Abstract
Despite many years of effort from cancer biologists and clinical oncologists, pancreatic ductal adenocarcinoma (PDAC) remains a thoroughly recalcitrant disease, resisting both conventional forms of cancer treatment and radical innovative therapies. Perhaps driving the lack of success in PDAC is the underappreciation of the primary hallmark of the tumour: a fibrotic extracellular matrix (ECM) that composes a majority of the highly rigid solid carcinoma. In recent years we have come to understand that the homeostasis of ECM mechanics is pivotal for the homeostasis of cells and the progression or initiation of a malignant phenotype. This can be understood by way of mechanobiology, a field that attempts to understand how physical forces like ECM stiffness alter cell behaviour. In this review, we provide an overview of our understanding of PDAC from this perspective and the recent advances in biophysics and engineering that allow for new tools with which to investigate the mechanobiology of PDAC.
Export citation and abstract BibTeX RIS
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the most common type of pancreatic cancer and the fourth leading cause of cancer-related death in the developed world with more than 300 000 new cases diagnosed each year worldwide. The 5 year survival-rate of pancreatic cancer is only 3% in the UK and this figure has not changed over the past four decades largely due to lack of effective therapies and inability of early detection [1, 2].
For many years, research in the field of pancreatic cancer focused on targeting cancer cells, but during the last decade the PDAC tumour microenvironment has attracted significant attention. PDAC is characterised by an extensive desmoplastic reaction or fibrosis that surrounds the tumour comprising up to 90% of the total tumour mass [3]. The contribution of the desmoplastic reaction to tumour progression is currently a topic of intense debate. Indeed, studies have shown that it hampers drug delivery and promotes tumour growth and metastasis [3–11]; however, while some works showed that decreasing fibrosis improved drug delivery and prognosis [12], other stroma targeting approaches that attempt to completely eliminate the desmoplastic stroma have yielded underwhelming results in the clinic as exemplified by the recent failure of Hedgehog inhibitors or radical efforts to delete all stromal myofibroblasts in genetically engineered mouse models [13, 14].
A hallmark of PDAC is the remarkable stromal stiffness, one of the highest of all human tumours [3, 4, 15]. This suggests that PDAC initiation and progression should be highly influenced by mechanical factors, and intuitively leads to the idea that disrupted mechanical communication of cells with their microenvironment could promote tumourigenesis. Thus, PDAC could be considered to some extent a multifaceted disease of altered mechanobiology. Mechanobiology is an emerging field that studies the effects of mechanical forces in biology and holds a plethora of possibilities to answer pressing questions in medicine, specifically in diseases that are highly regulated by mechanical factors such as cancer and in particular PDAC.
Mechanical forces play key role in PDAC
Tumourigenesis is a multifaceted process initiated by genetic alterations and mediated by biochemical and biophysical cues from the tissue microenvironment. Mounting evidence highlight the emerging role of cell and tissue context as a key regulator of tumour behaviour and stress the importance of the mechanical microenvironment as a modifier of the malignant phenotype [16–18]. More specifically, the increasingly emerging paradigm in the field postulates that while the malignant potential is determined by the intrinsic genetic profile of the cells, the tumour phenotype is regulated by an evolving balance between the physical and biochemical properties of the cells and the extracellular matrix (ECM), which synergistically modifies cellular behaviour by engaging actomyosin contractility and stimulating invasion, survival, and proliferation.
PDAC is a highly desmoplastic tumour. Persistent activation of stromal cells results in excessive ECM deposition, particularly of tensile-resistant fibrillar collagen and water-imbibing, compression-resistant hyaluronan [19]. The fibrotic microenvironment along with the mechanical pressure exerted by the number of proliferating stromal and cancer cells, facilitates a mechano-pathology known as growth-induced solid stress, resulting in collapsed or compressed intratumoural blood vessels and lymphatics, which respectively lead to increased hypoxia and elevated interstitial fluid pressure (IFP). Both hypoxia and IFP attenuate chemosensitivity via separate pathways. The former promotes epithelial-to-mesenchymal transition (EMT) in tumour cells, and the latter impairs intratumoural drug perfusion. Intriguingly, enzymatic targeting and depletion of stromal hyaluronan in PDAC with PEGPH20 (PEGylated recombinant human hyularonidase) normalises tumour IFP, re-expands the microvasculature and improves chemotherapy (gemcitabine) delivery in mouse PDAC models [9, 20]. PEGPH20 is on the shortlist of promising stromal-targeting agents and is now in Phase II clinical trials with the scientific and clinical community eagerly awaiting its results [19].
The pronounced tumour-associated fibrosis in PDAC alters the mechanical microenvironment by increasing matrix stiffness. This can in turn alter force transmission and shift the tensional homeostasis of resident tumour (and stromal) cells by way of engaging their mechanosensing machinery. Forces are detected and transduced into biochemical signals by force- bearing molecular elements at the cell surface, most notably via integrin-mediated adhesion complexes (figure 1). While integrins have no intrinsic enzymatic activity, force-induced integrin clustering can trigger downstream signalling pathways via focal adhesion kinase (FAK), demonstrating their ability to influence mechano-chemical signalling [21].
In this light, a study led by DeNardo et al reports elevated FAK activity in human PDAC tissues that correlates with high levels of fibrosis and immunosuppressive tumour microenvironment via reduced CD8+ cytotoxic T cell infiltration [7]. Notably, pharmacologic inhibition of FAK decreased fibrosis and tissue stiffness, reduced tumour growth and metastasis, and decreased the number of tumour-infiltrating immunosuppressive cells, including myeloid-derived suppressor cells (MDSCs), tumour-associated macrophages (TAMs) and regulatory T cells (Tregs). FAK inhibition also rendered previously unresponsive mice with PDAC responsive to T cell immunotherapy and PD-1 antagonists. FAK is a key mechano-chemical signalling nexus, and disrupting FAK signalling can perhaps unsurprisingly, induce pleiotropic beneficial effects, including increased immune surveillance by overcoming the fibrotic and immunosuppressive PDAC tumour microenvironment, while secondarily highlighting potential links between altered tissue tension, the fibroinflammatory response and immunosurveillance.
PDAC, as with most tumours, is also remarkably heterogeneous with its mutational landscape differing not only among patients but also within the same patient. Genomic analyses of PDAC have revealed four common oncogenic events in well-known cancer genes (KRAS, TP53, SMAD4 and CDKN2A). Recent efforts with whole genome and exome-sequencing have attempted to categorise PDAC in four distinct molecular subtypes (squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine –ADEX) underpinning their different gene mutational mechanisms and underlying transcriptional networks as well as their distinct histopathological characteristics and differential survival [22, 23].
Tumour-associated fibrosis also varies across cancers, tumour subtypes and even within the same tumour; the stromal cells that contribute to ECM deposition and remodelling are also themselves heterogeneous. Very recent research suggests genetic sequencing of tumours is insufficient for determining personalised drug targets; thus patient derived models need to be empirically tested [24]. Molecular characterisation of tumours also may open new avenues for personalised treatment but naturally raises the question whether stroma-targeting strategies in PDAC are more or less effective depending on the underlying mutational spectrum and subtype of the tumour.
Consistent with this notion, Weaver and colleagues have recently demonstrated that stromal contribution to PDAC progression may be in fact context-dependent [8]. They found that human PDAC tumours with disrupted TGF-β signaling associated with the SMAD4 mutation exhibit increased periductal fibrosis, tissue stiffness, and poor prognosis. Further analysis of this particular tumour genotype with transgenic mice carrying the SMAD4 mutation revealed enhanced STAT3 activity—a transcription factor that regulates expression of pro-inflammatory genes. STAT3 depletion attenuated tumour progression by reducing stromal stiffening and epithelial contractility induced by loss of TGF-β signalling. Linking tumour mechanics and aggressiveness under the context of specific tumour genotypes could also provide a reasonable explanation of the somewhat conflicting results previously reported with anti-stromal therapies focusing on genetic ablation of stromal sonic hedgehog (SHH) [14], or depletion of alpha-smooth muscle actin positive stromal cells in mouse models of PDAC [13].
It has become increasingly appreciated that altered mechanotransduction signalling can function as a potent tumour promoter. Mice harbouring the KRAS oncogene in the pancreatic epithelium rarely develop invasive disease [25]. However, when these mice carry an additional mutation in β1-integrin that recapitulates tension-dependent integrin clustering and focal adhesion signalling, they quickly develop lethal PDAC accompanied by STAT3-dependent inflammation, fibrosis, and altered immune infiltrates [8]. This highlights that deregulated mechanosignalling can override the need for additional inactivation in tumour suppressor genes (e.g. TP53) or pre-existing inflammation (e.g. repeated bouts of pancreatitis) that are usually required for invasive PDAC to develop [26].
Similarly, elevated lysyl oxidase (LOX) collagen crosslinking has been found to elevate tissue stiffness and promote invasive breast cancer in vitro and in vivo. This also involves integrin mechanosignalling that amplifies epidermal growth factor receptor (EGFR) dependent PI3 kinase (PI3K) signalling [16]. Furthermore, the periductal collagen in PDAC exhibits architectural and structural differences in terms of linearization, alignment, and thickness, which can serve as an independent prognostic factor but might also facilitate cancer invasion through topographical contact guidance [27, 28]. New imaging modalities such as second harmonic generation (SHG) imaging and elastography enable visualisation of cell-matrix interactions in 3D environments (in vitro) [29] and assessment of mechanical properties of tissues (in vivo) [30] and will help advance our understanding how structural, topographical, and mechanical changes of the microenvironment can impact cancer cell behaviour.
Figure 1. Relationship between PDAC progression and matrix rigidity. PDAC is initiated most commonly from precursor lesions called pancreatic intraepithelial neoplasia (PanIN), characterised by increasing (across three stages) disorganisation of the duct and more columnar and de-differentiated epithelial cells. Ductal disorganisation is concomitant with increasing desmoplasia culminating in invasion beyond the basement membrane. Fibrosis, consisting of increased ECM deposition and differentially organised ECM architecture, perpetuates the chronic activation of stromal cells via mechanosensitive focal adhesion (FA) proteins that complex with integrins and activate downstream partners such as pFAK and pMLC-2.
Download figure:
Standard image High-resolution imageReprogramming cell mechanics induces quiescence in PSCs, suppresses matrix remodelling, fibrosis, and cancer cell invasion
Pancreatic stellate cells (PSCs) are the main resident cells in the pancreatic stroma and the key driver of the desmoplastic reaction or fibrosis [4]. In healthy conditions, PSCs are quiescent, contain lipid (vitamin A) storing vesicles in the perinuclear area, and contribute to the dynamic and balanced tissue homeostasis of the normal pancreas. In PDAC, PSCs are activated and adopt a myofibroblast-like morphology that entails profound changes in the cytoskeletal machinery and increased production of ECM proteins and cytokines [31].
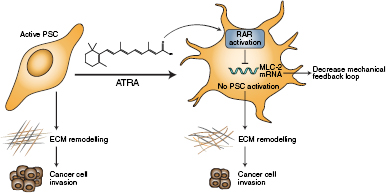
Similar to other cancer associated fibroblasts (CAFs), PSCs need to promote and sense a stiff microenvironment to sustain their activation [32–34]. Reprogramming PSCs to restore physiological tissue homeostasis seems to offer an attractive avenue for adjunct PDAC therapy. For example, the activation of vitamin D receptors in PSCs returned them to quiescence, reduced inflammation and fibrosis, and enhanced chemotherapy efficiency in PDAC tissues [6]. Similarly, all trans-retinoic acid (ATRA), the physiological active metabolite of vitamin A, restores mechanical quiescence in PSCs via activation of the retinoic acid receptor beta (RAR-β) and down regulation of actomyosin contractility, which suppresses the ability of PSCs to apply forces and mechanosense matrix rigidity. Mechanically quiescent PSCs are unable to remodel and stiffen the matrix, and this creates a microenvironment that is not favourable for pancreatic cancer cell invasion (figure 2) [35]. Notably, PSCs were removed from remodelled collagen/matrigel matrices before cancer cell invasion in these in vitro experiments; thus, the reduced ability of cancer cells to invade was related to the mechanical and/or topological changes in the ECM. Both vitamin A and vitamin D analogues work by manipulating global transcriptional control through interactions between super-enhancers and transcriptions factors and could open avenues to normalise both the cellular and acellular components of the PDAC-associated stroma.
Figure 2. All trans retinoic acid (ATRA) restores mechanical quiescence to PSCs. ATRA reduces cancer cell invasion by inhibiting activation of PSCs via a mechanism involving RARβ and MLC-2.
Download figure:
Standard image High-resolution imageIn addition to matrix stiffness, the cytokine TGF-β is a potent activator of PSCs and fibrosis [31, 36]. TGF-β is secreted to the ECM in an inert/latent form complexed with much larger proteins that encapsulate TGF-β, the latency associated peptide (LAP) and latent TGF-β binding protein-1 (LTBP-1), which interface TGF-β between the fibronectin molecule in the ECM and the integrin receptors in the PSCs surface [37, 38]. A spatiotemporal organisation of cellular and ECM mechanical factors including PSC contractility release TGF-β in its active form [39, 40], which can further activate PSCs and perpetuate fibrosis. Notably, ATRA hampers the capacity of PSCs to mechanically activate TGF-β [41], and by doing this, ATRA breaks the autocrine and paracrine loop that sustains PSC activation and a tumour-favouring microenvironment.
ATRA has been used in combination with gemcitabine in PDAC mouse models that reproduce with good approximation the repertoire of processes occurring in human PDAC in a known time scale [42]. There is a significant improvement in outcome when ATRA is combined with gemcitabine compared to control vehicle or gemcitabine alone. ATRA reduced fibrosis, enhanced tumour necrosis, increased the number of blood vessels, and reduced hypoxia in PDAC tissues to induce an overall reduction in tumour size [43]. Interestingly, the authors observed increased levels of expression of RARβ, whose activation by ATRA promotes mechanical quiescence of PSCs. Currently ATRA is undergoing Phase 1 clinical trials.
Mechanobiology platform to study processes underpinning PDAC
The relevance of mechanical factors in PDAC progression underscores the need of a fresh look of PDAC from a mechanotransduction perspective. Mechanotransduction refers to the biophysical process through which cells sense and respond to biomechanical inputs (for instance ECM rigidity) from their physical microenvironment and transduce them into biochemical signals that ultimately influence cellular behaviour. This approach requires combining tools and concepts borrowed from biophysics, engineering, and cancer biology to investigate if/how mechanobiology may be the missing piece of the puzzle that links the three hallmark features of PDAC: desmoplasia, inflammation, and immune suppression.
Historically, our knowledge about how mechanical forces influence cell behaviour lags well behind the very active research in molecular genetics and cellular biochemical signalling. This is due, among other factors, is due to the dearth of appropriate methodologies to investigate these fundamental parameters. However, over the past two decades, the advent of powerful techniques in high resolution microscopy and mechanobiology opened the possibility to study the effect of mechanical forces in medicine and cancer with unprecedented detail. We will present below some of the techniques and applications available in the field of mechanobiology (summarised in figure 3).
Figure 3. Tools for investigating mechanobiology and their applications.
Download figure:
Standard image High-resolution imageMagnetic tweezers
Magnetic tweezers have been used to manipulate biological entities (cells, sub-cellular structures, and molecules). With this technique, forces can be tightly controlled and applied remotely without touching the sample using different angles and directionality. Forces can be static or pulsatile in a wide range of frequencies up to 1 kHz, and the magnitudes can range from several picoNewtons (physiological range of forces at the single molecule level) to tens of nanoNewtons (physiological range of forces in cells) [44].
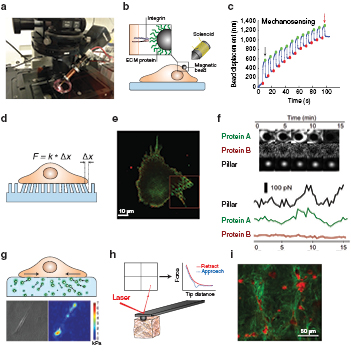
The working principle for this technique is based on the forces that can be generated with electromagnets. This apparatus consists of a solenoid wrapped around a ferromagnetic core that forms the magnetic needle (figure 4(a)). A known current circulates through the solenoid to create a magnetic field gradient in the vicinity of the ferromagnetic tip. Any magnetic particle placed within this magnetic field gradient is subjected to a force directed towards the source of the tip [45]. Then, if micron size magnetic beads are coated with the ligand of interest (for instance ECM molecules such as fibronectin, or collagen), and bound to specific receptors in the cell surface, organelle, or to a terminal end of a molecule; a controlled and known force can be applied to them (figure 4(b)) [46–48]. This setup is mounted on an inverted microscope, which allows the visualisation of the behaviours of cells and the attached beads (figure 4(a)). A second channel in the microscope can be used to monitor the recruitment of fluorescent proteins after the application of mechanical forces [48–51].
Figure 4. Methods and tools to study the mechanobiology of pancreatic cancer. (a) Magnetic tweezers probes mechanical properties at a single cell level and can assess the functionality of a cell's response to applied forces. (b) Magnetic beads coated with ECM protein can be driven by a pulsatile force. (c) The attenuation of bead displacement is a result of cellular reinforcement. The black and red arrow indicate the first and last oscillation pulses of a bead attached to a cell that has a robust mechanosensory machinery exemplified by the decrease in the oscillation of the bead over time, which indicates local stiffening of the cell surface. Panel reproduced from [35]. CC BY 4.0. (d) Elastic micropillar sensors determine the traction forces of cells on their substrate by the deflection of a PDMS pillar with a known mechanical elasticity. F is force applied by cells on the pillar and x is pillar displacement. (e) and (f) This can be combined with live immunofluorescent staining of focal adhesion proteins at the pillar interface to track protein recruitment and their relationship to cell-generated stress. Panel reproduced from [59]. (g) Traction force microscopy also reveals the forces cells generate on their substrates by using fluorescent markers that are displaced by the strain in the polyacrylamide gel. Top, schematic representation. Bottom, bright field images of PSC and its corresponding force map. Panel reproduced from [35]. CC BY 4.0. (h) Atomic force microscopy at the tissue level determines the elastic modulus of a tissue or gel from the deflection of a cantilever in contact with the sample, which can be used as a measure of stiffness associated to fibrosis. (i) Second harmonic generation imaging allows label-free visualisation of collagen fibre (green) architecture and alignment when remodelled by stromal cells (red). Panel reproduced from[29]. CC BY 4.0.
Download figure:
Standard image High-resolution imageCurrent applications for magnetic tweezers in biology can be classified into three main categories: (i) adhesion strength, (ii) response to mechanical stimuli, and (iii) microrheology. See diagram below. Adhesion strength can be tested for cell–cell [49, 50], or cell–ECM [47] interactions provided that the bead is coated with the cell adhesion molecule ligand or antibody, or with an ECM molecule, respectively. In this case, high static forces (1–5 nN) are applied, and the percentage of the beads that remained attached, or the time needed for detachment are quantified. To investigate cell responses to external mechanical stimuli, oscillatory pulses are applied and the oscillatory movement of the bead is monitored. Cells with robust mechanosensory machinery will respond to the applied force by generating local stiffening, also known as reinforcement, which will reduce the oscillation of the beads over time (figure 4(c)). Finally, using beads strongly attached to the cell (or organelle), it is possible to apply microrheology to quantify the viscoelastic properties of cells, such as cell compliance (reciprocal of stiffness) and fluidity [52].
Elastic micropillar sensors
Elastic micropillars are useful tools that have been employed for several years to quantify the forces that cells exert on their substrates. These pillars consist of an ordered array of an evenly spaced and flexible elastomers commonly made of polydimethylsiloxane (PDMS). These pillars are moulded from microfabricated silicon wafers that contain a repeating pattern of micron-wide holes of varying depth. Pillars are coated with the ECM molecule of interest, for instance fibronectin, and cells are plated on top of the pillars. Cellular forces are determined by analysing the deflection of the vertical pillars in image sequences obtained via brightfield microscopy. Through the application of Hooke's Law, the force is directly proportional to the deflection of the pillar and thus can be calculated using pillars of a known dimension/spring constant, which informs how much pillars deflect under the application of a known force (figure 4(d)) [53–56].
In recent years, technological advances have created new platforms to expand the possibility of these pillar sensors to investigate cellular forces. For example, Saez and colleagues developed an elastic pillar array that recapitulated the mechanical environment of epithelial sheets that allowed the study of the mechanical factors influencing mitosis with unprecedented detail [57]. Also, Ghassemi et al extended the applicability of these sensors by fabricating pillars of submicron sizes, which enabled many cellular processes that could not be observed with the micropillars [58]. Elastic pillars can be combined with fluorescent microscopy to correlate the force applied on the pillar at a particular time with the molecule involved in mechanosensing that is being recruited at this particular pillar (figures 4(e) and (f) [59].
Traction force microscopy
Traction force microscopy (TFM) is another technique used to quantify traction forces that cells apply to substrates. This assay uses polyacrylamide (PAA) elastic gels approximately 30 µm thick formed on a glass coverslip and embedded with micron sized fluorescent beads. Once the gels are coated with the ECM molecule (commonly fibronectin), cells are placed on top and are allowed to spread and deform the gels, displacing the fluorescent beads from their initial position. Afterwards, cells are removed and by comparing the initial and final position of the beads, the traction forces can be quantified following the Butler method (figure 4(g)) [60–62].
More recently Steinwachs and colleagues have developed a physio-mimetic biopolymer based system that allows the direct quantification of the mechanical forces applied by cancer cells fully embedded within physiological relevant 3D network [63]. Another recent study has reported the use of stimulated emission depletion (STED) microscopy with TFM to solve one of the major shortcomings of this technique, the spatial resolution. This improvement allows a greater than 5-fold higher sampling of the forces generated by cells compared to conventional TFM [64].
Atomic force microscopy
Atomic force microscopy (AFM) is a powerful technique that can be used to measure the biophysical properties of structures in a wide length scale in biology from atoms, to molecules and cells, to tissues or matrices. AFM is commonly used when combined with inverted microscopes for simultaneous imaging of the processes occurring in the samples. AFM uses a cantilever with a very sharp tip (typically around 10 nm radius of curvature) to probe the mechanical properties of the surface under study. The deflection of a laser beam on top of the cantilever head is detected by a photodiode, which transforms this deflection into a voltage signal that is directly proportional to the force at which the cantilever was submitted during the indentation of the solid surface (figure 4(h)).
For cellular studies, micrometre-scale beads are commonly attached to regular AFM tips to indent the cell surface and obtain an accurate measurement of the overall cytoskeletal tension [65]. Using these beads minimizes erroneous contact between the cantilever beam and the cell surface and permits greater spatial averaging of the elastic properties. AFM can be used also to probe the tension of subcellular structures such as the plasma membrane by limiting the cell indentation to decouple the plasma membrane tension from the overall cytoskeletal tension [66, 67].
Using indentation of biopsy samples, AFM provided the first mechanical profiling of normal and cancer breast tissues [68]. AFM has been also extensively used to assess the mechanics of physiological relevant matrices that recreates different types of tissues. Combining AFM with high resolution microscopy techniques, such as multi-photon SHG facilitates not only to assess the matrix mechanics but also to correlate this with the topological changes in the tissue or matrix architecture, such as the thickness, alignment, and length of the ECM fibres (figure 4(i)) [29].
Concluding remarks
Progress in PDAC research would be very limited without a holistic approach that integrates both the biochemical and biophysical interactions between cells and their microenvironment. Thus, it is necessary to approach the unsolved puzzle of PDAC through the innovative lens of mechanobiology and biophysics combined with cancer biology techniques to abolish the boundaries in these fields and provide solutions to an unmet clinical need. The broad use of the mechanobiology techniques showcased here will expand our capability to push the boundary of our knowledge in basic science concepts underpinning PDAC initiation and progression, which may have direct application in early diagnosis and development of novel therapies.
Acknowledgments
This work was supported by the European Research Council (grant agreement 282051). TJL is supported by the Whitaker International Program and James Dyson Foundation.