Abstract
The wastewater produced by the large-scale usage of antibiotics worldwide was more harmful to the ecological environment and human health. In the research, the coupling technology of 'adsorption-plasma regeneration' was taken to treat tetracycline in aqueous solution. The pollutants were adsorbed by the resins firstly and then regenerated ectopically by dielectric barrier discharge plasma. The discharge parameters, such as discharge voltage and frequency were researched to achieve the optimal regeneration efficiency and energy efficiency. Meanwhile, the analyses of the surface functional groups and microstructure were also investigated. After the five 'adsorption-regeneration' processes, the results showed that the optimal discharge parameters of voltage and frequency were 20 kV and 1 kHz, respectively. The regeneration efficiency and energy efficiency were above 80% and 115 g kW−1·h−1, respectively. The tetracycline adsorption by virgin resin and regenerated resin nearly followed pseudo-second order kinetics, and there was no fatal damage to the surface characteristics and physicochemical properties of the resins through the multiple plasma regeneration processes. Finally, according to the intra-particle diffusion model and the degradation products detected by GC-MS, the adsorption and degradation mechanisms of tetracycline were deduced.
Export citation and abstract BibTeX RIS
1. Introduction
The antibiotics have been widely used in the world. However, there are currently no strict laws and regulations to regulate the use of antibiotics in many countries, whether in the pharmaceuticals or the livestock industry [1, 2]. The overuse of antibiotics has become a widespread phenomenon. As one of the most important antibiotics, especially tetracycline is widely used or even abused around the world, which has caused many adverse effects on the ecological environment and human health. Therefore, it is imperative to develop effective degradation technologies [3, 4]. At present, the conventional treating methods of antibiotic wastewater can be divided into three categories [5]: biological methods [6], physical-chemical methods [7], and advanced oxidation processes [8]. Among them, non-thermal plasma (NTP) [9, 10] has become a research hotspot in recent decades, whose advantages include simple operation, strongly oxidizing components, and less secondary pollution. NTP can be generated through a variety of discharge forms, such as dielectric barrier discharge (DBD) [11], glow discharge [12], corona discharge [13]. During the discharge process, DBD can produce a large amount of oxidative active components (·OH, ·O, O3, H2O2) in the discharge area. However, when DBD plasma is directly used to treat organic pollutants in aqueous solution, due to the limitation of gas–liquid mass transfer, the utilization rates of strong oxidizing radicals and high-energy electrons are low. With the development of plasma coupling technology, a method of using NTP to regenerate adsorbents has been proposed [14]. This method refers to firstly using adsorbents to enrich organic pollutants, and then through plasma to regenerate the saturated adsorbents in certain discharge time and limited space. The concentrated pollutants on the adsorbents can directly react with strong oxidizing radicals or high-energy electrons to improve the processing effect, and simultaneously enhance the service life of the adsorbents. So far, the regenerated adsorbents are mainly the carbonaceous adsorbents [15], such as GAC, ACF, and AC. Whereas, the regeneration effects and mechanisms of other adsorbents are still unknown. The research on gas phase products and degradation mechanisms of tetracycline are also lacked in the 'adsorption-plasma degradation' system.
In view of the existing research problems, tetracycline is chosen as the target antibiotic, and the resin is used as the regenerated adsorbent. A total of five regeneration times were performed. Taking the regeneration efficiency and energy efficiency as evaluation parameters of the system, the optimal discharge conditions are settled down. Simultaneously, the reaction kinetics of the adsorption process under different discharge parameters and the influences of the DBD plasma regeneration process on the physical-chemical properties of the resin are also investigated. Finally, the adsorption and regeneration mechanisms of tetracycline solution by resin through DBD plasma are proposed.
2. Materials and analysis methods
2.1. Materials
The resins in this study were provided by Purolite (China) Co., Ltd. The resins were pretreated by the following steps, which were given in text S1 (available online at stacks.iop.org/PST/23/115506/mmedia).
2.2. Experimental setup
The 'adsorption-plasma regeneration' system was applied, which was shown in figure 1(a), the specific structure of the regeneration reactor in figure 1(b), and the actual discharge picture in figure 1(c). The experiment process consisted of two parts:
- (1)Static adsorptionFirstly, 0.100 g of the resins and 150 ml of tetracycline solution with the concentration of 50 mg l−1 were poured into 250 ml conical flask in an oscillating incubator. It was operated at 180 rpm for 24 h, during which the tetracycline concentrations were analyzed at regular intervals. Finally, the saturated resins were taken out, filtered, dried, and ready to be used in DBD ectopic regeneration experiments.
- (2)DBD regeneration
Figure 1. (a) Bench-scale apparatus of DBD plasma for tetracycline decomposition, (b) the specific structure of DBD plasma regeneration reactor, (c) physical discharge picture.
Download figure:
Standard image High-resolution imageThe schematic diagram of the DBD regeneration system is shown in figure 1(a), the specific structure of the regeneration reactor in figure 1(b), and the actual discharge picture is in figure 1(c). For the DBD regeneration system, it mainly includes pulse power supply, gas cylinder, oscilloscope, and DBD reactor. For the type of plate DBD reactor, copper sheets (length 50 mm, width 22 mm) were as the discharge electrodes, and the discharge electrodes are closely attached to the outer wall of the quartz dielectric. The two electrodes were connected to a pulse power supply with an uttermost peak voltage of 35 kV and adjustable pulse frequency range of 100 Hz–10 kHz. The discharge gap was 3 mm. Oxygen was the carrier gas with the flow rate of 3 l min−1. However, the reactor is open and filled with only a small amount of quartz wool at the outlet of the reactor to prevent the resin from flowing out with the gas, so the plasma space contains a certain amount of air.
When the resins had completed an adsorption test, and then filled into the regeneration reactor until the regeneration test was over. The regenerated resins would continue the next 'adsorption-plasma regeneration' test. This above process was called a test cycle. Under the same condition, five cycles of 'adsorption-plasma regeneration' were conducted. In order to ensure the systematicness of the analysis, the resins regenerated after the first, third and fifth tests were analyzed, respectively. Among them, the first 'adsorption-regeneration' test and the next adsorption test were defined as 'DBD1', the third 'adsorption-regeneration' test and the next adsorption test were defined as 'DBD3', the fifth 'adsorption-regeneration' test and the next adsorption test were defined as 'DBD5'.
2.3. Analysis methods
2.3.1. Adsorption amount
The adsorption amount of tetracycline on the resins can be calculated by equation (1).
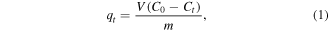
where C0 and Ct are the concentrations of tetracycline in the solution at the initial time and time t, mg l−1; qt is the adsorption amount at time t, mg g−1; V is the volume of the solution, l; m is the mass of the resins, g.
2.3.2. Regeneration efficiency
The calculation method of the regeneration efficiency is in equation (2):
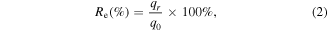
where q0 and qr refer to the adsorption amount of virgin resin and the rth regenerated resin, respectively, mg g−1.
2.3.3. Discharge power and energy efficiency
The discharge voltage and current waveforms were recorded with the oscilloscope (KEYSIGHT DSOX3024T, USA), with a voltage probe (Tektronix P6021, Beaverton, USA) and a current probe (Tektronix P6021, Beaverton, USA). The storage capacitance Creactor and the reaction time were 3.8 pF and 15 min, respectively. The detail calculation methods of discharge power and energy efficiency were presented in text S2.
2.3.4. Adsorbent characterization and products analysis
The characterization analysis of the virgin and regenerated resins, the detection of gas phase intermediate products, and the adsorption kinetic models are introduced in detail in text S3.
3. Discussion and results
3.1. Effects of discharge voltage on regeneration efficiency
In the DBD plasma regeneration process, the discharge voltages investigated in the experiment were 18 kV, 19 kV, 20 kV, and 21 kV, respectively. The relationship between discharge voltage and regeneration efficiency was shown in figure 2.
Figure 2. Regeneration efficiency after different regeneration times under different voltages (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, frequency = 1 kHz).
Download figure:
Standard image High-resolution imageThe results showed that the regeneration efficiency of the resins increased firstly and then decreased with the increase of the discharge voltage. When the discharge voltage was from 18 to 21 kV, the regeneration efficiency in DBD1 process was 82%, 83%, 92%, 90%, respectively. While the regeneration efficiency in DBD5 process was 71%, 74%, 83%, 81%, respectively. It could be seen that when the discharge voltage was 20 kV, the regeneration efficiency of the resins was the highest above 80%. Therefore, it is not conducive to the regeneration process under the lower or higher pulse voltage.
One reason for this result was that when the discharge voltage was too low, the effective plasma channel could not be formed, and the concentration of the active material generated during the discharge was too low. It was not enough to degrade the adsorbed tetracycline completely, and the inactivated adsorption sites could not be reused, which had a negative effect on the next cycle of adsorption test. On the other hand, when the discharge voltage was too high, too strong plasma discharge might change the microscopic morphology of the resins, the functional groups, and active sites, which caused the decrease of the adsorption performance. The physical-chemical properties of the resin would be discussed in detail later.
In order to study the effect of the regeneration process on the adsorption kinetics of the resin, the adsorption data after different regeneration times under different voltages were investigated by fitting with the pseudo-first order and pseudo-second order kinetic models, which were described in text S4. The fitting results were shown in figures 3(a)–(f), and the fitting parameters were shown in table S1.
Figure 3. The adsorption kinetics of virgin resins and regenerated resins under different regeneration times at different voltages (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, frequency = 1 kHz).
Download figure:
Standard image High-resolution imageIn figures 3(a)–(f), it could be concluded that the adsorption processes were more in line with the pseudo-second order kinetic model, and the fitted equilibrium adsorption amount of qe,cal was closer to the experimental value of qe,exp. As the regeneration times increased, the adsorption reaction rate constant k2 gradually decreased. It is shown that the plasma regeneration process would affect the adsorption performance of the resin at a certain extent, which was consistent with the change trend of the adsorption amount. However, it could be seen that multiple regeneration processes followed the same order kinetic model, which indicated that it did not have a significant impact on the resins.
3.2. Effects of discharge frequency on regeneration efficiency
The experiments with discharge frequencies of 0.5, 0.75, and 1 kHz under the discharge voltage of 20 kV were done, which were shown in figure 4. The results showed that as discharge frequency increased, regeneration efficiency increased. This was because under the same discharge voltage, the higher discharge frequency, the higher input energy, which increased the number of high-energy electrons and the yield of strong oxidizing particles (such as ·OH, O3, H2O2 and ·O) to further accelerate the regeneration of inactivated resin. When the discharge frequency was 1 kHz, the regeneration efficiency of the saturated resin was maintained above 80% after five regeneration times. Therefore, 1 kHz was the optimal discharge frequency.
Figure 4. Regeneration efficiency of adsorbed resin after five times under different frequencies (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, discharge voltage = 20 kV).
Download figure:
Standard image High-resolution image3.3. Effects of discharge voltage on energy efficiency
Under different discharge voltages, the results of the single pulse energy and average discharge power were shown in table S2. Based on the discharge power, the energy efficiencies under different discharge voltages were calculated, and the results were shown in figure 5.
Figure 5. Energy efficiency of resin after five regeneration times under different discharge voltage (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, frequency = 1 kHz).
Download figure:
Standard image High-resolution imageAs the discharge voltage increased, the energy efficiency continued to decrease, showing the opposite trend to the regeneration efficiency. This was because the discharge voltage increased the regeneration efficiency of the resin and the discharge power, and the proportion of discharge power increased much greater than the regeneration efficiency. For example, regeneration efficiency in the DBD1 process increased by 8% at the discharge voltage from 18 to 21 kV, while the increase in discharge power reached 67.8%, so the energy efficiency was reduced from 158.5 to 103.6 g kW−1·h−1. Therefore, if the regeneration efficiency was to be maintained above 80%, the ideal discharge voltage was 20 kV. After five regeneration times, the energy efficiencies still maintained above 115 g kW−1·h−1.
The trend of discharge frequency and discharge voltage was consistent with energy efficiency, as shown in figure S1. It was found that the energy efficiency decreased with the increase of discharge frequency. Therefore, reducing the discharge power was an effective means to improve the energy efficiency of the plasma treatment system, while the deterioration of the regeneration efficiency was also a problem. Taking this system as an example, in order to maintain 80% after multiple regenerations, it is necessary to keep the discharge frequency at 1 kHz.
3.4. Resin characterization analysis
3.4.1. FT-IR analysis
The FT-IR spectra of the above six samples had ten main spectral peaks shown in figure 6. As shown in figure 6, different legends stood for different samples. Among them, the sample of virgin resin was only pretreated. The sample of saturated adsorption was the resin that was saturated tetracycline. The sample of plasma refers to the one that obtained from the pretreated resin directly disposed by DBD plasma. All of the DBD1, DBD3 and DBD5 samples had been introduced in section 2.2. Compared with the virgin resin, after the plasma treatment, the functional groups nearly had not changed significantly, which could be seen in figure 6(a). However, for the sample of saturated adsorption, the intensities of some functional groups were reinforced obviously, which were corresponding to the functional groups of tetracycline and the absorption peaks of the resins. From DBD1, DBD3 to DBD5 processes, the adsorbed contaminants were degraded, which weakened the intensity of the adsorption peaks. Nevertheless, the intensities were still stronger than that of the virgin resin, which indirectly indicated that the adsorbed pollutants were not completely degraded, resulting in the decrease of adsorption amount. The specific spectral peaks were analyzed in detail.
Figure 6. FT-IR spectra of six resin samples: (a) the full spectrum, (b) the part spectra of 2800–1800 cm−1 (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, discharge voltage = 20 kV, frequency = 1 kHz).
Download figure:
Standard image High-resolution imageThe peak at ∼3426 cm−1 was probably formed by the superposition of two peaks caused by O–H and N–H stretching vibrations. Meanwhile, with the increase of the regeneration times, the absorption peak of 3426 cm−1 gradually tended to widen in the direction of the lower wavenumbers. This was mainly because the tetracycline molecule contained the amide functional group and the two –NH2 group. The symmetrical and anti-symmetrical vibration bands of each NH were located at the spectral range of 3350–3250 cm−1, leading to the vibration band wider.
The molecular skeleton structure of the resin was formed by the cross-linking polymerization of styrene and divinyl benzene, ∼3016 cm−1 was the stretching vibration peak of olefin in resin molecule. The stretching vibration peak of CH2 peaks at ∼2922 cm−1 (antisymmetric) and ∼2855 cm−1 (symmetric), and CH3 and CH2 might overlap at the absorption summit between 2970 and 2850 cm−1 [16]. The peak intensity of ∼2922 and ∼2855 cm−1 might be caused by the collective impact of the stretching vibration peak of =C–H of the resin, tetracycline molecule, and the products.
The peaks of ∼1604, 1511, and 1449 cm−1 were the skeletal vibration of the heteroaromatic ring. For the saturated resin, the peak of ∼1604 cm−1 was sharp and broad, which might be due to the presence of the C=O stretching vibration peak on the tetracycline (corresponding to ring C) [17], and the latter two peaks were mainly caused by the stretching vibration of the benzene ring after the hydrogen atoms on the benzene ring were substituted. The hydrogen on ring D, which was seen in figure S2, the molecular structure of tetracycline adsorbed on the surface was replaced by –OH.
In figure 6(b), the saturated resins had five adsorption peaks compared to the other five samples, at the wavenumbers of ∼2735 cm−1, ∼2333 cm−1, ∼1901 cm−1, ∼1357 cm−1, ∼1179 cm−1, respectively. These peaks were corresponding to the partially infrared spectra of the tetracycline molecule, which could be seen in the infrared spectra of standard tetracycline (Spectrum No.122) [18]. After several regeneration processes, the intensities of those peaks were obviously weaken or even disappeared, which demonstrated that the functional groups corresponding to tetracycline were degraded.
The peaks below ∼1300 cm−1 was the fingerprint area of the infrared spectra, and many peaks were difficult to be identified. For the region below 900 cm−1, the absorption peaks of ∼815 and ∼700 cm−1 were the infrared spectra of benzene ring due to substitution.
3.4.2. SEM
In order to observe the microstructure of the resin, SEM images were shown in figure 7. The four samples included virgin resin, DBD1, DBD3, and DBD5, respectively. It could be seen that as the regeneration times increased, some defects and cracks appeared on the surface of the resin, while the damaged area was not large and the depth was relatively shallow. The overall surface appearance of the resin was still in a relatively complete state.
Figure 7. (a)–(d) The virgin, DBD1, DBD3, and DBD5 resin samples magnified 500 times, (e)–(h) the virgin, DBD1, DBD3, and DBD5 resin samples magnified 5000 times (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, discharge voltage = 20 kV, frequency = 1 kHz).
Download figure:
Standard image High-resolution image3.5. Adsorption behavior of tetracycline by resin
3.5.1. The adsorption process of tetracycline by resin
The fitting of the intra-particle diffusion model [19] for a complete adsorption process can be divided into three parts in figure 8. The corresponding kinetic parameters were in table S3.
Figure 8. Fitting results of internal diffusion in the adsorption processes of DBD1, DBD3, and DBD5 resins under different discharge voltages (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, discharge voltage = 20 kV, frequency = 1 kHz).
Download figure:
Standard image High-resolution imageAccording to the first part of the intra-particle diffusion fitting results, the external diffusion was mainly in the early adsorption process, and then most of the tetracycline diffused into the interfacial membrane in the active sites of the resin surface. With the adsorption process, the outer surface of the resin tended to be saturated, and the tetracycline molecules began to enter the internal pores of the resin, which was corresponding to the second part of the intra-particle diffusion fitting results. The large pores were saturated firstly, and tetracycline deeply diffused into the narrower pores in the resin. When the main area of internal diffusion changed from large pores to small pores, the adsorption process entered in the third part, it could be seen that the adsorption rate of this part was reduced significantly, and the resin tended to be saturated completely.
The parameters of the linear fitting in the second part of the adsorption process were listed in table S3. In figures 8(a) and (b), those lines did not pass through the origin, indicating that adsorption reaction was not controlled by the only internal diffusion under the DBD1 and DBD3 processes. The adsorption rates by the resin were controlled by both external diffusion and internal diffusion. The reason might be that the resins had a small particle size and low dosage [20]. While the DBD5 process, the second part of the adsorption process passed through the origin in figure 8(c), which was the opposite compared to the first two regeneration processes. The reason might be that the adsorption sites on the surface were destroyed, leading to the outer surface of the resin saturated quickly.
3.5.2. Adsorption mechanisms of tetracycline molecules by resin
The adsorption of tetracycline by the resin was also related to the property of the tetracycline. Considering that tetracycline solution in the experiment was neutral or weakly alkaline, the main adsorption mechanisms under neutral conditions were analyzed.
The resin had a styrene-based skeleton, which was non-polar. The surface property of the resin was hydrophobic. Based on the FT-IR analysis in figure 6, it could be seen that there were nearly no new functional groups between the resin and tetracycline, which indicated that there was no obvious covalent bond to be formed. Therefore, the resin mainly relied on the hydrophobic interaction to adsorb tetracycline, which was the amphiphilic compound. It proved that the strong force promoting the adsorption behavior was not by the ionic bond or covalent bond, rather than by van der Waals forces.
In addition to the hydrophobic adsorption, π–π EDA interaction might be one of the important non-hydrophobic forces. The conjugated enone structure of the tetracycline had the strong electron withdrawing ability, which made the aromatic ring D become π electron acceptor [21]. It strongly interacted with the benzene ring of the polystyrene resin, which contained the large π bond (π electron donor) composed of p electrons contributed by sp2 hybridized carbon atoms [22], shown in figure 9. The π–π EDA mechanism made the adsorption of tetracycline enhanced obviously.
Figure 9. π–π electron donor-acceptor (π–π EDA) mechanism.
Download figure:
Standard image High-resolution image3.6. Regeneration mechanism of tetracycline
3.6.1. Spectral diagnosis
In order to monitor the active particle components produced by the plate reactor during DBD discharge, a three-channel emission spectrometer (PG2000) was used to diagnose the plasma emission spectroscopy. The three channels of the spectrometer form a spectrum probe through an optical fiber line, which can simultaneously measure the emission spectra of 179–393 nm (resolution 0.21 nm), 394–604 nm (resolution 0.20 nm), and 595–791 nm (resolution 0.16 nm). The emission spectra of the discharge area were measured, as shown in figure 10.
Figure 10. Spectroscopic diagnosis results of plate reactor (concentration = 50 mg l−1, volume = 150 ml, resin = 0.1 g, oxygen flow = 3 l min−1, discharge voltage = 20 kV, frequency = 1 kHz).
Download figure:
Standard image High-resolution imageWhen the discharge voltage reached the breakdown voltage, the molecules in the discharge area were ionized, and the electron density increased greatly. These electrons were accelerated under the action of the high-voltage electric field and could obtain a large amount of kinetic energy in a short time to become the high-energy electrons. The high-level particles could spontaneously transit to a more stable and lower energy level, and release the energy in the form of photon or thermal motion. The radiation transition of releasing photons could produce the emission spectrum. The emission spectrum of DBD was mainly related to N2 molecules due to a certain amount of air. After the gas crashed by high-energy electrons, N2 molecule in a ground state would be excited to different energy levels based on the following reactions:
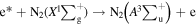
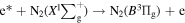
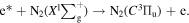
N2 molecules transitioned from a high-level excited state to a ground state or a lower-level excited state, resulted that the emission spectra were mainly the second positive system ( ), the first positive system (
), the first positive system ( ), and Vegard–Kaplan system (
), and Vegard–Kaplan system ( ). Among the above three excited states of N2 molecule, the C3Πu state was the most unstable, from whose transition to
). Among the above three excited states of N2 molecule, the C3Πu state was the most unstable, from whose transition to  state was also the strongest. The specific corresponding relationship was shown in table S4. In table S4, ν' is the principal quantum number of the initial state energy level before the transition (C3Πu), ν'' is the principal quantum number of the final state energy level after the transition (
state was also the strongest. The specific corresponding relationship was shown in table S4. In table S4, ν' is the principal quantum number of the initial state energy level before the transition (C3Πu), ν'' is the principal quantum number of the final state energy level after the transition ( ), and Aν'ν
'' is the probability of Einstein transition.
), and Aν'ν
'' is the probability of Einstein transition.
In addition to the second positive system of N2 molecule, the DBD process also produced other transition radiations. However, due to the low concentration of the relevant excited state particles, the radiation intensity generated by the transition was too weak to be detected by spectral diagnosis. Taking this system as an example, molecules in air were mainly involved in the discharge, and a series of gas–liquid mass transfer reactions would occur in the tetracycline solution through the discharge space. The dissociation limit of O2 molecules is 7.047 eV, which is much lower than the excitation energy required for the N2 molecules ( ), thus the dissociation products of O2 molecules were usually the metastable O(1
D) and metastable O(1
S), which are 1.976 eV and 4.188 eV, respectively [23]. On one hand, the oxygen atoms produced by dissociation continued to react with O2 to generate ozone, which was the reason that DBD reactor was used to produce ozone in early research [24–26]. On the other hand, the reaction between oxygen atoms and water molecules can generate hydroxyl radical (·OH). The most common transition of ·OH in DBD process was from the excited state
), thus the dissociation products of O2 molecules were usually the metastable O(1
D) and metastable O(1
S), which are 1.976 eV and 4.188 eV, respectively [23]. On one hand, the oxygen atoms produced by dissociation continued to react with O2 to generate ozone, which was the reason that DBD reactor was used to produce ozone in early research [24–26]. On the other hand, the reaction between oxygen atoms and water molecules can generate hydroxyl radical (·OH). The most common transition of ·OH in DBD process was from the excited state  to the ground state
to the ground state  where ν'–ν'' was the strongest in the radiation of (0–0) and (1–1), and the emission spectra generated were around 309 nm and 314 nm, respectively. However, because the emission of the second positive system of the N2 molecule was too strong, it was submerged behind among the spectral lines of (2–1) and (1–0). There are many kinds of particle reactions in the air DBD or gas–liquid phase DBD. Some of the most important chemical equations that generated strong oxidizing particles were listed in table S5. These plasma chemical reactions played a very important role in the degradation of tetracycline, and the specific degradation mechanism would be discussed in detail later.
where ν'–ν'' was the strongest in the radiation of (0–0) and (1–1), and the emission spectra generated were around 309 nm and 314 nm, respectively. However, because the emission of the second positive system of the N2 molecule was too strong, it was submerged behind among the spectral lines of (2–1) and (1–0). There are many kinds of particle reactions in the air DBD or gas–liquid phase DBD. Some of the most important chemical equations that generated strong oxidizing particles were listed in table S5. These plasma chemical reactions played a very important role in the degradation of tetracycline, and the specific degradation mechanism would be discussed in detail later.
3.6.2. Analysis of products in regeneration process
In order to analyze the products of the entire regeneration process, the products of the virgin, regenerated resins during the DBD1, DBD3, and DBD5 processes were investigated in detail, which were demonstrated in figure S3. The main types of products produced during the regeneration of virgin resin were decomposed after plasma treatment, as shown in figure S3(a). The molecular formulas circled in red were the products newly generated in the DBD1 process, as shown in figure S3(b), and those in green were the main products newly generated during the DBD3 process, as shown in figure S3(c). The new substances did not appear in figure S3(d), which indicated that the new intermediate products were produced by the degradation of tetracycline. The molecular and structural formulas of these products obtained by GC-MS were summarized in table S5. As seen from figure S3, there were also some unmarked ion peaks in the total ion current diagram. Among them, the analyses of relatively abundance ion peaks, such as retention time of 3.64 and 5.40 min were mainly chains or rings of siloxane, which mainly caused by the incomplete aging of the chromatographic column or the loss of the silicone rubber septum in the instrument.
3.6.3. Analysis of tetracycline degradation process
A large number of studies had shown that extraordinary chemical phenomena can occur in the plasma, which were closely related to the electric field and high-energy electrons. Thus it can promote a certain of thermodynamically unfeasible reactions under ambient temperature and pressure [27, 28], such as the decomposition of CO2 [29], the conversion of methane to produce liquid hydrocarbons or C2 hydrocarbons [30, 31], methanol synthesis from methane and water [32, 33]. Based on the eight products listed in table S4, the joint effects of the strong oxidizing active particles with high-energy electrons were analyzed and derived the degradation process of tetracycline.
- (1)Ring-opening of tetracycline molecule
Tetracycline was an organic compound with hydrogenated naphthacene as the basic skeleton, which had four carbon rings of the molecule, while the products consisted of at most two carbon rings. Therefore, according to the molecular structure of the products, two ring-opening mechanisms for tetracycline molecules were proposed.
The products (2), (3), and (7) had a benzene ring, so they might be the products based on the ring D after the ring C of the tetracycline molecule was destroyed. It was inferred that the first ring opening process was shown in figure 11. The C–C bond of ring C was broken under the collision of high-energy electrons, and a ring-opening reaction occurred. At the same time, the hydroxyl group on ring D underwent a dehydration reaction under the combined action of the H+ and the high-energy electrons. There were also other ways to open ring C. Firstly, C(11)–C(11a) and C(6)–C(6a) were broken, and C=O at C(11) was added to hydrogen ions to form the hydroxyl group, and the product based on ring D (benzene ring) after ring opening was benzyl alcohol. Secondly, C(10a)–C(11) and C(6)–C(5a) were broken, and the hydroxyl group on C(6) was oxidized by oxygen atoms to generate α-phenylethanol. Thirdly, C(10a)–C(11) and C(6a)–C(6) were broken to generate benzene.
Figure 11. The first ring opening process of tetracycline molecule.
Download figure:
Standard image High-resolution imageThe ring C through the above three ways of ring opening generated benzyl alcohol, α-phenylethanol, and benzene. The benzyl alcohol was bombarded by high-energy electrons, the chemical bond between the methylene group and the hydroxyl group was broken, and the methylene benzene was combined with a H+, and finally toluene named the product (2) was formed; α-phenylethanol was oxidized by some oxidants (O, O2, and O3) to produce acetophenone. The carbonyl group on the acetophenone underwent a reduction reaction under the action of H+ to produce ethylbenzene named the product (3); benzene could be directly added to ·OH to produce phenol named the product (7).
In addition to the aromatic compounds with a single benzene ring in table S5, there were two biphenyl ring compounds, namely naphthalene and 1-methylnaphthalene, which were products (4) and (5), respectively. Early studies had found that through traditional chemical methods, tetracycline could undergo two types of degradation reactions: dehydration and epimerization. The tertiary alcohol hydroxyl group on C(6) had the properties of benzyl alcohol, and C(5a) had another hydrogen that was in the trans plane with the hydroxyl group, which could lead to the intramolecular dehydration between them. Moreover, the β-dicarbonyl system between C(11) and C(12) underwent the keto-enol interconversion to make the ring C with a C=C double bond, which can lead to the aromatization of ring C under the action of dehydration [34], as shown in figure 12.
Figure 12. The second ring opening process of tetracycline molecule.
Download figure:
Standard image High-resolution imageThe aromatization of ring C converted the tetracycline molecule into an aromatic compound containing a biphenyl ring, followed by the C(11a)–C(12) bond and C(5a)–C(5) bond on ring B in high-energy electrons. The hydroxyl groups on ring C and ring D were dehydrated under the action of H+ and high-energy electrons to form 1-methylnaphthalene named the product (5), which was bombarded by high-energy electrons. The methyl group was broken to produce naphthalene named the product (4).
- (1)Ring-opening of benzene ring
By inferring the ring-opening process of the tetracycline molecule, the possible ways to generate intermediate products (2)–(5), (7) were obtained, and the remaining intermediate products (1), (6), (8) were derived from the benzene ring to get by further opening the benzene ring.
The product (6) maleic anhydride was a typical ring-opening product of benzene epoxidation, which could be generated by the benzene oxidation method. The general industrial reaction condition was catalytic oxidation, and the chemical equation of the reaction [35] was:
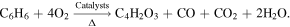
In addition to benzene molecules, there was also toluene, which could undergo a similar ring opening reaction in the plasma. The specific process was shown in figure 13. First, the H+ in the plasma adhered to the benzene ring, destroying its aromaticity, and then oxygen molecules could oxidize the benzene ring and add to the two opposite carbon atoms. For toluene, the oxygen atom could not be added to the carbon atom where the methyl group was located. The hydrocarbon ring continued to be oxidized by oxygen molecules to form a para-hydroxyl group, and the carbon atom where the hydroxyl group was located further added by oxygen molecules. The intra-ring –O–O– of the intermediates was very unstable, whose two oxygen atoms were very active. They could attack the C–C bond connected to them, causing the hydrocarbon ring to break. When the primary reactant was a benzene molecule, maleic acid and acetylene were formed by ring-opening, and maleic acid and propyne were formed by ring-opening of toluene. The two carboxyl groups of maleic acid underwent intramolecular dehydration and polymerization to produce maleic anhydride named the product (6); the addition of propyne and water molecules produces acetone named the product (1). After the benzene ring was opened, the small molecule products would be completely mineralized into carbon oxides by the oxidants O, O2, and O3 in the plasma.
Figure 13. The first ring opening process of benzene ring.
Download figure:
Standard image High-resolution imageIn addition to oxidative ring opening, the benzene ring might also be hydrogenated to generate cyclohexane, thereby destroying the aromaticity. As shown in figure 14, ethylbenzene was first added with ·OH to form 2-ethylphenol, and then the benzene ring was converted into cyclohexane by the addition of H+. Under the impact of high-energy electrons, a ring opening occurred at the carbon atom to which the hydroxyl group was connected to produce isooctyl alcohol named the product (8).
- (1)Comparison of products with different regeneration times
Figure 14. The second ring opening process of benzene ring.
Download figure:
Standard image High-resolution imageAfter analyzing the formation mechanism of the products detected by GC-MS, and then observing table S5, all the products detected in the DBD1 process were the ring-opening products of tetracycline, mainly diphenyl aromatic hydrocarbons. The tetracycline products detected in the DBD3 process were all aromatic compounds with a single benzene ring, and a variety of benzene ring opening products. The product types of the DBD5 process were almost the same as the DBD3 process, only the product (1) acetone no longer existed. This results indicated that the degree of mineralization increased with the process of regeneration times.
At the beginning of the experiment, the resins had the largest adsorption amount, and the concentration of enriched tetracycline molecules was the highest. The active particles produced in the plasma had a low concentration relative to the pollutants. After high-energy electron bombardment to open the ring, a large proportion of the intermediate products could not be quickly oxidized and degraded. The products at the initial stage of the reaction were continuously generated and difficult to convert effectively. They were collected by the sampler and detected in the DBD1 process. These larger molecular intermediate products would also accumulate on the resin surface and pores, resulting in a significant decrease in the adsorption amount of the resin. When the regeneration was carried out in the DBD3 process, the adsorption amount of the resin had been reduced to 85%, the concentration of tetracycline molecules was reduced, and the oxidative ability of the tetracycline ring-opening products was enhanced. Therefore, a variety of further ring-opening products of benzene rings were detected. When the regeneration was carried out in the DBD5 process, the active particles in the plasma had basically degraded the tetracycline molecules adsorbed by the resin, and the intermediate products were further reduced. Comparison of the ion peak intensities in figures S3(c) and (d), It could be seen that the concentration of the intermediate products of DBD5 was significantly lower than that of DBD3, indicating that the intermediate products produced during regeneration could basically be converted into carbon oxides, and the degree of mineralization of tetracycline molecules had reached a high level.
4. Conclusions
- (1)The 'adsorption-plasma regeneration' system can effectively treat the tetracycline in aqueous solution. The regeneration efficiencies keep above 80% and the energy efficiency was maintained 115 g kW−1·h−1 after five regeneration times.
- (2)The adsorption processes of the virgin resins and the regenerated resins fit well with the pseudo-second order kinetic model, and the adsorption properties and mechanisms of the resin samples have almost no change, which are mainly controlled by the external and internal diffusion. After multiple plasma regenerations, internal diffusion becomes the main control step of adsorption. The main mechanisms about adsorption of tetracycline include hydrophobic interaction caused by van der Waals forces and π–π EDA interaction.
- (3)The FT-IR spectra and SEM images indicate that multiple regeneration processes do not have a serious effect on the functional groups and microstructure of the resins. The plasma can already degrade the reduced content of tetracycline more fully, and the molecular structures of the intermediate products become smaller, which no longer accumulate on the surface of the resin, and the adsorption amount of the resin tends to be stable.
- (4)The degradation products measured by GC-MS are all produced after ring opening of tetracycline molecules, and small molecular compounds after benzene ring opening, which points out the possible reaction paths for the complete mineralization of tetracycline molecules into carbon oxides.
Acknowledgments
This research has been financed by National Natural Science Foundation of China (No. 51877046). The financial support is gratefully acknowledged.
















