Abstract
To find the phase change microcapsule material (MEPCM) with suitable temperature and high heat storage energy. The phase change microcapsules with sodium sulfate decahydrate (Na2SO4·10H2O) as core material and polystyrene (PS) as wall material were prepared by emulsion polymerization. The microcapsule is fixed on the cloth of the overalls by coating method, and the thermoregulation fabric is obtained. The microcapsules were characterized by scanning electron microscopy (SEM), differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and infrared spectroscopy (FTIR). Fabrics are tested for durability, air permeability and temperature control. The DSC results show that the Tc and Tm of Na2SO4·10H2O/PS microcapsules are 26.0 °C and 58.0 °C, respectively, and the ΔHc and ΔHm are 64.0 J g−1 and 121.1 J g−1, respectively. The TG results show that the first weight loss temperature range is 104.1 ∼136.7 °C, and the second weight loss temperature range is 395.5 ∼434.6 °C. The infrared spectral characteristic peaks of microcapsules include all the characteristic peaks of Na2SO4·10H2O and PS. In the range of microcapsule phase transition temperature, the cloth treated with 0.5 g MEPCM and 1.5 g MEPCM delayed the temperature change by 16.0 ∼23.0 °C and 10.0 ∼18.0 °C, respectively, compared with the blank cloth. The fabric treated with 0.5 g microcapsules was subjected to 100 and 300 heating/cooling cycles. Compared with before and after the cycles, the fabric after thermal shock cycles showed a 4.15% and 3.56% reduction in delayed temperature changes rate in a rising and falling temperature environment. Therefore, Na2SO4·10H2O/PS microcapsule material can achieve the goal of heat storage and energy storage, and can be used as a low-temperature operation protection material.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The sustainability and recyclability of energy is the cornerstone of social security and stable development. The report of the Party's 20 th National Congress pointed out that 'strengthen the construction of the energy production, supply, storage ,and marketing system to ensure energy security' and further promote the energy revolution. Latent heat storage of phase change materials (PCM) is one of the most effective energy storage methods, which can absorb or release energy with the change of external temperature and maintain a constant local ambient temperature [1]. In addition, PCM has the characteristics of sustainable and recyclable energy saving [2, 3]. The PCMs have been widely applicable in construction, textiles, refrigeration systems, solar energy storage and other fields, and are an important research direction for future green thermal energy [4–9]. In the continuous study of PCM, it was found that the direct use of PCM would lead to problems such as leakage of PCM, phase separation and corrosion [10]. Researchers apply microencapsulation technology to PCM, that is, PCM is wrapped in a polymer film by physical or chemical methods to form a core–shell structure and produces MEPCM, which can not only extend its service life, but also improve its thermal properties through shell materials [11]. Phase change microcapsules are added to ordinary fabrics to make thermoregulation fabrics, which can absorb or release heat by sensing changes in ambient temperature and can be used as personal protective clothing materials [12–16].
Rui X [17] prepared paraffin phase change microcapsules by in situ polymerization, which melted at about 45.0 °C and solidified at about 60.0 °C. The mass loss between 200.0 °C and 340.0 °C was caused by the decomposition of paraffin in the core, and the loss between 340.0 °C and 700.0 °C was caused by the high-temperature decomposition of urea resin in the wall material. Jianhua Z [18] prepared nanocapsules using microemulsion polymerization and successfully fixed them on cotton fabric, and the results show that the fabric has temperature control and durability. Ayan P [19], Xi L [20] applied the prepared PCM to fabrics and tested its temperature regulation performance through heating/cooling cycles. According to the requirements of the human body for thermal comfort, protective materials with a phase change temperature of 23.0∼26.0 °C can enhance human comfort and are easy to recover after phase change [21–24]. The latent heat value of encapsulated salt hydrate is higher than that of encapsulated organic PCMs [25]. At the same time, phase change microcapsules prepared by Na2SO4·10H2O are suitable for textile applications [26]. In this study, for the first time, Na2SO4·10H2O microcapsules were prepared by emulsion polymerization using Na2SO4·10H2O as core material and PS as wall material. The phase change temperature of the PCM was equal to the comfort level of human body. The ΔHc and ΔHm of Na2SO4·10H2O/PS microcapsules are 64.0 J g−1 and 121.1 J g−1, respectively. Through the performance characterization of microcapsules and the test experiment of thermoregulation fabric, the personal protective material used to regulate human temperature was explored. The protective clothing made of this material can provide a suitable microclimate environment for the human body.
2. Materials and methods
2.1. Matterials
The core material used for the preparation of microcapsules is crystal sodium sulfate decahydrate (AR) provided by Shanghai Zhanyun Chemical., Ltd The initiator is azodiisobutyronitrile (AIBN, AR), the wall material is styrene (St, AR) and polystyrene amide (PAM, AR), all provided by Tianjin Fuchen Chemical., Ltd The emulsifier is Span80 (CP) provided by Tianjin Hengxing., Ltd The dispersion medium is n-octane (C8H18, AR) provided by Tianjin Fuyu Fine Chemical., Ltd Sodium borax tetraborate (Na2B4O7, AR) provided by Tianjin Beichen Founder., Ltd Homemade deionized water from our lab. Emulsion adhesive purchased by Harbin Green Times Rubber Industry Share., Ltd Fabric purchased by Guangzhou Zhongda International Textile City., Ltd.
2.2. Preparation of Na2SO4·10H2O/PS microcapsules
This study adopts the emulsion polymerization method, which is a catalytic reaction of monomers in a specific form (i.e. in the form of insoluble dispersion in water). This method will not cause environmental pollution, the reaction time is short, the monomer consumption rate is fast, the final product has a large molecular weight, and the reaction can be carried out at a lower temperature [27, 28].
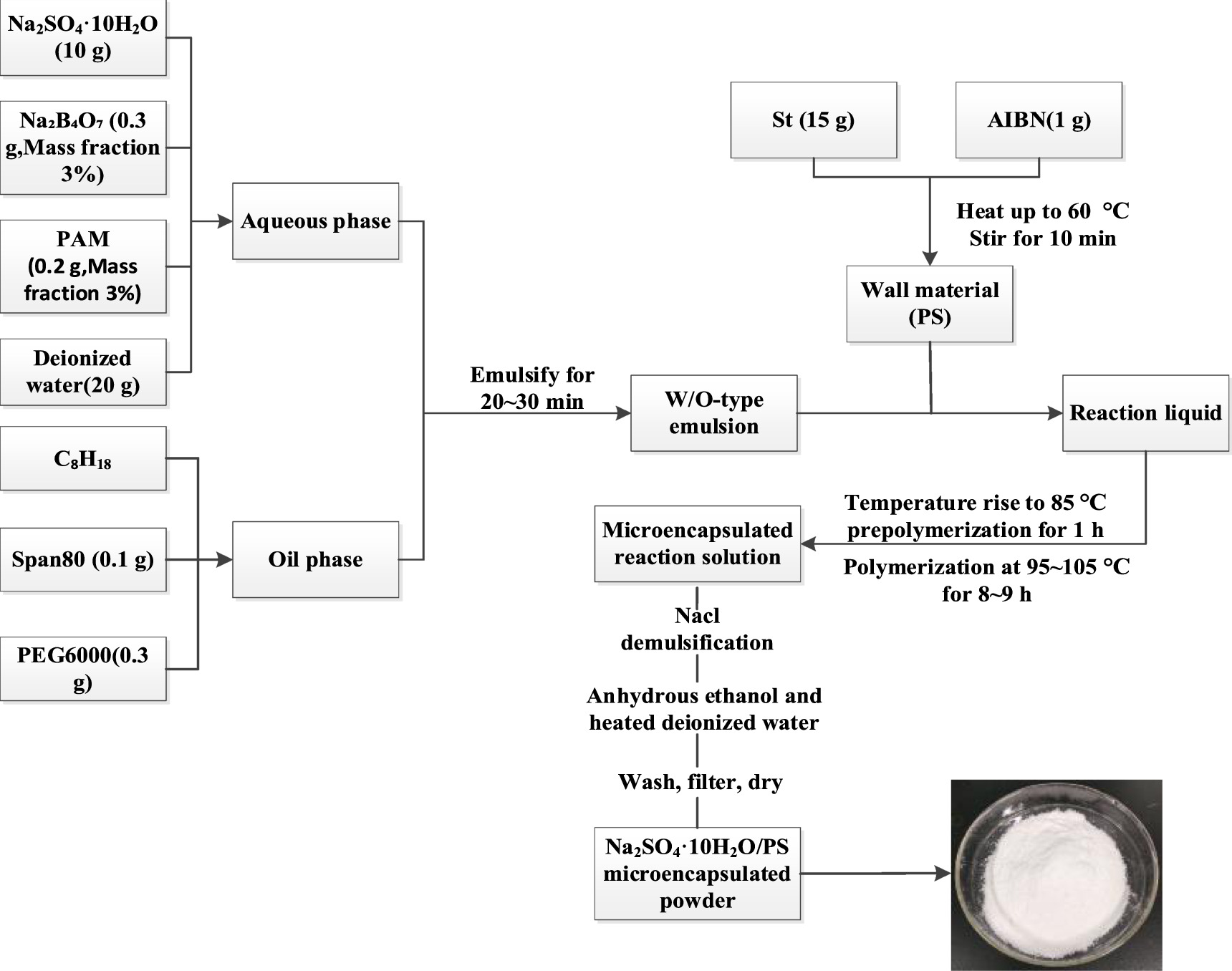
In this experiment, Na2SO4·10H2O was used as the phase change core material and PS as the phase change wall material. After many experiments, when the ratio of core material to wall material is 2:1, the performance of phase change microcapsules is the best. The test process is complicated, and the correlation with this paper is not high, so it is not detailed here. The microcapsules were synthesized using the optimal experimental scheme. Subsequently, microcapsules were studied and analyzed. The process for preparing Na2SO4·10H2O microcapsules is shown in figure 1.
Figure 1. Process flow diagram of Na2SO4·10H2O/PS microcapsules.
Download figure:
Standard image High-resolution image2.3. Preparation of thermoregulation fabric
The fabric is treated using the coating method. Add microcapsule sample powder into the adhesive solution and stir well to prepare a mixed coating solution. In this study, four kinds of fabrics, pure cotton, polyester cotton, combed cotton and canvas, were cut into a fixed size of 5 × 5 cm. The coating liquid was evenly applied to the front and back of the four kinds of fabrics with a self-made coating rod, and the coating was pre-baked in a drying oven at 50.0 °C for 20 min. Drying at 80.0 °C for 20 min, after drying can obtain the thermoregulation fabric.
2.4. Characterization methods
2.4.1. Surface topography testing (SEM)
The microcapsule powder was directly glued to the conductive adhesive, and gold was sprayed by a sputtering coater. The surface morphology of the sample was observed by SEM (Reguius8100, Japan).
2.4.2. Thermal performance test (DSC)
The test samples were dried and placed in aluminum POTS filled with nitrogen. The test temperature range is set to −80.0 ∼ 150.0 °C, and the temperature is heated at a rate of 10.0 °C min−1. Measure the phase transition crystallization temperature(Tc), melting temperature(Tm), crystallization enthalpy value(ΔHc), and melting enthalpy value(ΔHm) of the samples were measured by DSC (DSC25, USA). The samples were tested in a high and low temperature impact test chamber (PVS-3KP, ESPEC, Japan), as shown in figure 2, and the heating/cooling cycles impact temperatures were set at 25.0 °C and 60.0 °C, and the thermal shock cycles were 100 and 300 times. DSC of samples before and after thermal shock were measured 10 times.
Figure 2. High and low temperature impact test chamber (PVS-3KP).
Download figure:
Standard image High-resolution image2.4.3. Thermogravimetric test (TG)
The test samples were dried and placed in ceramic a crucible and filled with nitrogen. Set the temperature range of 30.0∼600.0 °C for the analysis process, and heat up at a rate of 10.0 °C per minute. TG (TG209F1Libra, Germany) was used to test the thermal stability of the sample.
2.4.4. Infrared spectrum test (FTIR)
For dry grinding samples, take 1∼2 mg sodium sulfate decahydrate, polystyrene, and microcapsules, and then take the appropriate amount of potassium bromide, mixed grinding. The test wave number ranges from 4000 to 500 cm−1. The chemical compatibility of the samples was tested by FTIR (NicoletiS20, USA).
2.5. Performance characterization of thermoregulation fabric
2.5.1. Durability
The four types of cloth were cut into a fixed size of 5 × 5 cm, and the thermoregulation fabric and blank cloth were weighed. According to the quality difference, judge the adhesion strength of the cloth to MEPCM. According to GB/T3921–2008 washing temperature control cloth, according to the quality loss rate before and after washing to determine the cloth washable performance. The durability of the treated fabric is considered by the two indexes of adhesion and washable resistance.
2.5.2. Air permeability
Drop the water droplets on the blank cloth and the thermoregulation fabric cloth respectively, and record the time for the cloth to absorb all the water droplets. The breathability of the fabric is judged by the length of the water drop permeation time. The difference in water penetration time between the two groups of cloth was calculated to determine the effect of microcapsules on the permeability of cloth.
2.5.3. Thermoregulation
Take the blank fabric, 0.5 g MEPCM, and 1.5 g MEPCM coated thermoregulation fabric respectively. They were placed in ice water for 30 min, and then taken out to a constant temperature water bath of 60.0∼70.0 °C to simulate the performance changes of the cloth under a heating environment. The cloth with the same indexes was taken and heated in a 70.0 °C drying oven for 30 min, and then taken out and placed in ice water respectively to simulate the performance changes of the cloth in a cooling environment. The 0.5 g microcapsule coated thermostatic fabric was subjected to 100 and 300 thermal shock cycles, and the thermal shock temperature was set at 25.0 °C and 60.0 °C.The temperature control performance of the fabric before and after thermal shock was tested in the heating and cooling environment.
3. Results and discussion
3.1. Performance analysis of microcapsule
3.1.1. Surface topography analysis (SEM)
After spraying gold on the surface of the microcapsule, the photos of the microcapsule were taken by scanning electron microscope, as shown in figure 3. As can be seen from the figure, the microcapsule presents a spherical shape, the spherical surface is smooth as a whole, with slight fine marks, and a small amount of anhydrous sodium sulfate fine slag (circled part in figure 2) sticks to the outer wall of the microcapsule. The reason for anhydrous sodium sulfate is that the core material of sodium sulfate decahydrate was not successfully coated during the polymerization process and was transformed after water loss.
Figure 3. SEM diagram of Na2SO4·10H2O/PS microcapsules.
Download figure:
Standard image High-resolution image3.1.2. Thermal properties analysis (DSC)
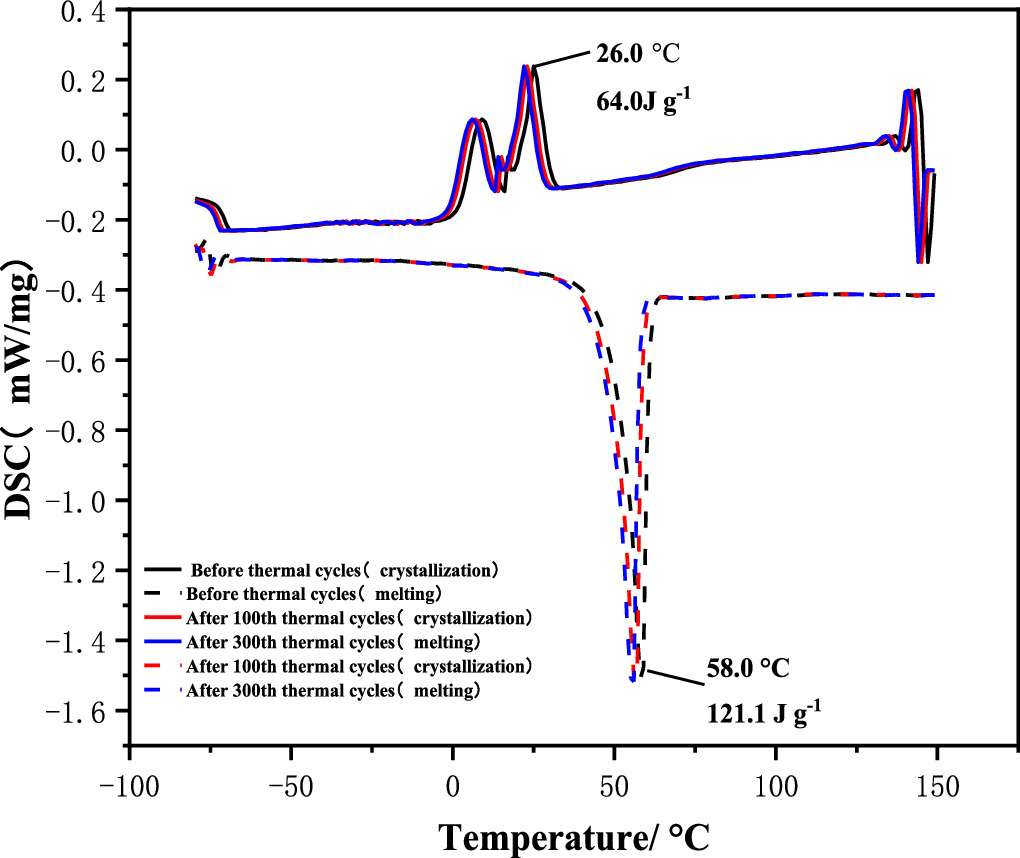
The phase transition temperature and enthalpy of Na2SO4·10H2O/PS microcapsule were tested. Calculate the average value of multiple tests. As shown in figure 4, the Tc and Tm of Na2SO4·10H2O/PS microcapsules are 26.0 °C and 58.0 °C, respectively, and the ΔHc and ΔHm are 64.0 J g−1 and 121.1 J g−1, respectively. The DSC results showed that after 100 and 300 consecutive thermal shock cycles, the melting phase transition temperature decreased by 2.03% and 2.21%, and the crystallization phase transition temperature decreased by 1.68% and 1.71%. The enthalpy of melting decreased by 1.21% and 1.58%, and the enthalpy of crystallization decreased by 1.54% and 1.77%. Compared with the standard melting phase transition temperature of Na2SO4·10H2O at 32.0 °C [29], the phase transition temperature of microcapsules increased by 26.5 °C, because the melting point of PS was 206.0 °C [30], which was much higher than the phase transition temperature of Na2SO4·10H2O. After reaching the phase transition temperature of Na2SO4·10H2O, the microcapsule was still in the endothermic process. In summary, the microcapsule wall material successfully played a coating role, and the thermal properties of the core material did not change.
Figure 4. DSC diagram of Na2SO4·10H2O/PS microcapsules.
Download figure:
Standard image High-resolution image3.1.3. Thermogravimetric analysis (TG)
TG was used to characterize the thermogravimetric properties of Na2SO4·10H2O, PS, and Na2SO4·10H2O/PS microcapsules. As shown in figure 5, the weight loss starting temperature of sodium sulfate decahydrate is 31.0 °C, and the weight loss ending temperature is 95.0 °C, after which the thermal weight loss rate remains at about 60%. This is because only the crystalline water in it is decomposed during the heating process, and sodium sulfate decahydrate is transformed into anhydrous sodium sulfate. The weight loss starting temperature of polystyrene is 371.6 °C, and the weight loss ending temperature is 428.7 °C. Two weightlessness occurred in the microcapsule. The temperature range of the first weightlessness process was 104.1∼136.7 °C, and the thermogravimetric rate was 3.17%, due to the evaporation of crystal water in the core material Na2SO4·10H2O. The temperature range of the second weight loss process is 395.5∼434.6 °C, and the thermal weight loss rate is 95.31%, which is caused by the thermal decomposition of the shell. The initial weight loss temperature of the microcapsule was higher than the initial weight loss temperature of Na2SO4·10H2O, and the temperature difference was 73.1 °C, indicating that PS had a coating and protection effect on Na2SO4·10H2O. The results are in good agreement with those obtained by DSC.
Figure 5. TG diagram of Na2SO4·10H2O, PS and Na2SO4·10H2O/PS microcapsules.
Download figure:
Standard image High-resolution image3.1.4. Infrared Spectral Analysis (FTIR)
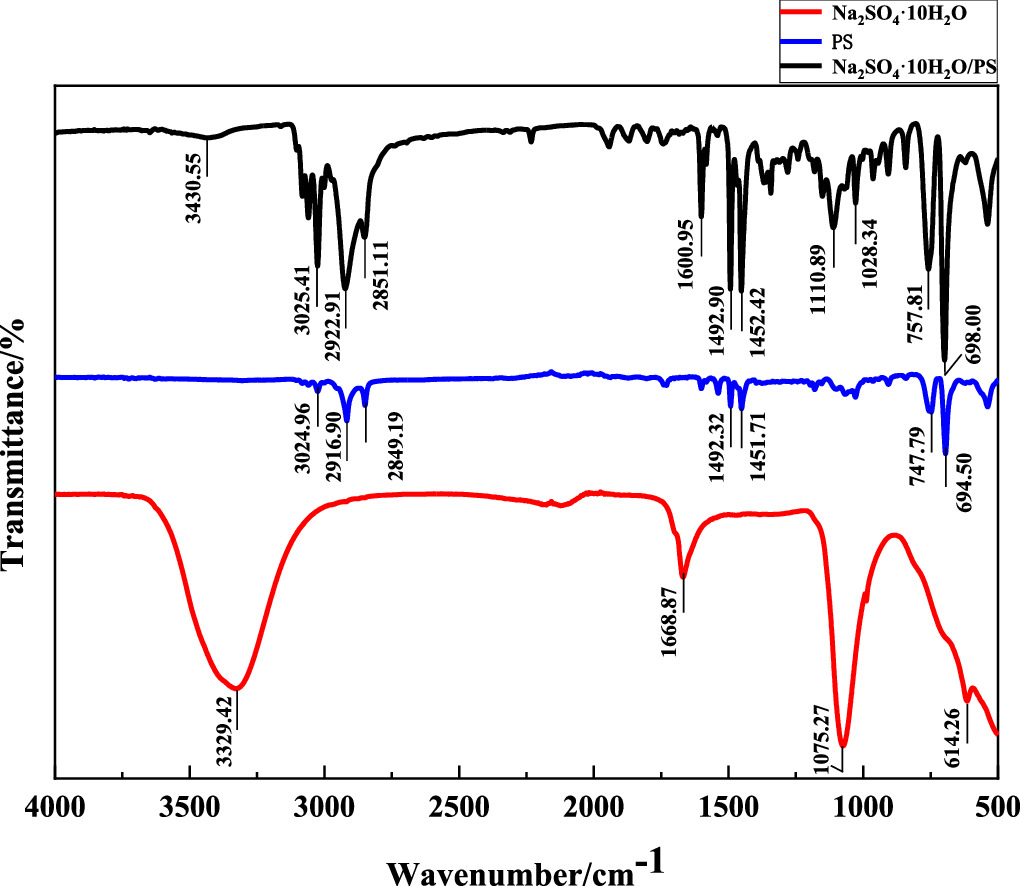
The structural components of Na2SO4·10H2O, PS, and Na2SO4·10H2O/PS microcapsules were respectively detected by FTIR, and the results were shown in figure 6.The infrared spectrum of Na2SO4·10H2O shows that the O-H bond and SO4 2− stretching vibration peaks in the coordination water molecules are 3329.42 cm−1 and 1668.87 cm−1, 1075.27 cm−1 and 614.26 cm−1, respectively. According to the infrared spectrum of PS, the stretching vibration peaks of = C–H bond, −C–H bond, and benzene ring skeleton were found at 3024.96 cm−1, 2916.90 cm−1 and 2849.19 cm−1, 1492.32 cm−1 and 1451.71 cm−1, respectively. 747.79 cm−1 and 694.50 cm−1 are the out-of-plane bending vibration peaks of the single substituted benzene ring and the C–H bond on the benzene ring, respectively. As shown by the external spectrum of Na2SO4 ·10H2O/PS microcapsule, the O−H bond, = C–H bond, −C–H bond, benzene ring skeleton, SO4 2- stretching vibration peaks in the coordination water molecules are 3430.55 cm−1, 3025.41 cm−1, 2922.91 cm−1 and 2851.11 cm−1, 1492.90 cm−1 and 1452.42 cm−1, 1110.89 cm−1 and 1028.34 cm−1, respectively. The out-of-plane bending vibration peaks of the C–H bond and single substituted benzene ring were found at 963.48 cm−1, 698.00 cm−1 and 757.81 cm−1, respectively. 1600.95 cm−1 may be the O-H stretching vibration peak of the coordinated water molecule. It may also be the benzene ring skeleton stretching vibration peak. Comparing the absorption spectral curves of the three substances, the absorption peak of the microcapsule included all the absorption peaks of the other two substances, and no new characteristic absorption peak appeared, indicating that no chemical reaction occurred between Na2SO4·10H2O and PS, indicating that Na2SO4·10H2O was successfully coated by PS. Na2SO4·10H2O/PS microcapsules were synthesized.
Figure 6. FTIR diagram of Na2SO4·10H2O, PS and Na2SO4·10H2O/PS microcapsules.
Download figure:
Standard image High-resolution image3.2. Thermoregulation fabric performance analysis
3.2.1. Durability analysis
Calculate the quality loss rate of the cloth before and after application and washing and drying. The test results are shown in table 1. Comparing the quality difference and quality loss rate of four kinds of cloth before and after MEPCM, the quality difference of pure cotton and combed cotton is higher than that of polyester cotton and canvas, the quality loss rate of pure cotton cloth after washing is 8.4% lower than that of combed cotton, and the quality loss rate of polyester cotton and canvas is only 0.9% and 2.0%.
Table 1. Washability test results.
| Fabric material | Blank fabric quality (mg) | Tempering fabric quality (mg) | ΔH (mg) | Quality after washing (mg) | Mass loss rate (%) |
|---|---|---|---|---|---|
| Pure cotton | 907.7 | 934.9 | 27.2 | 913.1 | 2.3 |
| Polyester cotton | 1013.9 | 1023.0 | 9.1 | 1008.6 | 1.4 |
| Combed cotton | 558.3 | 583.3 | 25.0 | 520.5 | 10.7 |
| canvas | 832.0 | 837.4 | 5.4 | 835.0 | 0.3 |
3.2.2. Air permeability analysis
Test the time for water droplets to completely penetrate the cloth before and after each cloth treatment, and the results are shown in table 2. The time for water droplets to fully penetrate pure cotton, polyester cotton and canvas is not more than 30.00 s, and the time for fully permeating combed cotton is more than 1 min.The time difference of water droplet penetration before and after MEPCM treatment was compared. The full penetration time difference of combed cotton is 51.25 s, and that of pure cotton is 0.04 s.
Table 2. Hydrophilic test results.
| Fabric material | Treatment method | Full penetration time (s) | Δt (s) |
|---|---|---|---|
| Pure cotton | coating | 17.49 | 0.04 |
| blank | 17.45 | ||
| Polyester cotton | coating | 16.59 | 0.12 |
| blank | 16.47 | ||
| Combed cotton | coating | 140.26 | 51.25 |
| blank | 89.01 | ||
| Canvas | coating | 17.49 | 3.06 |
| blank | 17.45 |
3.2.3. Temperature control performance analysis
Based on the results of durability and air permeability, the durability and air permeability of pure cotton cloth are better than the other three kinds of cloth, so choose pure cotton cloth is the research object to do the temperature control performance test.
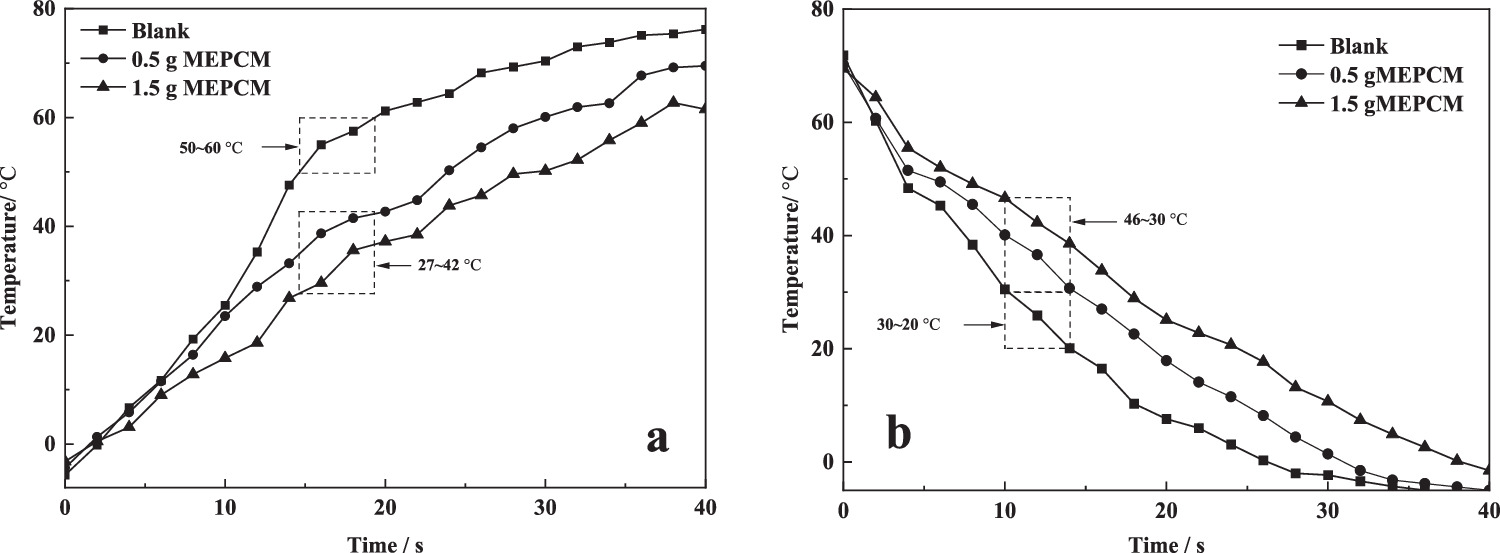
Heating test: the temperature rise performance test was performed on blank pure cotton cloth, 0.5 g MEPCM and 1.5 g MEPCM treated pure cotton cloth. As shown in figure 7(a), when the blank fabric is in the melting phase transition temperature region of microcapsule 50.0∼60.0 °C, the fabric temperature after MEPCM treatment is only 27.0∼42.0 °C, which delays the heating rate of the fabric. The delayed heating rate of 1.5 g and 0.5 g MEPCM were 2.6 °C s−1 and 3.6 °C s−1, respectively.
Figure 7. Heating test curve (a) and Cooling test (b) of blank cloth and MEPCM treated cloth.
Download figure:
Standard image High-resolution imageCooling test: The cooling performance of blank cloth, 0.5 g and 1.5 g MEPCM treated cloth was tested. As shown in figure 7(b), when the blank fabric is in the solidification phase transition temperature region of microcapsules at 30.0∼20.0 °C, the temperature of the fabric after MEPCM treatment is 46.0∼30.0 °C, which delays the cooling rate of the fabric. The delayed cooling rates of the fabrics treated with 1.5 g and 0.5 g MEPCM were 2.0 °C s−1 and 3.3 °C s−1, respectively.
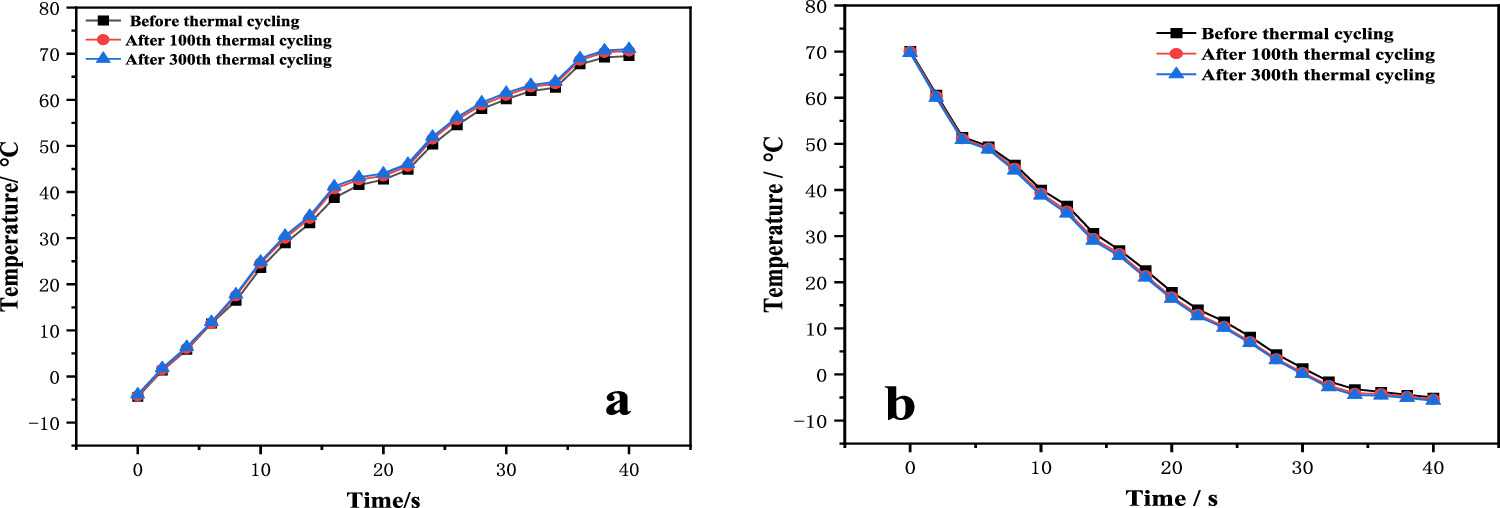
Heating/colling and cold cycle test: After 100 and 300 heating/cooling cycles of 0.5 g MEPCM treated fabric at 25.0 °C and 60.0 °C, the performance test results of fabric under heating and cooling environment are shown in figures 8(a) and (b). After the thermal shock cycle, the delayed temperature change rate decreased by 4.15% in the heating environment and 3.56% in the cooling environment compared with that before the cycle.
Figure 8. Heating test curve (a) and Cooling test (b) before and after 100 th and 300 th heating/cooling cycles.
Download figure:
Standard image High-resolution image4. Conclusions
In this paper, Na2SO4·10H2O/PS microcapsules were prepared with Na2SO4·10H2O as core material and PS as wall material, and the properties and characterization of the synthesized microcapsules were tested by SEM, DSC, TG, and FTIR. The prepared microcapsules were arranged on four common workwear fabrics, and the treated fabrics were tested for durability and permeability, and the optimal fabric was selected for heating/cooling cycle experiment to test the heating and cooling performance of the fabric before and after the cycle. The following conclusions are drawn: (1) Na2SO4·10H2O/PS microcapsules were prepared by emulsion polymerization with the spherical surface, and the characteristic peaks of the infrared spectrum included all the characteristic peaks of Na2SO4·10H2O and PS. The wall material PS successfully covered the core material Na2SO4·10H2O without affecting the thermal properties of the core material. (2)The Tc and Tm of Na2SO4·10H2O/PS microcapsules are 26.0 °C and 58.0 °C, respectively, and the ΔHc and ΔHm are 64.0 J g−1 and 121.1 J g−1, respectively. The microcapsule has two weight loss processes. The first weight loss is due to the weight loss of the core material sodium sulfate decahydrate, and the weight loss temperature range is 104.1∼136.7 °C. The reason for the second weightlessness is the thermal decomposition of polystyrene, and the weightlessness temperature range is 395.5∼434.6 °C. (3) Four kinds of common workwear fabrics were treated with microcapsules to form thermoregulation fabrics. The results showed that the durability and breathability of pure cotton fabrics were better than those of other fabrics. The cloth treated with MEPCM has the performance of delaying the temperature change, and the temperature change shows a decreasing trend with the increase of MEPCM content. Compared with the pre-cycle rising and cooling environment, the delayed temperature change rate of 0.5 g MEPCM treated cloth was reduced by 4.15% and 3.56% after 100 and 300 thermal shock cycles.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).