Abstract
The efficient utilization of coal gangue plays an indispensable role in reducing environmental pressure, improving resource utilization efficiency and promoting green development. As a kind of solid waste rich in silicon and aluminium, coal gangue can be used to prepare ceramic materials. Therefore, a new ceramic preparation process was proposed in this study to prepare ceramic binders for grinding wheels by geopolymerization. The SiO2-B2O3-Al2O3-RO-R2O ceramic binder, a green and low-cost material, was successfully prepared using this method. The effect of the concentration of NaOH on the mechanical properties and microstructure of the ceramic binder was also studied. The strength and microstructure of different ceramic binders were characterized by XRD, TG-DTG, SEM-EDS and FT-IR. The results show that the bending strength of the geopolymer ceramic binder increases proportionately with the increase of NaOH concentration. When the concentration of sodium hydroxide is 15 M, the bending strength reaches 19 MPa. N-A-S-H gel and zeolite formed in the geopolymerization reaction and the pores formed in the sintering process have a significant effect on the bending strength of the ceramic binder. This technology can significantly stimulate the chemical activity of coal gangue and provide innovative ideas for efficient utilization of coal gangue.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
With the accelerating process of industrialization and urbanization, industrial solid waste generated daily in various countries continues to accumulate. Improper disposal of these accumulated industrial solid wastes can lead to water, soil, and air pollution, causing irreversible damage to the local ecological [1]. Among industrial solid wastes, coal gangue has a huge production volume, with an average of 10% to 20% of coal gangue produced for every 1 ton of coal. In 2021, China's coal gangue production reached approximately 743 million tons, showing a 5.84% increase compared to 2020. Currently, coal gangue has become one of the most abundant industrial solid wastes in China [2, 3]. In the context of the current carbon-neutral era, finding harmless methods to treat coal gangue and achieve its resource utilization has become a global hot topic. Researchers are exploring alternatives to traditional adhesives such as lime and cement, seeking environmentally friendly options that produce less carbon emissions during production [4]. Geopolymers and alkali-activated materials are potential candidates for effective replacements, such as coal gangue, waste glass, etc
Coal gangue is a ceramic phase mineral rich in silicon dioxide (SiO2) and alumina (Al2O3), typically consisting of 80% SiO2 and Al2O3 [5]. Its chemical composition is similar to that of slag and fly ash, with the main mineral components being quartz, muscovite, and kaolinite. Currently, the utilization rate of coal gangue is relatively low [6], mainly in applications such as power generation, road filling, and construction materials that do not require extensive processing. The limited reactivity of coal gangue is a major obstacle to its large-scale application. However, in the geopolymerization reaction, the combination of sodium hydroxide and water glass (sodium silicate) can greatly enhance the reactivity of calcined coal gangue [7]. Geopolymers are green inorganic polymers comprising aluminosilicate constituents and are characterized by the semi-crystalline or amorphous form in a three-dimensional network, which has the advantages of high strength, high temperature resistance, corrosion resistance and solid sealing of heavy metal ions [8–13].
Research on coal gangue geopolymers is limited, with the majority of geopolymer studies focusing on their application in construction materials such as cement [14, 15]. Han et al [16]investigated the mechanism of coal gangue and the effect of activation on the properties of coal gangue geopolymers. The results showed that by adjusting the high calcium materials, alkaline activator content, Na2SiO3 modulus (n = (mSiO2)/(mNa2O)), and curing conditions, coal gangue geopolymers exhibited higher fluidity and mechanical strength compared to cement-based composites. Li et al [17] analyzed the influence of coal gangue particle size and activation temperature on the properties of coal gangue geopolymers and concluded that the optimal performance was achieved at a calcination temperature of 700 °C and a particle size of 200 mesh. Yang et al [18] studied the effects of single and mixed activators on the setting time, strength, and microstructure of coal gangue geopolymers. They found that mixed activators produced higher strength due to more intense hydration reactions and gel formation. In the study of preparing ceramic materials from solid waste such as coal gangue, Liew et al [19] used metakaolin to prepare single-component mixed geopolymers and sintered them to obtain geopolymer ceramic materials. The research revealed that the ceramic materials exhibited higher flexural strength after sintering at 1200 °C. Ji et al [20]. proposed a new method using high-alumina content fly ash as the main raw material for the preparation of ceramic tiles. Yang et al [21]. utilized coal gangue as the main raw material, with up to 70% coal gangue content, to prepare CaO-Al2O3-SiO2 glass ceramics. These ceramics showed high density, low water absorption, and improved chemical durability.
Overall, coal gangue has great potential for various applications in the preparation of geopolymer ceramics, such as refractory ceramics, corrosion-resistant chemical ceramics, grinding wheel ceramic binders [22], and biomedical ceramics. In this study, coal gangue was used as raw material and NaOH solution and water glass were chosen as alkaline activators to prepare coal gangue geopolymer. The geopolymer was pre-cured, crushed, and then sintered using a powder sintering method to obtain a coal gangue geopolymer ceramic binder. The NaOH concentration affects the extent of coal gangue geopolymerization reaction and the formation of crystalline phases during the sintering process. Therefore, this study conducted a detailed analysis of the flexural strength and microstructure of ceramic binder samples prepared under different NaOH concentrations. This research provides important theoretical basis and experimental support for the preparation of ceramic binder composites using coal gangue geopolymer.
2. Materials and methods
2.1. Material
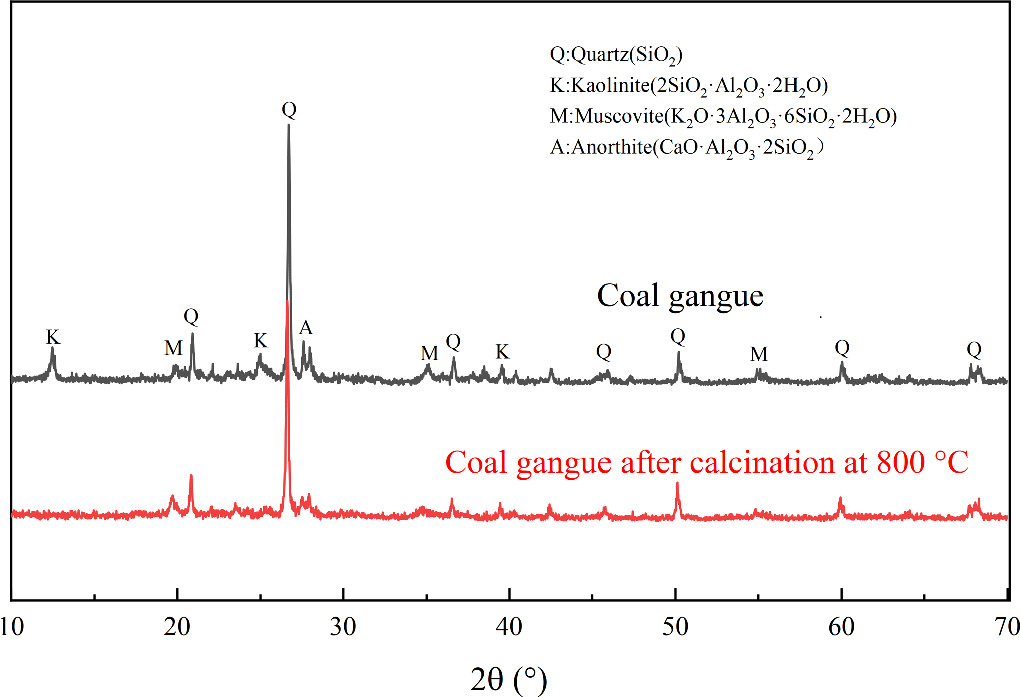
The coal gangue used in this study was sourced from Datong, Shanxi, China, and had a high content of silica and alumina. The x-ray fluorescence (XRF) analysis results are shown in table 1. To activate the coal gangue, a combination of mechanical and calcination methods was employed. The coal gangue was first milled in a planetary ball mill (Changsha Tianchuang Powder Technology Co., Ltd, China) at a rotation speed of 500 rpm for 20 min. Subsequently, it was calcined at 800 °C for 2 h in a box-type high-temperature furnace (Hefei Kejing Materials Technology Co., Ltd, China). After sieving, the calcined coal gangue powder with a particle size of 200 mesh was obtained. The x-ray diffraction (XRD) patterns of the coal gangue before and after calcination are shown in figure 1. From the figure, it can be observed that the main mineral components of the coal gangue are quartz, kaolinite, plagioclase feldspar, and muscovite. After calcination, the diffraction peaks of kaolinite and muscovite diminish significantly, while the diffraction peak of quartz enhances. This is attributed to the fact that at high temperatures, kaolinite and muscovite undergo dehydration and dehydroxylation, resulting in the transformation of kaolinite into metakaolinite and the formation of quartz due to the silicification of muscovite. These transformations enhance the reactivity of the coal gangue.
Table 1. XRF analysis results of coal gangue.
| Compound | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Mass (%) | 62.14 | 23.91 | 1.08 | 5 | 1.83 | 1.09 | 4 | 0.95 |
Figure 1. XRD patterns of coal gangue in original state and 800 °C calcination temperatures.
Download figure:
Standard image High-resolution imageFor the preparation of the geopolymer, a mixed solvent of NaOH solution and water glass (Na2SiO3 solution) was used as the alkaline activator. Four different concentrations of NaOH solutions of 8 M, 10 M, 12 M and 15 M (1 M = 1 mol l−1) were prepared. NaOH used had a purity of 99% analytical grade. High modulus water glass generates more free silicon bases during the geopolymerization process, resulting in less precipitation of hydrated sodium metasilicate crystals [23]. This makes it easier to form crystalline phases during the sintering process. A water glass solution with a modulus of 3.22 M was prepared, with a composition of SiO2 26.5%, Na2O 8.5%, and H2O 65%. The water glass and NaOH solutions were cooled and allowed to stand separately. They were then mixed in the required proportions as the alkaline activator for the preparation of the coal gangue-based geopolymer. In addition, Al2O3, SiO2, Na2CO3, B2O3, and CaO were prepared to adjust the composition of the geopolymer ceramic binder. These powder substances were all of 99% analytical grade.
2.2. Preparation of coal gangue geopolymers
Geopolymers were prepared from coal gangue powder using alkali activators with different NH moduli (NH modulus refers to the molarity of NaOH solution, which expresses the concentration of NaOH in moles per liter of solution.) through a water mixing method. The solid-to-liquid mass ratio was 0.8, and the Na2SiO3/NaOH mass ratio was 0.24. The pre-curing temperature was set at 80 °C, with a pre-curing time of 24 h. The experimental process is illustrated in figure 2. The activated coal gangue powder and the prepared alkali activator solution with the respective NH moduli were mixed in proportion until a homogeneous fresh paste was formed. The paste was then left to stand in a cup and placed in a thermostatic chamber for pre-curing. After pre-curing, the mold was removed to obtain solid geopolymer from coal gangue. Under the same experimental conditions and steps, four sets of geopolymer from coal gangue with different NH moduli were prepared.
Figure 2. Preparation process of coal gangue geopolymer.
Download figure:
Standard image High-resolution image2.3. Formulation and preparation of ceramic binders
The ceramic binder prepared in this study is a SiO2-B2O3-Al2O3-RO-R2O system, with a composition ratio of 48% SiO2, 12% Al2O3, 13% B2O3, 17% Na2O, 5% MgO, 3% CaO, and 2% K2O. Four different NH moduli coal gangue geopolymer samples were prepared, each with a different composition ratio after pre-curing. The precise calculation approach entails determining the mass of sodium (Na) in the sodium hydroxide solution based on its modulus. Subsequently, the mass of sodium silicate is calculated using the fixed ratio of Na2SiO3/NaOH. The molar ratio of Na to Si in the sodium silicate of the sodium glass solution is derived from the modulus of the sodium silicate. Finally, the masses of Na and Si in the sodium glass solution are determined. The alkali activator containing Na and Si is added to the original coal gangue components, and the percentage is recalculated. After removing the influence of water, any unreacted NaOH in the alkaline activator at high temperatures was fully converted to Na2O and H2O, while Na2SiO3 was fully converted to Na2O and SiO2. Based on the final composition of the four geopolymer samples, the necessary components were calculated and introduced to achieve the desired composition of the four samples. It should be noted that Na2O was introduced through Na2CO3. Table 2 shows the composition ratios of the pre-cured geopolymer samples and the set composition of the ceramic binder. The ceramic filaments prepared from geopolymer samples with NH moduli of 8 M, 10 M, 12 M, and 15 M are labeled as CB-1, CB-2, CB-3, and CB-4, respectively.
Table 2. The composition and preset ratio of each geopolymer sample after precuring.
| Mass (%) | |||||
|---|---|---|---|---|---|
| Compound | CB-1 | CB-2 | CB-3 | CB-4 | Set ratio |
| SiO2 | 54.46 | 52.01 | 52.11 | 50.27 | 48 |
| Al2O3 | 19.37 | 17.97 | 18.05 | 17.02 | 12 |
| CaO | 0.88 | 0.86 | 0.8 | 0.76 | 3 |
| MgO | 1.50 | 1.34 | 1.37 | 1.31 | 5 |
| Na2O | 20.69 | 24.76 | 24.68 | 27.97 | 17 |
| K2O | 3.20 | 3.06 | 2.98 | 2.61 | 2 |
| B2O3 | 0 | 0 | 0 | 0 | 13 |
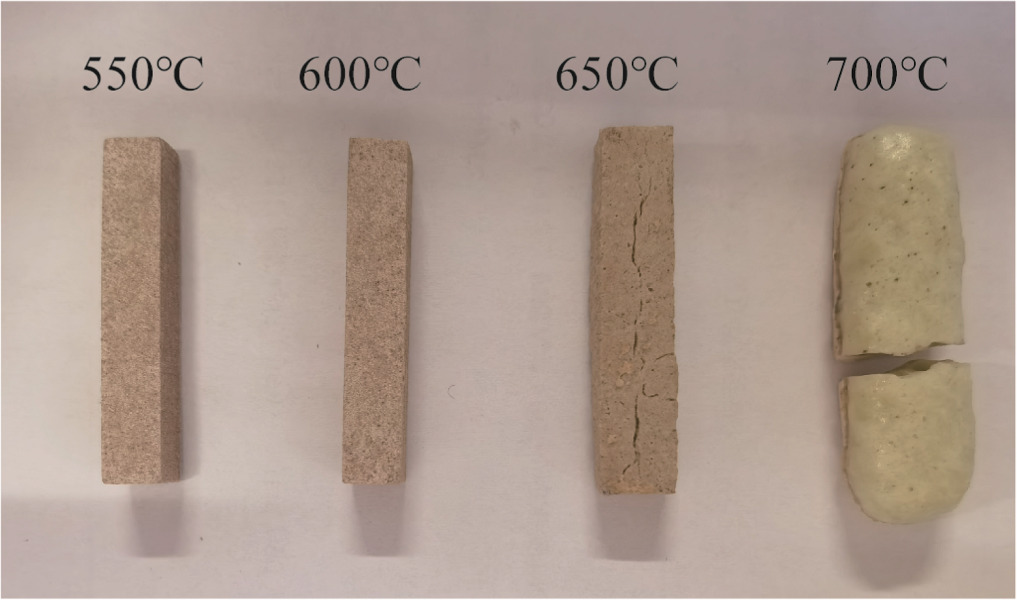
The specific steps for preparing ceramic binder rods are as follows: geopolymers were crushed and mixed uniformly with the required supplementary components (fillers) in a planetary ball mill, followed by drying to obtain a ceramic binder mixture with grain size smaller than 100 micrometers. The ceramic binder mixture was then pressed into rectangular rods (35 mm × 6 mm × 4 mm) using a powder compaction machine at a pressure of 20 MPa. These samples were sintered for 120 min at a predetermined sintering temperature, cooled naturally to room temperature, and the ceramic binder samples were obtained. Figure 3 shows the ceramic binder rods sintered at temperatures of 550 °C, 600 °C, 650 °C, and 700 °C. It was observed that the rods sintered at 700 °C exhibited severe cracking and deformation, while the rods sintered at 650 °C had some cracks. The rods sintered at other temperatures showed uniform and no significant cracking or deformation, indicating a refractoriness range between 600 °C. and 650 °C. The rods sintered at 630 °C show uniform texture without any cracks, suggesting a sintering temperature of 630 °C. At the same sintering temperature of 630 °C, four groups of ceramic binders CB-1, CB-2, CB-3 and CB-4 with different NH moduli were prepared.
Figure 3. Ceramic binder rods at different sintering temperatures.
Download figure:
Standard image High-resolution image2.4. Testing and characterization
2.4.1. Flexural strength testing
The flexural strength of the four groups of ceramic binder rods is determined using an INSTRON 6800 Universal Testing Machine (Instron Limited, USA) through three-point bending tests. The fracture load P (N) of the rods is obtained, and the flexural strength σ (MPa) is calculated using equation (1). The width of the fracture surface b is 6 (mm), the height of the fracture surface h is 4 (mm), and the span length L is 30 (mm). The loading rate is set to 0.5 MPa s−1.
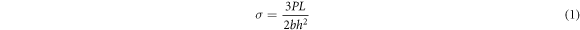
2.4.2. X-ray diffraction characterization
The crystallographic analysis was performed using a TK-XRD-201 x-ray diffractometer (Beijing Taikun Industrial Equipment Co., Ltd, China) with CuKα target radiation to obtain x-ray diffraction patterns of the ceramic binder samples. The scanning speed was set at 2°/min with a step size of 0.02°, and the scanning range was 5°–80° (2θ).
2.4.3. FT-IR characterization
FT-IR testing was conducted using a Nicolet 6700 FT-IR spectrometer (Thermo Fisher Scientific Technologies, USA) to evaluate the functional groups in the samples. The spectral range was 400–4000 cm−1, with a resolution of 0.09 cm−1, and the dynamic adjustment was set at 130,000 scans per second.
2.4.4. SEM-EDS testing
The microstructure of the ceramic binder was observed using an O-INSPECT scanning electron microscope (Carl Zeiss AG, Germany). X-ray energy-dispersive spectroscopy (EDS) analysis was performed on the ceramic binder using the scanning electron microscope to analyze the types and concentrations of elemental components in the material microregions.
3. Results and discussion
3.1. Flexural strength analysis
The flexural strength is an important indicator for evaluating the performance of ceramic binders and reflecting the quality of grinding wheels. Figure 4 presents the flexural strength curves of four groups of samples. From the graph, it can be observed that the flexural strengths of CB-1, CB-2, CB-3, and CB-4 are 5 MPa, 10 MPa, 11 MPa, and 19 MPa, respectively. The flexural strength is directly proportional to the NH modulus. Comparing CB-2, CB-3, and CB-4 with CB-1, the flexural strengths have increased by 200%, 220%, and 380% respectively. The main components of the SiO2-B2O3-Al2O3-RO-R2O ceramic binder system are SiO2, Al2O3, and B2O3. The alkali oxide and alkali-earth oxides in the system provide oxygen atoms for the formation of the glass network structure, such as [SiO4] tetrahedra, [AlO4] tetrahedra, and [BO4] tetrahedra, which affect the strength and hardness of the glass structure. Among the four groups of samples, B2O3 is supplemented in the same proportion. Therefore, the strength of the ceramic binders is mainly determined by [SiO4] and [AlO4] tetrahedra.
Figure 4. The flexural strength of ceramic binder samples.
Download figure:
Standard image High-resolution imageWhen coal gangue powder is dissolved in the alkali activator, [SiO4] and [AlO4] tetrahedra are initially formed [24], and then condensation and dehydration occur to produce Si-O-Al bonded polymeric aluminum silicate gel. When the NH modulus is between 8 M and 10 M and the NaOH concentration is low, the leaching of SiO2 and Al2O3 from coal gangue is less, and the content of Al2O3 in coal gangue powder is higher. At this time, there are more [AlO4] tetrahedra than [BO4] tetrahedra, and a small amount of [BO4] tetrahedra are transformed into [BO3] tetrahedra by the more stable [AlO4] tetrahedra, resulting in lower overall mechanical properties of the ceramic binder. When the NH modulus is between 12 M and 15 M and the concentration of sodium hydroxide is high, more SiO2 and Al2O3 are leached out, promoting the geopolymerization process. At this time, the content of Al2O3 in coal gangue powder is lower, and the [BO4] tetrahedra and [AlO4] tetrahedra formed by the interaction of free oxygen with Al2O3 and B2O3 constitute the main body of the glass network structure, presenting a spatial lattice structure. The mechanical properties of the binder are better at this stage. When the NH modulus is between 8 M and 12 M, the early precipitation of aluminum silicate gel hinders the condensation of [SiO4] and [AlO4] tetrahedra, resulting in slower strength growth.
3.2. The crystallographic analysis
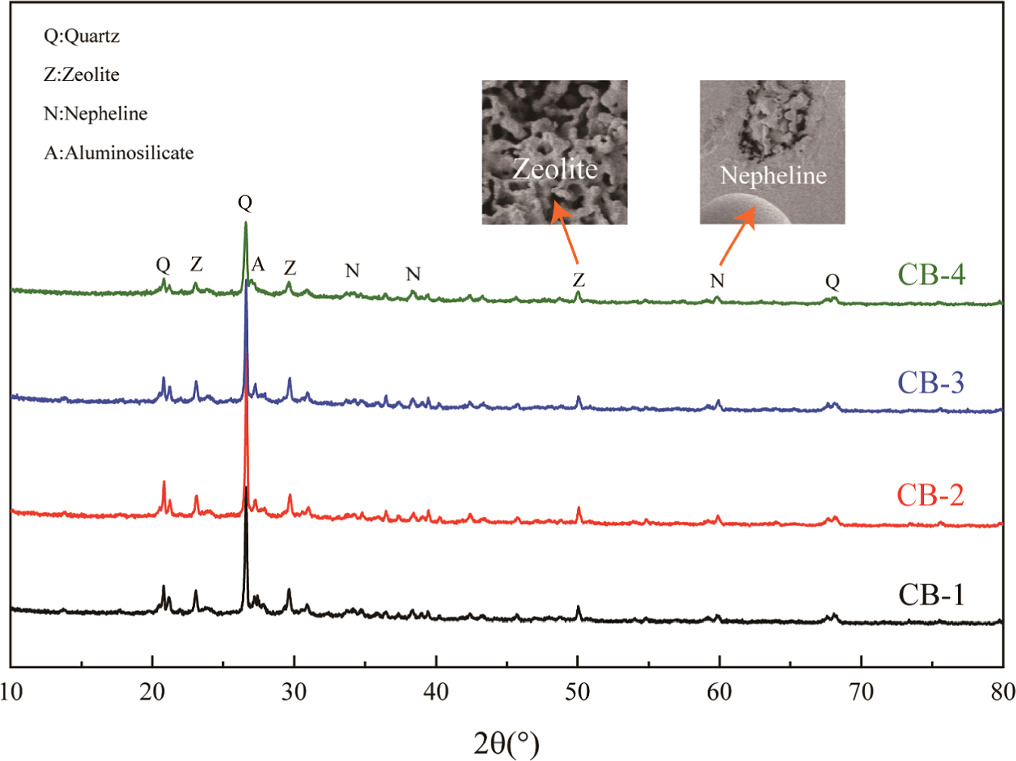
The XRD patterns of samples with different NH moduli are presented in figure 5. The identified mineral phases in the four sample groups include quartz, zeolite, and other aluminosilicates. The dominant peak in the XRD patterns of the ceramic binder is attributed to quartz, while the presence of zeolite is commonly observed in the geopolymerization reaction. Notably, distinct diffraction peaks are observed in the 22°–35° range in the XRD patterns, indicating an intermediate state between amorphous and semi-crystalline phases. The increase in NH modulus does not significantly affect the intensity of the main peak; however, it does reduce the peak intensity of SiO2 crystals, thereby enhancing the flexural strength of the ceramic binder [25, 26]. Zeolite peaks are observed in all sample groups. The porous and low-strength nature of zeolite limits the development of compressive strength. As the temperature rises, certain amorphous and zeolite phases undergo transformation into crystalline diffraction peaks. The zeolite structure undergoes collapse due to the breakage of Si–O–Si and Si-O-Al bonds in the temperature range of 600 °C–800 °C [27], with some zeolite phases transforming into mullite. Mullite contains a substantial amount of silica-rich polymeric species [28], which significantly contributes to the enhancement of mechanical strength. The high-water content in the geopolymer reaction favors the formation of zeolite phases [29], and a lower occurrence of zeolite phases is observed under increased NH modulus, leading to an overall higher strength. Additionally, during the sintering process, some zeolite phases transform into mullite, further augmenting the flexural strength of the ceramic binder.
Figure 5. XRD patterns of ceramic binder samples.
Download figure:
Standard image High-resolution image3.3. FT-IR analysis
The phase transition of geopolymer, which refers to the changes in functional groups, can be detected using FT-IR absorption peaks. Figure 6 presents the FT-IR characterization of ceramic binder at different NH moduli. According to figure 6, the main band appears as a broad peak, and the peak occurring at 3500 cm−1 is attributed to the stretching vibration of the hydroxyl groups (O-H) in the bound water of coal gangue geopolymer. The absorption peak at 1620 cm−1 corresponds to the bending vibration of the O–H–O group, providing further evidence for the presence of bound water in the samples. The absorption peak at 1420 cm−1 can be attributed to the stretching vibration of the O–C–O bond (CO3 2− group), and the absorption peaks for CB-1, CB-2, CB-3, and CB-4 are observed at 1413 cm−1, 1417 cm−1, 1422 cm−1, and 1426 cm−1, respectively. This indicates the possibility of CO2 gas generation during the geopolymerization process, and the shift of the absorption peak towards higher wavenumbers with increasing sodium hydroxide concentration can be attributed to the atmospheric carbonation of the NaOH solution [30]. The absorption band around 1000 cm−1 is associated with the asymmetric stretching vibration of Si-O-Al/Si–O–Si [31, 32]. The bands below this frequency indicate a higher infiltration of tetrahedral aluminum during the geopolymerization process. The spectral band from 880 cm−1 to 870 cm−1 corresponds to tetrahedral aluminum, and the spectral band at 720 cm−1 represents the stretching vibration of Si–O–Si or Si–O–Al, which is attributed to the transformation of 6-coordinated aluminum through the hydration reaction. These results indicate the formation of N-A-S-H gel with more tetrahedral aluminum structures during the geopolymer reaction, thereby enhancing the mechanical properties of the material. The analysis suggests that the studied samples contain incompletely reacted coal gangue, as well as aluminum silicate and sodium aluminate formed through the hydration reaction. Additionally, the stretching vibration of the hydroxyl group of kaolinite, located around 3695 cm−1, does not exhibit significant absorption peaks in any of the samples, confirming the removal of hydroxyl groups from kaolinite during the calcination and activation process of coal gangue.
Figure 6. FT-IR characterization of ceramic binder samples.
Download figure:
Standard image High-resolution image3.4. Thermogravimetric analysis
The thermal behavior of ceramic binder powder was determined using TG-DTG analysis. Figure 7 shows the TG-DTG curves of the CB-1, CB-2, CB-3, and CB-4 samples. The total weight loss percentages for CB-1, CB-2, CB-3, and CB-4 are 9.389%, 18.062%, 15.271%, and 7.515%, respectively. The weight loss is relatively small for the 8 M and 15 M samples, which can be attributed to the evaporation of physically adsorbed water, the loss of chemically adsorbed water, and partial decomposition of crystalline phases. The weight loss from 25 °C to 100 °C is mainly due to the evaporation of adsorbed water and pore water in the geopolymer, with CB-1 sample showing the highest weight loss in this stage. The additional weight loss between 100 °C and 600 °C is attributed to the release of water from the condensation/polymerization of Si-OH and Al-OH groups. After 600 °C, the weight loss of all samples is minimal, indicating a reduction in carbon and carbonates in the coal gangue after pre-activation. At lower NH moduli, due to the lower amount of gel phase and the lower content of non-evaporable water in the gel, the overall weight loss is mainly concentrated on the evaporation of physically adsorbed water, resulting in a smaller weight loss. On the other hand, at higher NH moduli, the premature precipitation of aluminosilicate gel hinders the condensation/polymerization of [SiO4] and [AlO4] tetrahedra, leading to a reduced release of water from the condensation/polymerization of Si-OH and Al-OH groups, resulting in a smaller weight loss as well. With an increase in NH modulus, the corresponding ceramic binder exhibits higher thermal decomposition temperatures, and the peak of the DTG curve (maximum weight loss temperature of free water in the gel phase) shifts to higher temperatures. The weight loss of coal gangue-based geopolymer mainly occurs in the range of 50 °C to 150 °C, mainly attributed to the dehydration of the N-A-S-H gel and zeolite crystalline phase (hydrated aluminosilicate).
Figure 7. TG-DTG curves of ceramic binder samples.
Download figure:
Standard image High-resolution image3.5. Microstructure analysis
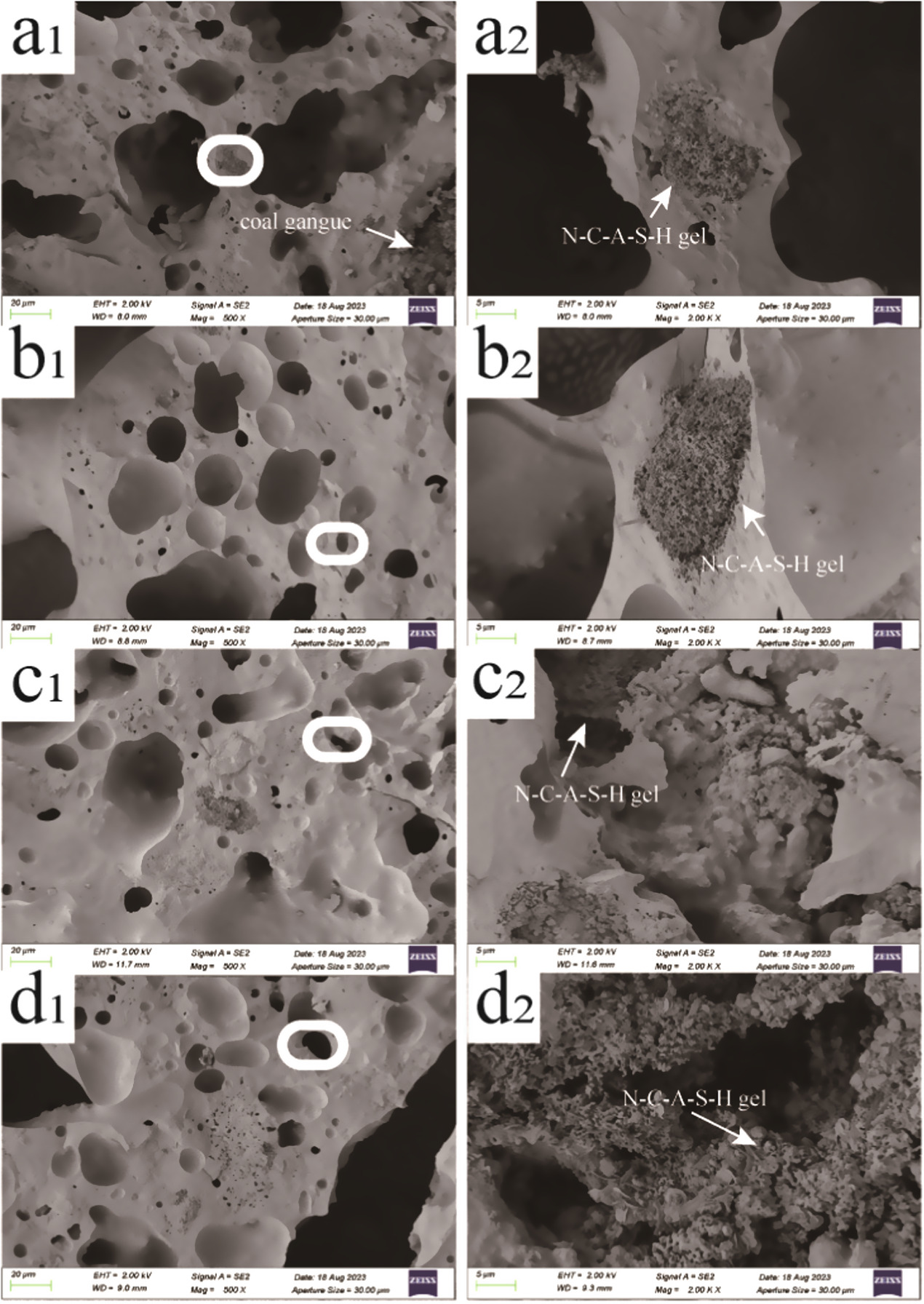
The microstructural differences in ceramic binder prepared at different NH moduli can be determined based on the SEM images. Figure 8 shows the SEM images of the ceramic binder cross-sections, where (a1), (b1), (c1), (d1) represent the microsurface images of CB-1, CB-2, CB-3, CB-4, respectively, and (a2), (b2), (c2), (d2) show the magnified pore images of the boxed regions in CB-1, CB-2, CB-3, CB-4, respectively. At low NH moduli, the microstructure exhibits a porous and rough morphology (figure 8(a1)). As the NH modulus increases, the pores become smaller and more uniform, and the surface becomes denser and smoother, with an increased amount of gel phase. Many of the pores that form in the sample may be due to foaming effects during high-temperature melting and solidification, removal of organic content, and release of gases (such as CO2 gas produced during heating). At lower NH moduli, incomplete reaction between the low concentration of -OH and coal gangue results in a lower degree of N-Si hydration, and the hydration products only cover a part of the surface of the raw material particles. Due to the lack of sufficient binder filling the pores between particles, the surface pores of the samples with low NH moduli are relatively large. As the NH modulus increases, the hydration process accelerates, and the iron-rich and calcium-rich phases of coal gangue diffuse in all directions, leading to an increased amount of hydration products and the formation of various types of gels. These gels coat the coal gangue raw material particles, resulting in a smooth and dense microstructure surface.
Figure 8. SEM image of ceramic binder samples.
Download figure:
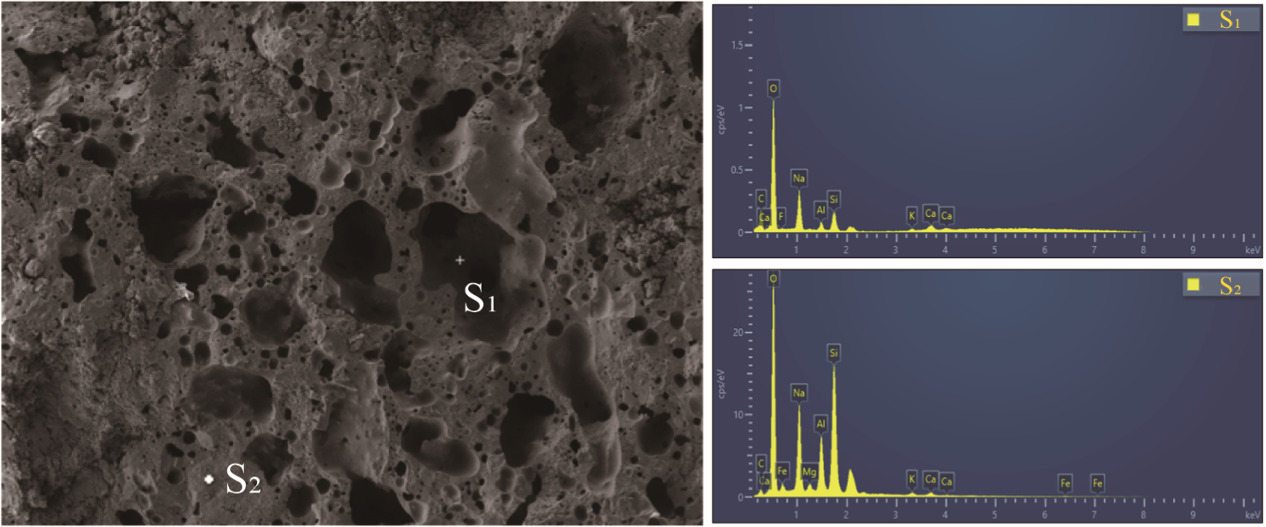
Standard image High-resolution imageFigure 9 shows the EDS spectrum of sample CB-3 with an NH modulus of 12 M. S1 and S2 represent the points in the pore and on the surface, respectively. From figure 9, it can be observed that the main elements in S1 and S2 are Na, Al, Si, Ca, and O, indicating the formation of gel phase and the presence of N-A-S-H and C-A-S-H gels. Table 3 presents the element ratios in S1 and S2, where the Na/Si ratio is much higher than the Ca/Si and Al/Si ratios in both the pore point S1 and the surface point S2. This indicates that the hydration products in the ceramic binder are mainly composed of N-A-S-H gel. There is also a certain amount of Fe content in S2, suggesting the formation of N-A(Fe)-S-H or C-A(Fe)-S-H gels. This is because during the depolymerization-polycondensation reaction, Fe3+ can replace Al3+ to form Fe-Si gel (-Fe-O-Si-O-Al-O), while Ca2+ acts as a network modifier to balance the charge and generate a small amount of C-Si gel (C-(Fa)-Si-H) in localized regions. It is worth mentioning that the carbon and oxygen content in S1 is significantly higher than that in the surface point S2, indicating that the release of CO2 by the geopolymer is one of the reasons for pore formation. The above analysis indicates that an increase in NH modulus leads to a decrease in unreacted coal gangue particles, resulting in a denser and more uniform structure with smaller pores, thereby enhancing the strength of the binder. Furthermore, an increase in NH modulus intensifies the hydration reaction, resulting in an increased amount of geopolymer gel. These gels have good binding ability and can coat the coal gangue particles to form a well-cured structure. At higher NH moduli, the structure becomes denser and more compact, thereby improving mechanical properties such as flexural strength.
Figure 9. SEM-EDS spectra of ceramic binder sample (NH = 12 M).
Download figure:
Standard image High-resolution imageTable 3. Ratio of elements of scanning dots in EDS spectrum.
| Scan dot | C | O | Na | Al | Si | K | Ca | Fe | F | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 21.12 | 53.59 | 10.83 | 2.10 | 5.70 | 1.51 | 4.69 | 0 | 0.47 | 0 |
| S1 | 4.01 | 42.05 | 12.79 | 7.96 | 21.22 | 0.98 | 1.42 | 8.68 | 0 | 0.89 |
4. Conclusion
In this paper, the coal gangue was successfully transformed into geopolymer gel and the low temperature ceramic binder was further prepared by the geopolymerization reverse method. The mechanical properties and microstructure of the samples prepared with different concentrations of NaOH were analyzed. The role of NaOH concentration in the geopolymerization and sintering process was determined. The results are as follows:
- (1)The method of geopolymerization can effectively activate the chemical activity of coal gangue, and can prepare the ceramic binder with mullite crystal from geopolymer as raw material.
- (2)The flexural strength of the ceramic binder is proportional to the NaOH concentration and reaches 19Mpa when the concentration of sodium hydroxide is 15 M. However, the strengthening rate will decrease after 12 M, because too much NaOH concentration will lead to early gel precipitation, which will hinder the geopolymerization reaction and slow the strength growth.
- (3)The reason for the increase in strength is that the N-A-S-H gel and C-A-S-H gel formed in the geopolymerization process provide the main body of the tetrahedral structure, and part of the zeolite phase is converted into mullite during the sintering process, which improves the strength of the ceramic binder.
- (4)The increase of the NaOH concentration can improve the distribution of the internal pores of the ceramic binder and make them more uniform and finer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51902294) and China Postdoctoral Science Foundation (Grant No. 2020M670699).
Data availability statement
No new data were created or analysed in this study.