Abstract
Immunomagnetic nanoparticles (IMNPs) have been widely applied for the capture and concentration in the rapid detection of target bacteria. In this research, the focus was on studying the changes in magnetic properties changes of the IMNPs when they were attached to bacterial cells. These alterations in properties could facilitate an even rapid detection of the target bacteria and eliminate the need for culturing on plating media. The variation of magnetizing values, including saturated magnetization (Ms), remanent magnetization (Mr), coercivity force (Hc), and magnetic susceptibility (χm), was analysed through M-H loops. It was observed that the magnetizing properties of the IMNPs underwent changes based on the concentrations of Salmonella Typhimurium cells in the test solution. The correlation of this phenomenon was confirmed by the results of synchrotron x-ray absorption spectroscopy (XAS), which revealed electronic transition changes in the IMNPs after capturing the bacteria cells. Additionally, the electronic bands of the magnetite nanoparticle [Fe(II) and Fe(III)] were detected, indicating an electronic transformation between the Salmonella cells and the bound IMNPs. The XAS change was further verified using different cell types, such as Campylobacter jejuni which also showed electronic transformation after attaching to IMNPs. These findings suggest that IMNP-cell attachment triggered the change in the magnetic properties of IMNPs. Such insights could serve as valuable information for the development of novel rapid bacteria detection assays/devices using magnetic sensing techniques.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Conventional methods for the detection of foodborne pathogenic bacteria typically require more than 5 days [1]. The process involves a nonselective pre-enrichment step of a defined weight or volume of the sample, followed by a selective enrichment stage, plating onto selective agars, and subsequent biochemical and serological confirmations of the suspicious colonies [2]. Although these methods effectively identify microorganisms, they are time-consuming and labor-intensive before obtaining results [3]. Iron-oxide-based magnetic nanoparticles (MNPs) have been favorably utilized for various applications, including cell separation, cell concentration, bio imaging, and drug delivery [4, 5]. The magnetic nanoparticles coated with antibodies specific to the target pathogen form immune-magnetic particles (IMNPs). These IMNPs can be specifically bound to the target bacteria, creating an IMNP-bacteria complex that can be easily separated from the food matrix and background bacteria. Subsequently, the complex can be concentrated into a smaller volume through exposure to a magnetic field [6]. This approach significantly shortens the detection time. However, there have been few research works focusing on the change of magnetic properties of the magnetic nanoparticles after IMNPs-cell interaction, which is crucial for their applications [7]. This includes the study of surface effect [8], structure and magnetism behaviour [9], and phase transformation [10] after charge doping and surface modification. Additionally, the magnetism changes of MNPs MNPs when attached to a bacterium cell have been neglected. This work hypothesized that the magnetic property of MNPs would be alternated when they conjugate to the bacteria cell. Therefore, the magnetic properties of the magnetic nanoparticles were studied after surface modification and target bacteria cell attachment (Salmonella Typhimurium as sample bacteria). The magnetism variation of MNPs after the bacteria cell-attached and surface modification steps were analyzed and explained by the analyzed results of XANES spectrums using the synchrotron XAS. Additionally, Campylobacter jejuni was implemented as a different bacterial cell to confirm the result of XAS analysis. The results of this research would be considered a possibility for a new food-borne rapid detection platform based on the response of MNPs magnetic property.
2. Materials and methods
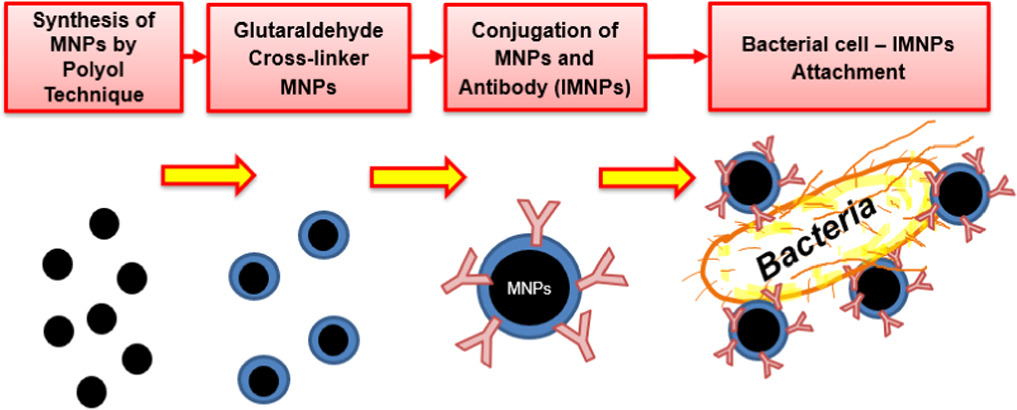
The synthesized magnetic nanoparticle (MNPs), immune-magnetic nanoparticle (IMNPs), and the magnetic nanoparticle attached to the target bacteria cell (Cell-IMNPs) were prepared as described previously [11] and shown diagram of the sample preparation in figure 1. The samples were used for investigating the magnetic properties and their natures. The analytical techniques utilized in this work are described in subsections below.
Figure 1. Diagram of sample preparation steps.
Download figure:
Standard image High-resolution image2.1. Preparation of magnetic nanoparticles
The synthesized MNPs were prepared using the polyol technique with amino functional groups provided by ethylenediamine. This followed the description in the previous work by Songvorawit et al [11]. The expected size of Fe3O4 would be around 30–50 nm in cubic shape. Briefly, 2 g of ferric chloride (FeCl3·6H2O) was dissolved in 40 ml ethylene glycol and mixed until the solution was clear yellow. Then, 6 g of sodium acetate (CH3COONa), 1.68 g of sodium hydroxide (NaOH), and 20 ml of ethylenediamine were added and stirred for 30 min. After mixing, the solution was further heated in an autoclave at 1210 °C, 105 kPa, and 2 h per cycle for 3 cycles. After each cycle, the mixed solution was shaken for 5 min to complete the reaction. Next, the amino-functionalized ferromagnetic nanoparticles were separated by a strong magnet and washed five times with deionized water and five times with 95% ethanol to remove the solvent. Ultrasonication was used while washing to remove the solvent from the particle surface. The MNPs were dried in an oven at 50 °C for 24 h, milled with mortar, and kept in air-tight amber bottles until use.
2.2. Surface modification and antibody conjugation of magnetic nanoparticle
The preparation of antibody-conjugated magnetic nanoparticles (Ab-MNPs or Immune-MNPs) was prepared. Glutaraldehyde (GA) was used as the cross-linker to connect antibodies to the nanoparticle surface. The aldehyde group reacts with MNPs, while the aldehyde group on the other end could link to amine groups of the antibodies [12]. The reaction was used to improve the immobilization capacity of antibodies [13]. For the reaction, firstly, 0.1 g of MNPs were washed and re-suspended in 50 ml of phosphate-buffered saline (PBS) pH 7.4 at a concentration of 2 mg ml−1. Then, 5 ml of glutaraldehyde (25% v/v) was added and stirred at room temperature for 2 h. The modified MNPs were separated by an external magnetic field and washed three times with PBS (pH 7.4) to remove free glutaraldehyde. It was resuspended in PBS before being used in the next step.
A polyclonal Anti-Salmonella Typhimurium antibody (pAb-Salmonella) (S & A Reagent Lab, Bangkok, Thailand) and a monoclonal Anti-Campylobacter jejuni antibody (mAb-Campylobacter) [7721] ab33023 (abcam) were used in this work. Glutaraldehyde-modified FMNs were prepared into 2 groups of samples for each antibody conjugation which were the conjugation with the polyclonal anti-Salmonella Typhimurium antibody and the monoclonal anti-Campylobacter jejuni antibody by incubation at 25 °C overnight with gentle shaking. Then, the particles were washed three times with 1 × PBS (pH 7.4) to remove free chemicals and antibodies. They were stored in an air-tight amber bottle at 4 °C until use.
2.3. Preparation of bacterial samples
S. Typhimurium was used as a sample bacterium to study the changing of MNPs magnetism in the step of surface modification, antibody conjugation, and call attachment with cell concentration variation. In addition, C. jejuni bacterium sample was also used to verify the change. XAS analysis results were used to confirm the hypothesis of the changes due to the target organisms.
A loop full of fresh 24 h old colonies of S. Typhimurium was suspended in normal saline (0.85% NaCl) diluent. Appropriate dilutions with statistically acceptable counts of 30–300 colonies/plate on plate count agar (PCA) were made and used for the calculation to confirm the concentration of the suspension. The initial concentration of the suspension was 6.0 × 107 cell/ml. Ten-fold serial dilutions were made from this initial suspension to obtain 107, 105 and 104 cell/ml suspensions for this experiment.
C. jejuni ATCC 33560 was cultured on mCCDA and incubated at 42 °C for 2 days and then resuspended in PBS pH 7.4 before use. The culture of bacterial cells at 108 cell/ml was prepared and subjected to serial ten-fold dilutions to obtain the target concentrations.
2.4. Attachment of antibody-conjugated magnetic nanoparticles to the bacterial cells
The antibody-conjugated magnetic nanoparticles (IMNPs) were reacted with each bacteria cell by mixing 500 μl of cell suspension with 8 μl of the IMNPs and incubated at 37 °C for 30 min in a shaker incubator. After that, the particle-bacterial complexes were separated from the suspension by a strong magnet. At each preparation step, samples were washed three times with PBS (pH 7) buffer to wash away free glutaraldehyde and antibodies. The particles and cells, after attachment steps, were analyzed with Synchrotron radiation-based Fourier-transform infrared (SR-FTIR) spectroscopy, field emission scanning electron microscopy (FESEM) and field emission transmission electron microscopy (FETEM) techniques.
2.5. Characterization techniques
The morphologies of the cell-IMNPs binding were observed under the field emission scanning electron microscopy, FESEM (TESCAN MIRA 3), and the field emission transmission electron microscopy, FETEM (Thermo Scientific TALOS F200X). Results were used for approval of the functional group binding of the bacteria cell and IMNPs. The IMNPs with and without bacterial cell attachment were investigated by using Fourier transform infrared (FTIR) spectroscopy at the BL4.1 station of the Synchrotron Light Research Institute (Public Organization), Thailand (SLRI) using the transmission mode, with conventional internal IR source of a Bruker Ver- tex 70 spectrometer connected to the Bruker Hyperion 2000 microscope (Bruker Optics Inc., Ettlingen, Germany).
The magnetic property analyses of Magnetizations (M) versus magnetic fields (H) were monitored using a vibrating sample magnetometer (VSM). The measurement was carried out using Lake Shore 7403 VSM (Department of Chemistry, Faculty of Science, Khon Kaen University, Thailand) under a maximum Field of 20 kOe. Steps of surface modification and bacterial cell attachment of nanomagnetic particles were investigated in the Fe L edge and Fe K edge XANES spectra by using X-ray absorption near-edge structure in transmission mode at the BL2.2 station at SLRI, Thailand.
X-ray Absorption Near-Edge Structure Spectroscopy (XANES) analysis of all standard compounds including FeO, Fe2O3, Fe3O4,[14] as well as various ferritic oxide samples: MNPs, Ab-MNPs (IMNPs), and IMNPs-Cell (either attached with Salmonella or Campylobacter), were conducted at the Fe K-edge. The energy spectrum analysis took place at beamline 2.2 of the electron storage ring at the SLRI, Nakhon Ratchasima, Thailand [BL2.2]. The experimental setup involved using electron energy of 1.2 GeV, a bending magnet, and a beam current ranging from 80 to 150 mA, resulting in photon rates of 1.1 to 1.7 × 1011 photons s−1. For each sample, a finely ground and homogenized powder was spread to form a thin film (2.0 cm × 0.5 cm area) on Kapton tape (Lanmar Inc., Northbrook, IL, USA) and mounted on a sample holder. Care was taken to disperse the particles evenly and avoid hole effects. Multiple spectra (at least five) were obtained from each standard compound and oxide sample. The XANES spectra were measured in the transmission mode using ionization chamber detectors. During the acquisition of spectra, a Ge (220) double crystal monochromator with an energy resolution (∆E/E) of 2 × 10−4 was utilized. The energy range of 4936–5824 eV was scanned with energy steps of 2, 0.2, and 0.05 keV for Ti K-edge spectra. The photon energy was calibrated against the K-edge of Ti foil at 4966 ± 0.2 eV. Subsequently, the normalized XANES data were processed and analyzed after subtracting the background in the pre-edge and post-edge regions. The software used for XAS data processing was ATHENA (Chicago, IL, USA), which is based on the IFEFFIT library of numerical and XAS algorithms. ATHENA is written in the Perl programming language and utilizes the Perl/Tk graphics toolkit.
3. Results and discussion
3.1. Characterization of magnetite MNPs, IMNPs, and bacterium conjugation
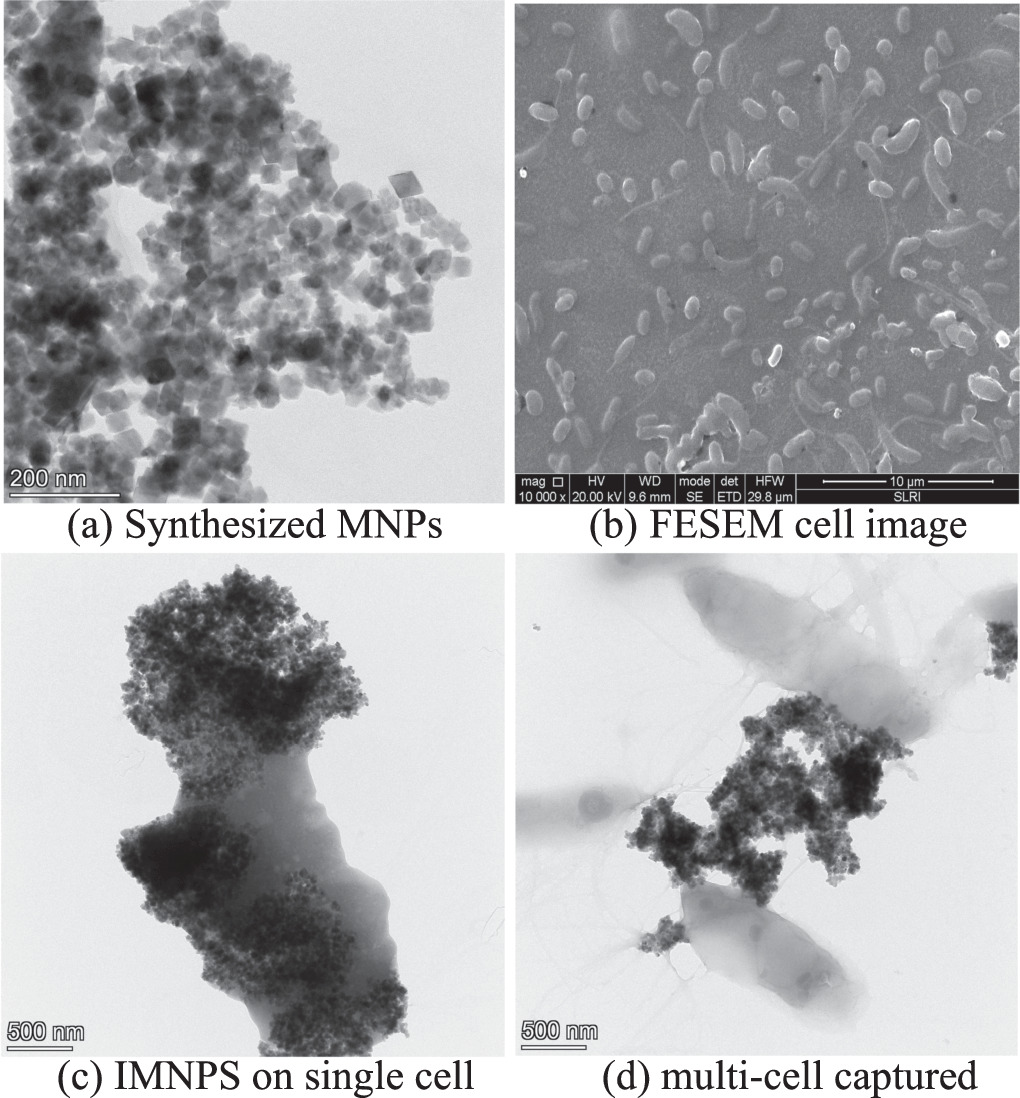
The cubic shape and 30–45 nm size of synthesized magnetite magnetic nanoparticles were explored by FETEM (figure 2(a)) before surface modification to be IMNPs. Their XRD pattern could be found in Songvorawit et al [11] showing diffraction peaks of synthesized MNPs from the polyol protocol matching well with the XRD pattern of standard for face center cubic (FCC) magnetite [Fe3O4] according to the database (JCPD 088–0315, ICSD collection code: 084611). The IMNPs were capable of conjugating to the cell through antigen–antibody binding, forming noncovalent bonds, which involved van der Waals, hydrogen bonds, hydrophobic or ionic bonds [15]. To verify the attachment of IMNPs to the target bacteria cell, FE-SEM images displayed a uniform distribution of target bacteria and IMNPs in the mixed solution (figure 2(b)), and the close-up images of the IMNPs-Cell sample by FE-TEM (figures 2(c) and (d)).
Figure 2. Characterization of synthesized MNPs and IMNPs with Salmonella cells captured by FESEM and FETEM.
Download figure:
Standard image High-resolution imageThe closed-up image illustrated that IMNPs can aggregate together after attaching to any one of the bacterial cells due to their magnetic force as shown in figure 2(c). Therefore, the de-magnetization must be utilized with a sample after the step of cell-IMNPs attachment.
The FTIR spectra in figure 3 compared the spectra peaks of IMNPs with and without Salmonella cells conjugated to confirm functional binding. The spectra peaks of 1645 to 1652 cm−1 represent the N-H bending of the IMNPs binding site, which shifted after the bacteria cells were conjugated with IMNPs. Additionally, the spectra peak at 1723 cm−1 (C=O stretching) was observed for the protein on the gram-negative cell surface at [16–18].
Figure 3. FTIR spectra of IMNPs and bacteria captured.
Download figure:
Standard image High-resolution image3.2. Magnetic property of MNPs, IMNPs, and bacterium captured by VSM results
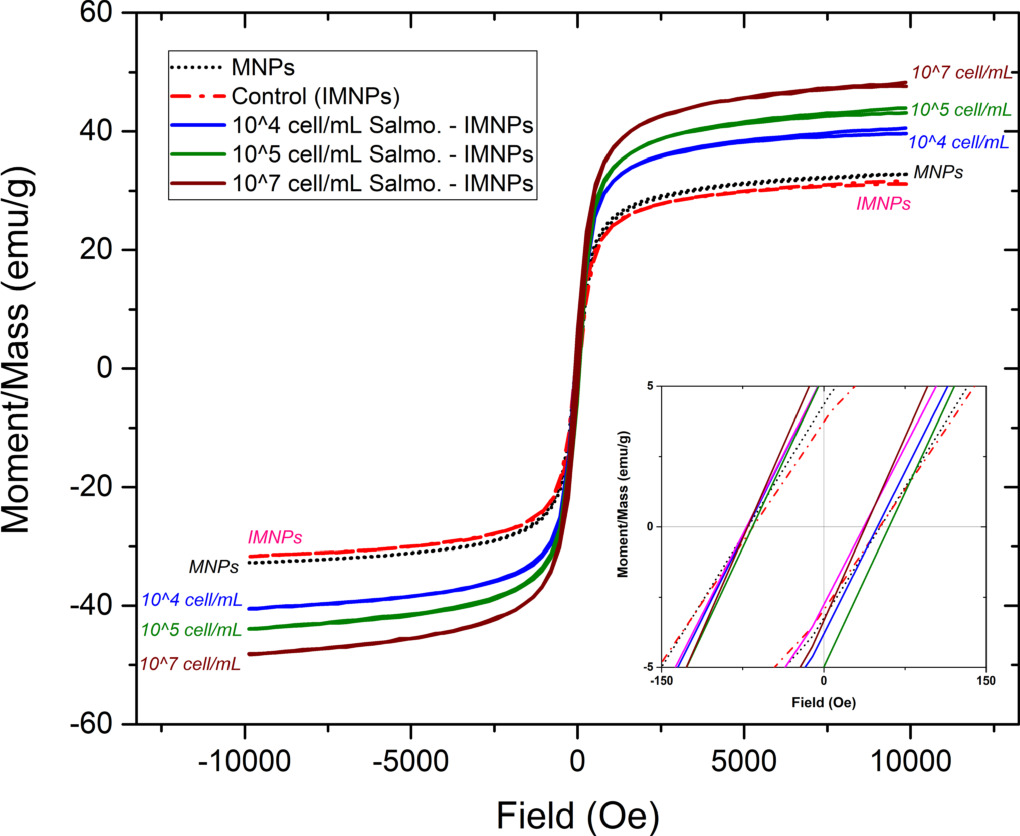
The magnetic behaviour of the samples, including MNPs, IMNPs, and Salmonella-IMNPs was investigated by plotting magnetization (M) versus magnetic field (H) loop. The M-H loops of IMNPs without call attachment (named as 'control sample') and IMNPs with 104, 105, and 107 cell ml−1 of S. Typhimurium cell concentration solution attached, showed a well-defined hysteresis nature of ferro-magnetics in figure 4, which allowed the magnetic nature of each sample to be described by the numerical value of the magnetic parameters in terms of saturation magnetization (Ms), retentivity (Mr), coercivity (Hci) and magnetic susceptibility (χm) as presented in table 1.
Figure 4. M-H curve of Cell-IMNPs: Control('zero'), 104, 105, and 107cell ml−1.
Download figure:
Standard image High-resolution imageTable 1. Magnetic behavioural parameters of magnetic nano-particles.
| Magnetic parameter | Synthesized amino-MNPs | Control (IMNPs without cell-attached) | 104 cell/ml of Salmonella | 105 cell/ml of Salmonella | 107 cell/ml of Salmonella |
|---|---|---|---|---|---|
| Ms (emu/g) | 32.794 | 31.708 | 40.529 | 43.924 | 44.801 |
| Mr (emu/g) | 4.590 | 7.668 | 10.574 | 11.818 | 10.242 |
| Hci (Oe) | 61.062 | 59.927 | 61.027 | 61.607 | 61.467 |
| χm (emu/g/Oe) | 3.33E-4 | 3.29E-4 | 4.49E-4 | 4.61E-4 | 5.36E-4 |
After MNPs' surface was modified by antibody conjugation to become IMNPs ('control'), all magnetism parameters did not show significant differences (P >.05). Therefore, the study focused on magnetism changes of IMNPs and Cell-IMNPs samples. The relative plots of magnetism parameters and a sample of 'control' including varied cell concentrations with IMNPs attached were displayed in figure 5. The increase in saturation magnetization (Ms) and magnetic susceptibility (χm) had a relationship with Salmonella cell concentrations, as shown in figures 5(a) and (d), respectively. The increase of Ms and χm value indicated that the material was easier to align its magnetic dipole to the applied external magnetic field (H).
Figure 5. The average value of M-H curve parameters of IMNPs related to varied concentrations of attached Salmonella cells.
Download figure:
Standard image High-resolution imageThis could be attributed to a change in electron configuration, electronic band, or local structure after IMNPs conjugation with the bacterial cell [7]. Consequently, to understand these phenomena of magnetic properties that increased after bacteria cell-IMNPs attachment, the Fe-O structure, Fe K-edge energy, and an electron transition were analyzed by the XAS analysis in the next section.
3.3. Characterization and analysis of MNPs, IMNPs, and IMNPs-bacterium captured by Synchrotron x-ray absorption spectroscopy
XAS analysis of the MNPs, IMNPs, and IMNPs-Cell attachment aimed to investigate the local structure and electronic band changes in each sample preparation step. The hypothesis was that the local structure or electronic band of the sample could be altered when the sample surface was modified and attached to objects on the cell surface.
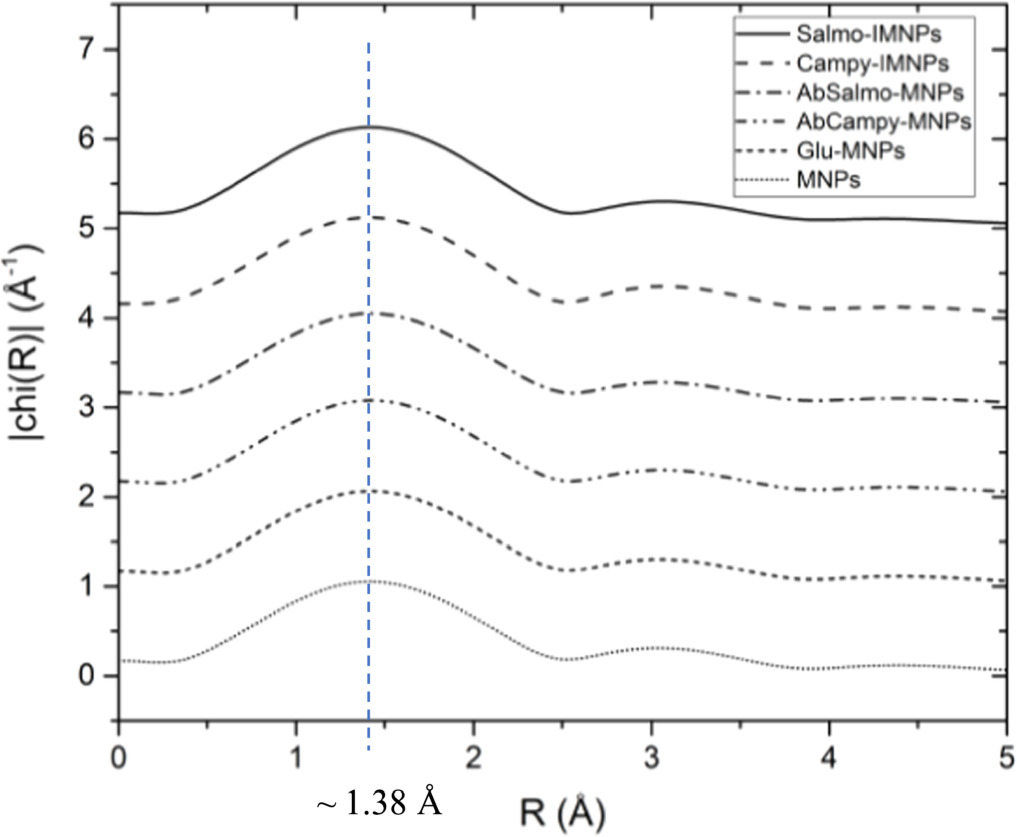
For analysis of local structure, the MNPs with a modified surface by antibody (to become IMNPs) and bacteria cell conjugation (either S. Thypimurium or C. jejuni), showed the local structure of MNPs Fe3O4 face-centered-cubic (FCC) [19] could be around 1.38 Å which comparable to Huie et al [20]. The R-space of the Fe-O peak for all sample steps was not significantly different. This showed that the MNPs Fe3O4 was not transformed to Fe2O3 as shown in figure 6.
Figure 6. Local structure analysis by considering the R-space value of both bacteria-conjugated protocols.
Download figure:
Standard image High-resolution imageResults of the investigation of Fe K-edge XANES spectrum of MNPs, IMNPs (pAb-Salmonella), and Salmonella-IMNPs attachment samples showed no difference in the absorption edge value (E0) of 7130 eV for all samples (figure 7(a)). Similarly, the spectral analysis of MNPs with a specific antibody (mAb-Campylobacter) conjugated and IMNPs with Campylobacter cells attached revealed no significant differences (figure 7(b)). The hypothesis that the oxidation state of MNPs would change when attached to bacterial cells was approved and corresponded well to the changing of magnetic properties from VSM results in the previous section.
Figure 7. Absorption spectra of each modified step and IMNPs—bacterial cell attached.
Download figure:
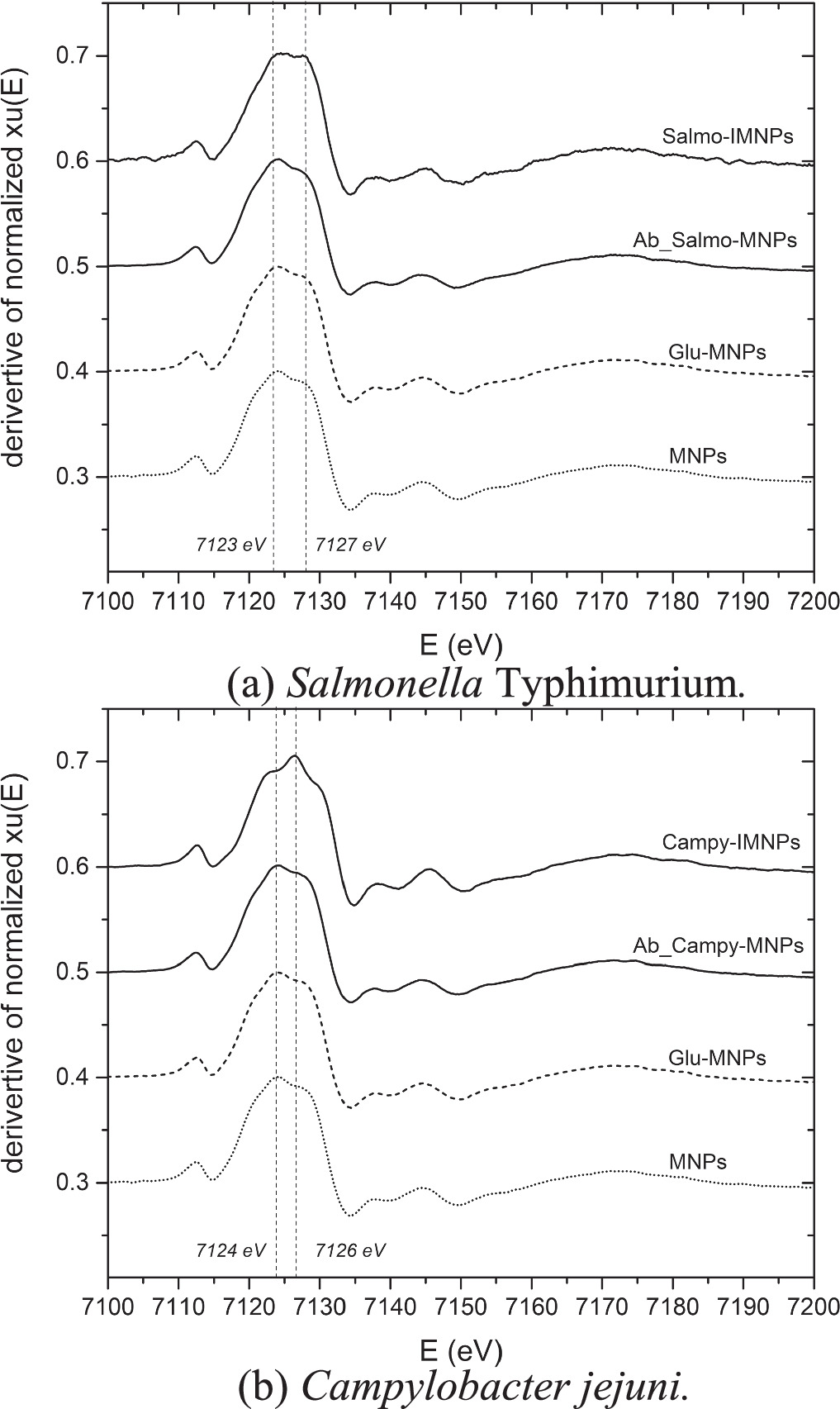
Standard image High-resolution imageTherefore, the absorption spectra were derivatised with a focus on the edge position of the Fe oxidation state were shown in figure 8. Both of  derivatized spectra showed that the oxidation state of MNPs, glutaraldehyde-MNPs, and IMNPs (of both antibodies conjugated) showed the same spectrum shape and oxidation state which identity at about 7123–7124 eV from a maximum of the derivate
derivatized spectra showed that the oxidation state of MNPs, glutaraldehyde-MNPs, and IMNPs (of both antibodies conjugated) showed the same spectrum shape and oxidation state which identity at about 7123–7124 eV from a maximum of the derivate  Thus, it was clearly approved that the oxidation state of MNPs after surface modification was not changed from Fe3O4 [Fe(II, III)].
Thus, it was clearly approved that the oxidation state of MNPs after surface modification was not changed from Fe3O4 [Fe(II, III)].
Figure 8. Derivative spectra of IMNPs with bacterial cell attached.
Download figure:
Standard image High-resolution imageOn the other hand, the sample of Cell-IMNPs, showed the shift of oxidation peak from 7123 eV to 7127 eV of Salmonella-IMNPs, and 7124 eV to 7126 eV of Campylobacter-IMNPs as shown in figures 8(a) and (b), respectively. These phenomena were due to cell-IMNPs attachment to bacterial cells. The electron transfer between IMNPs and the cell surface was processed. To indicate the shifted peaks and explain the electron transition, the Fe2O3 [Fe(III)] and Fe3O4 [Fe(II, III)] standard peaks from standard foil experiments were added for referring to the oxidation state of ferrous oxide [Fe(II)] and ferric oxide [Fe(III)] [14] (figure 9(a)). Consequently, the Campylobacter-IMNPs spectrum was derived to compare the oxidation peak shift with the Salmonella-IMNPs derived spectrum (figure 9(b)).
Figure 9. Illustration of XAS characteristic comparison of IMNPs with Salmonella and Campylobacter captured, and standardized spectrum Fe2O3 [Fe(III)] and Fe3O4 [Fe(II, III)].
Download figure:
Standard image High-resolution imageIn figure 9(b), the standard peaks of Fe3O4 [Fe(II), Fe(III)] showed the edge energy around 1723–1724 eV representing the combination of Fe(II) and Fe(III) while the peaks of FeO[Fe(II)] showed the edge energy around 7126–1727 eV representing the majority of Fe2O3[Fe(III)].
MNPs and IMNPs samples showed higher peaks of Fe(II) than Fe(III). This represented the characteristic of Fe3O4 as the combination between Fe(II) and Fe(III). As the conjugation to bacteria occurred, the electrons could be transferred out of the samples resulting in a lesser amount of Fe(II). This was due to the Fe(II) being less stable whereas in the Fe(III) state, the outermost shell exhibits a half-filled configuration of d-orbitals, leading to enhanced stability compared to alternative forms of Iron, thus, the oxidation transition from Fe(II) to be Fe(III) had more possibility. To explain the oxidation transition, the comparison of the derivative value of Fe(II) and Fe(III) as a ratio of Fe(III)/Fe(II) was used as the comparable methods used in other literature [21–23]. The Fe(III)/Fe(II) ratio of the prepared MNPs and IMNPs (figure 9(b)) were the same as Fe(II, III) standard peaks were about 0.075/0.1 = 0.75. The phenomena of electron donation to the cell wall and the electronic transition of Fe(II) to Fe(III) were sketched in figure 10 for more understanding.
Figure 10. Phenomena of electron donation to cell wall where Fe3+ ion increasing.
Download figure:
Standard image High-resolution imageThe focus was on the absorption peak shift of Salmonella-IMNPs which could explain the oxidation transition that absorption Fe(II) oxidation was transited to Fe(III) oxidation after IMNPs attached to Salmonella cell. Notice, the relative ratio of absorption intensity of Fe(III): Fe(II) was about 1. On the other hand, the peak shift of Campylobacter-IMNPs, the Fe(III) to Fe(II) transition was higher with a ratio of about 0.110/0.08 = 1.375. The Fe(III)/Fe(II) ratio related to the magnetic properties of MNPs' modified surface which included the saturated magnetization (Ms) and magnetic susceptibility (χm) whereas the higher Fe(III)/Fe(II) ratio indicated higher value of saturated magnetization (Ms) and magnetic susceptibility (χm) [24].
However, the difference in Fe(III)/Fe(II) ratio between Salmonella cells and Campylobacter cells with IMNPs attached, was shown in figure 9(b). This could be caused by the larger size of Campylobacter cells compared to the Salmonella cells [25, 26]. The larger cell surface could be attached by the greater amount of IMNPs, resulting in a higher electron transition. Additionally, the quantity of Campylobacter cell samples for the XAS experiment was 108 cells/ml while the Salmonella sample was 107 cells/ml.
It was important to note that the transfer of free electrons from a magnetic nanoparticle to a bacterial cell surface, and the oxidation transition depended on several factors, including the properties of the nanoparticle [27], the nature of the bacterial cell, and type of gram-stain [28–30], and the surrounding environment [23] were evident. The precise mechanisms and pathways involved in the electron transfer process may vary in different systems and require further investigation for a comprehensive understanding.
4. Conclusions
Nano-sized iron oxide particles, upon synthesized into magnetite (Fe3O4) and subjected to surface modification, exhibited a specific affinity for bacteria cells through antibody conjugation. In this research, it was found that the magnetic properties of the iron oxide did not undergo significant changes. However, when these modified iron oxide nanoparticles were specifically targeted to bacteria cells, their saturation magnetization, remanent, and susceptibility showed a significant change. Moreover, the concentration of bacteria cells used in preparing cell-IMNPs samples (fixed amount of IMNPs for all samples) also influenced these magnetic properties. This indicated the probable development of a new rapid target bacteria detection technique using the changes in magnetic properties as the research goal.
The reason for the changes in magnetic properties could be explained by an oxidation transition phenomenon from Fe2+ of Fe(II) to Fe3+ of Fe(III) in the magnetite (Fe3O4) nanoparticles. It was hypothesized that the free electrons at the outermost layer of Fe(II) transferred to the bacteria cell when IMNPs were specifically attached to the bacteria cell surface. X-ray Absorption Spectroscopy (XAS) results confirmed this hypothesis, showing an increase in the Fe(III)/Fe(II) ratio in Fe3O4 MNPs, which was attributed to the increase of Fe3+ in the Fe3O4 structure.
Moreover, in this research, two types of gram-negative species, Salmonella and Campylobacter, were used as examples to confirm the analysis results and explain the changes in the oxidation state of the iron oxide nanoparticles when interacting with bacteria cells. Nevertheless, the research did not conduct a detailed investigation to identify or differentiate specific types of bacteria strains. This aspect will be explored in future studies including a study and application of gram-positive species.
Acknowledgments
The authors would like to thank the Nation Research Council of Thailand (NRCT) for research funding, S&A Reagents Lab Ltd for supporting the antiserum and antigen, all staff of BL 2.2 W:TR-XAS and BL 4.1:IR at Synchrotron Light Research Institute for best advises and supports, and those of Food Safety Centre of KMUTT for kindly supports.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Funding statement
This thesis has received research support from the National Research Council of Thailand (NRCT) for the Graduate Education Development Fund, for the fiscal year 2020 [12462].