rate. On the other hand, Ar plasma modification leads to the generation of more active sites due to the broken coordination bonds between the metal ions and BDC linker. Consequently, the equilibrium adsorption capacity (40.6 mg·g−1) of UiO-66 (Ar) is higher than that of the pristine UiO-66 (37.0 mg·g−1). In the case of UiO-66 (H2), H2 plasma treatment has a slightly negative effect on the adsorption activity of UiO-66 for MB adsorption. A possible reason is that H2 plasma contains numerous active species, such as H and excited H2, which are able to react with BDC linker to generate many more missing-linker defects in comparison to Ar plasma treatment, as verified by ICP, XPS, TGA and Pydine-IR measurements. The missing-linker defects have positive and negative effects on the UiO-66 adsorption performance for MB. A small number of defects is able to improve the adsorption activity. In contrast, a large number of defects will suppress the number of active sites, and make the adsorption capacity of UiO-66 for MB decrease.
Figure 8. Adsorption isotherms of MB on UiO-66, UiO-66 (Ar) and UiO-66 (H2) different Zr/HBDC (a) 1:2 and (b) 1.5:1.
Download figure:
Standard image High-resolution imageThe dependence of the adsorption of MB on the contact time for UiO-66, UiO-66 (Ar) and UiO-66 (H2) with fZr/HBDC = 1.5:1 is shown in figure 6(b). In this case, the amount of Zr in UiO-66 is much more than BDC, indicating that numerous missing-linker defects exist within the synthesized UiO-66. Consequently, the equilibrium adsorption capacity of UiO-66 with fZr/HBDC = 1.5:1 is much lower than that of the sample with fZr/HBDC = 1:2. In addition, the effects of the plasma treatments of UiO-66 on the adsorption for MB are unobvious since the pristine UiO-66 possesses a large number of missing-linker defects.
4. Conclusion
In summary, we report a plasma process to modify UiO-66 MOFs. The process uses Ar or H2 as the discharge gas to modify the synthesized UiO-66 for 5 s. Although the main crystal structure of UiO-66 with plasma treatments is maintained, the partial BDC linkers within UiO-66 are removed due to Ar+ bombardment or reaction between H species with UiO-66. As a result, the defective UiO-66 framework is generated. The adsorption of methyl blue is carried out on UiO-66, UiO-66 (Ar) and UiO-66 (H2). The defective UiO-66 (Ar) displays enhanced adsorption activity for methyl blue adsorption, indicating that the broken coordination bonds between the metal ions and BDC linker increase the number of adsorption sites. Compared with Ar plasma modification, H2 plasma treatment of UiO-66 shows the negative effect on the adsorption of MB. These results suggest that the missing-linker defects can be easily tuned by plasma treatment and are expected to extend to other MOF-based applications.
Acknowledgments
This study is financially supported by National Natural Science Foundation of China (Nos. 12075032 and 12105021), the Natural Science Foundation of Beijing Municipality (Nos. KZ202010015022 and 8222055), the Yunnan Police College Project (Nos. YNPC-S2021002 and YJKF002) the and Beijing Institute of Graphic Communication Project (Nos. Ec202207 and S202210015021).