Abstract
It is presented a 'shocking thermal treatment' methodology as a modification to the molten salts process to rapidly obtain pure phase nickel ferrite nanoparticles within a high-scalable green synthesis scheme. Analysis from x-ray diffraction and electron microscopy (SEM and TEM) confirms the formation of the nanostructures, with the most frequent crystallite sizes between 55–85 nm. Room temperature magnetic characterization, from Mössbauer spectroscopy and magnetic measurements M(H), evidences their superparamagnetic distribution with a magnetic saturation of 47.9 emu g−1. An approximated stoichiometry of  was proposed from Rietveld refinement, Mössbauer and energy-dispersive x-ray spectroscopies. The effect on the electronic structure due to the shocking thermal treatment was elucidated by estimating the optical band gap with values between 1.78 and 2.38 eV.
was proposed from Rietveld refinement, Mössbauer and energy-dispersive x-ray spectroscopies. The effect on the electronic structure due to the shocking thermal treatment was elucidated by estimating the optical band gap with values between 1.78 and 2.38 eV.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nickel ferrite nanoparticles (NiFe2O4) are under continuous investigation due to their potentiality in recording and information storage devices, magneto-optic effects, catalysis, gas sensor and anode materials for lithium batteries [1–5] or as a magnetic recoverable catalyst with plausible enhanced ferroelectric behavior if NiFe2O4 is a component of a nanocomposite with multiferroic materials like TbFeO3 or BiFeO3 [6, 7].
According to previous reports in the literature, nickel ferrite nanoparticles are typically synthesized by bottom—up chemistry approaches like co-precipitation, the sol-gel route (conventional or modified), assisted reverse micellar synthesis and combustion synthesis (comprising modified combustion methodologies) at which the main procedure involves the employment of transition metal salts as chemical reagents (Ni(NO3)2 6H2O, Fe(NO3)3 9H2O, FeCl3 6H2O or NiCl2 6H2O) dissolved in polar liquids like water or ethylene glycol. In most cases, the pH was controlled by adding specific concentrations of NaOH, citric acid or ammonia solutions. Generally, the thermal treatments used for nanoparticle production go from 500 °C to 1000 °C with time intervals from 2 h to 8 h, excluding microwave methodologies, which are short time procedures of around 20 min [8–14]. Nevertheless, this kind of chemical route is industrially low-scalable with high-cost processes in which waste-water reagents remain after nanoparticle production.
However, using nickel ferrite for technological purposes requires low-cost and highly scalable production methods with a reduction of waste reagents. The molten salts synthesis is a green chemistry approach [15], which has been successfully tested in the formation of complex oxides [16–21] comprising the combination of simple oxide reagents in the desired stoichiometry into an adequate proportion of salts that entails a time-saving and highly scalable synthesis process, with non-toxic reagents involved since the by-product after the reaction process is saline water.
The molten salts synthesis uses water-soluble salts as the reaction medium, promoting the rapid diffusion of the reagent materials (typically oxides and carbonates) to enhance the mobility of the reactants and act as the solvent. The standard procedure for the molten salt method consists of a mixture of reagents and salts heated above the melting temperature of the salts; then, the reaction takes place and the product is formed. The obtained powder is washed with distilled water to remove the salt from the sample. The characteristics of the sample product, like morphology and particle size, depend on the selected heating temperature, the time thermal treatment lasts, the molar ratio between reagents and salts (due to the concentration of the diffusion medium) and the molar proportion between salts because the melting temperature of the salt mixture is dependent on the concentration [16]. However, once the synthesis conditions are well identified, a scaling process could be applied if thermal conditions and the ratio between salts and precursors are fixed [22, 23]
There are some reports on the synthesis process of nickel ferrite by molten salts, for example, Senthilkumar and co-workers [21] reported the synthesis of nickel ferrite by the molten salt method at 900 °C with 3 h of thermal treatment with different molar ratios between NaCl—KCl (4:0, 3:1, 1:1 and 0:4) and NiO2 / Fe2O3 as chemical reagents but with no control in the proportion between salts and reactants. Under these conditions, Senthilkumar achieves the formation of NiFe2O4 particles in the bulk regimen with sizes between 400 nm and 1000 nm.
Huang et al [19], also synthesized NiFe2O4 by the molten salts route at 800 °C during 4 h of thermal treatment. Huang employed Ni(NO3)2 / Fe(NO3)3 as chemical reagents and NaCl-KCl in a 3:1 molar proportion ratio, again with no control in the proportion between reagents and salts; under these conditions, the size of the NiFe2O4 particles was around 200 nm.
Jianxiang Liu et al [20], in their work entitled -structure, morphology, and magnetic properties of NiFe2O4 powder prepared by the molten salt method- report the use of NiCO3 2Ni(OH)2 4H2O and Fe2O3 as chemical reagents. At this time, the Fe2O3 precursor was ball milled for 10 h as a previous treatment. The reaction occurs at 1000 °C for 2 h in a mixture of salts NaCl-KCl in eutectic molar proportions between salts and a 6:1 molar ratio between the mixture of salts and the chemical reagents. The size distribution for the NiFe2O4 particles was between 400 nm—1000 nm.
The present work describes the synthesis conditions necessary to obtain the formation of nickel ferrite nanoparticles in 8 min with crystallite sizes between 55–85 nm. The process is highly scalable and a considerable time saver, in accordance with the green chemistry framework.
2. Materials and methods
NiO (Sigma-Aldrich >99%) and Fe2O3 (Sigma-Aldrich 99.99%) were mixed into stoichiometric proportion with an equimolar mixture of salts NaCl-KCl (Sigma-Aldrich 99.9%) with a total molar ratio of 4:1 between the salts and oxide powder reactants. The salts and the precursors were ground in an agate mortar until a fine homogeneous powder was attained, which was placed in an alumina crucible heated in a conventional electric furnace at 1000 °C for 8 min, and then quickly removed from the oven to be cooled in air, in something similar to a 'shocking thermal treatment'.
The product obtained was washed and stirred in deionized water to dissolve the salts, filtered with a 0.22 μm pore nitrocellulose filter and finally dried in air.
The x-ray diffraction pattern (XRD) was measured using a Siemens D5000 diffractometer with Cu-Kα radiation (λ = 0.154 nm) from 15° to 90° with steps of 0.02° in 2 Rietveld refinement was done with the MAUD software [24]. Transmission electron microscopy (TEM) images were acquired with a JEOL TEM-2010 FEG with 200 keV of accelerating voltage and a point resolution of 0.19 nm. Interplanar distance measurements were performed in high resolution transmission electron configuration (HRTEM) using the Fast Fourier Transform (FFT) within Digital Micrograph software from GATAN. SEM images were acquired with an ultra-high-resolution electron microscope (JSM-7800F) at 5 kV acceleration voltage with a 30,000 magnification. The room temperature transmission Mössbauer spectrum was acquired in constant acceleration configuration, using a 57Co source in a rhodium matrix and was fitted with the Recoil 1.05 software [25]. Magnetic measurements were performed in a superconducting quantum interference device (SQUID—MPMS 3, Quantum Design). Optical absorption spectra were obtained with a UV–vis spectrophotometer Thermo-Fisher GENESYSTM 150 in a wavelength range of 190–700 nm. The diffuse reflectance spectroscopy (DRS) was carried out in a UV–vis-NIR spectrophotometer Agilent Technologies Cary 5000 in a 200–800 nm wavelength range.
Rietveld refinement was done with the MAUD software [24]. Transmission electron microscopy (TEM) images were acquired with a JEOL TEM-2010 FEG with 200 keV of accelerating voltage and a point resolution of 0.19 nm. Interplanar distance measurements were performed in high resolution transmission electron configuration (HRTEM) using the Fast Fourier Transform (FFT) within Digital Micrograph software from GATAN. SEM images were acquired with an ultra-high-resolution electron microscope (JSM-7800F) at 5 kV acceleration voltage with a 30,000 magnification. The room temperature transmission Mössbauer spectrum was acquired in constant acceleration configuration, using a 57Co source in a rhodium matrix and was fitted with the Recoil 1.05 software [25]. Magnetic measurements were performed in a superconducting quantum interference device (SQUID—MPMS 3, Quantum Design). Optical absorption spectra were obtained with a UV–vis spectrophotometer Thermo-Fisher GENESYSTM 150 in a wavelength range of 190–700 nm. The diffuse reflectance spectroscopy (DRS) was carried out in a UV–vis-NIR spectrophotometer Agilent Technologies Cary 5000 in a 200–800 nm wavelength range.
3. Results and discussion
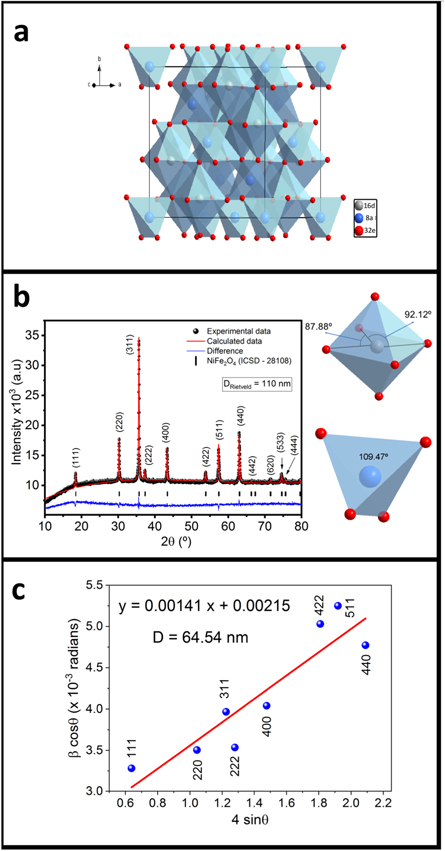
NiFe2O4 is a face centered cubic crystal structure ( space group) with eight formula units and three non-equivalent Wyckoff atomic positions; tetrahedral A site [8a(0, 0, 0)]; octahedral B site [16d(5/8, 5/8, 5/8)] and oxygen 32e(x, x, x), figure 1(a). The general formula is of an inverse spinel of the form
space group) with eight formula units and three non-equivalent Wyckoff atomic positions; tetrahedral A site [8a(0, 0, 0)]; octahedral B site [16d(5/8, 5/8, 5/8)] and oxygen 32e(x, x, x), figure 1(a). The general formula is of an inverse spinel of the form ![${\left[{{Ni}}_{1-\zeta }^{2+}{{Fe}}_{\zeta }^{3+}\right]}_{A}{\left[{{Ni}}_{\zeta }^{2+}{{Fe}}_{2-\zeta }^{3+}\right]}_{B}{O}_{4}^{-2},$](https://content.cld.iop.org/journals/2053-1591/10/7/076102/revision2/mrxace593ieqn4.gif) where
where  is known as the degree of inversion parameter and it is related with the amount of divalent ions occupying the octahedral and tetrahedral sites; and thus, it is assigned as fully inverse spinel ferrite for
is known as the degree of inversion parameter and it is related with the amount of divalent ions occupying the octahedral and tetrahedral sites; and thus, it is assigned as fully inverse spinel ferrite for  while a normal spinel corresponds with
while a normal spinel corresponds with  [26]. Figure 1(b) shows the x-ray diffraction associated reflections of NiFe2O4 phase with
[26]. Figure 1(b) shows the x-ray diffraction associated reflections of NiFe2O4 phase with  space group without any secondary phase. The Rietveld refinement suggests a small amount of Ni atoms occupying the tetrahedral A site, while the occupancy in octahedral sites is 49% and 51% for Ni and Fe atoms, respectively. The octahedral coordination has a slight distortion with two different angles 87.88° and 92.12° as consequence of the ionic radio difference between Fe (64 pm) and Ni (78 pm), suggesting a potential ferroelectric behavior, as it was reported in [27]. The Rietveld analysis suggest a stoichiometry for the nickel ferrite of the form
space group without any secondary phase. The Rietveld refinement suggests a small amount of Ni atoms occupying the tetrahedral A site, while the occupancy in octahedral sites is 49% and 51% for Ni and Fe atoms, respectively. The octahedral coordination has a slight distortion with two different angles 87.88° and 92.12° as consequence of the ionic radio difference between Fe (64 pm) and Ni (78 pm), suggesting a potential ferroelectric behavior, as it was reported in [27]. The Rietveld analysis suggest a stoichiometry for the nickel ferrite of the form  with a lattice parameter a = 8.3438 Å and an estimated density by x-ray diffraction of
with a lattice parameter a = 8.3438 Å and an estimated density by x-ray diffraction of  (table 1); where M is the molar mass and N = 6.023 × 1023 is the Avogadro´s number.
(table 1); where M is the molar mass and N = 6.023 × 1023 is the Avogadro´s number.
Figure 1. (a)–(b) Crystal structure and Rietveld refinement of nickel ferrite. (c) Williamson-Hall plot.
Download figure:
Standard image High-resolution imageTable 1. Refined parameters of the nickel ferrite.
Latice parameters: space group 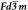
| ||||
| a (Å) | 8.3438(2) | |||
| Volume (Å3) | 580.83(5) | |||
Density (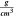 ) ) | 5.359 | |||
| Atomic parameters (Å) | ||||
| Atom | x | Y | z | Occupancy |
| Ni (16d) | 0.625 | 0.625 | 0.625 | 0.488 |
| Fe (16d) | 0.625 | 0.625 | 0.625 | 0.513 |
| Ni (8a) | 0.000 | 0.000 | 0.000 | 0.025 |
| Fe (8a) | 0.000 | 0.000 | 0.000 | 0.975 |
| O (32e) | 0.3795(2) | 0.3795(2) | 0.3795(2) | 1.000 |
| Angles in polyhedral around transition metal | ||||
| O-Ni/Fe(16d)-O | 87.88° | 92.12° | ||
| O-Ni/Fe(8a)-O | 109.47° | |||
| Interatomic distances (Å) | ||||
| Ni/Fe (16d) - O | 2.0491 | |||
| Ni/Fe (8a) - O | 1.8715 | |||
| Refinement parameters χ2 = 1.33 | ||||
Considering crystallite size  and microstrain
and microstrain  as the only two contributions responsible of the broadening in the full width half maximum
as the only two contributions responsible of the broadening in the full width half maximum  it is plausible to estimate an average linear dimension
it is plausible to estimate an average linear dimension  value through a linear least square fitting using the relation
value through a linear least square fitting using the relation  [28]; were
[28]; were  is the Bragg angle, K = 0.9 is a constant,
is the Bragg angle, K = 0.9 is a constant,  is the wavelength of the x-ray radiation and where the slope of the linear fitting would be related to the micro-strain (
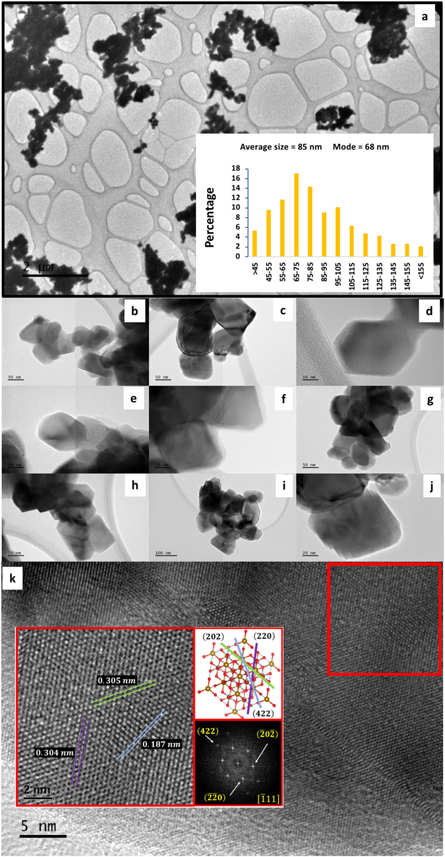
is the wavelength of the x-ray radiation and where the slope of the linear fitting would be related to the micro-strain ( ) while the crystallite D it would be associated to the interception. Measuring the adjusted interception, a value of D = 64.54 nm (figure 1(c) is obtained, while the Rietveld refinement estimates an average size of DRietveld = 110 nm; the employment of both approximations leads into a mean value of 87 nm for the crystallite size. The size distribution revealed by TEM/SEM, inset figure 2(a), shows that particles below 105 nm are 75% of the total contribution while sizes greater than 105 nm are 25%. Therefore, the estimated calculation performed by XRD is in good concordance with electron microscopy measurements. Since the reaction takes place in a very short time, it is conceivable that the dissolution rates between Fe2O3 and NiO are comparable and, as a consequence, the fast formation of a saturated solution occurs inside the molten salts. Because both reagents interact in the liquid medium, a precipitation process is followed during the particle-growth stage [16] promoting the rapid formation of the NiFe2O4 nanoparticles in the high stable [111] direction with faceted morphologies, figure 2(b) to (k) and figure 3(a). Figure 2(k) shows the HRTEM micrography and the FFT analysis with identified planes
) while the crystallite D it would be associated to the interception. Measuring the adjusted interception, a value of D = 64.54 nm (figure 1(c) is obtained, while the Rietveld refinement estimates an average size of DRietveld = 110 nm; the employment of both approximations leads into a mean value of 87 nm for the crystallite size. The size distribution revealed by TEM/SEM, inset figure 2(a), shows that particles below 105 nm are 75% of the total contribution while sizes greater than 105 nm are 25%. Therefore, the estimated calculation performed by XRD is in good concordance with electron microscopy measurements. Since the reaction takes place in a very short time, it is conceivable that the dissolution rates between Fe2O3 and NiO are comparable and, as a consequence, the fast formation of a saturated solution occurs inside the molten salts. Because both reagents interact in the liquid medium, a precipitation process is followed during the particle-growth stage [16] promoting the rapid formation of the NiFe2O4 nanoparticles in the high stable [111] direction with faceted morphologies, figure 2(b) to (k) and figure 3(a). Figure 2(k) shows the HRTEM micrography and the FFT analysis with identified planes 
 and
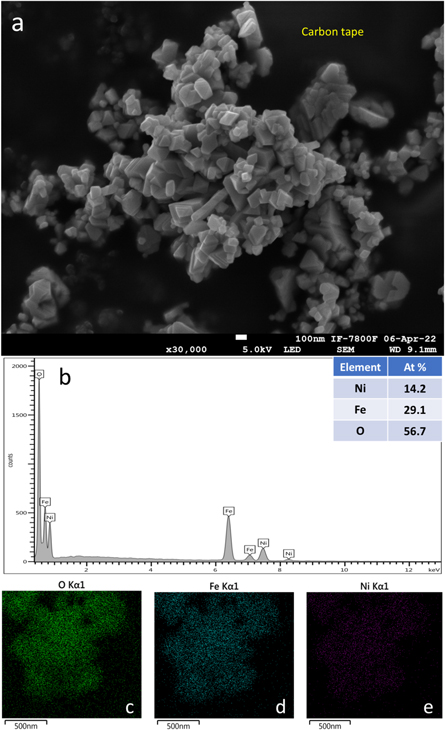
and  in agreement with Bragg reflections and the interplanar distances in NiFe2O4 phase. The energy dispersive x-ray spectroscopy (EDS) together with the surface element identification, figure 3(b)–(e), corroborates the nickel ferrite formation with an approximated 2:1 iron to nickel ratio.
in agreement with Bragg reflections and the interplanar distances in NiFe2O4 phase. The energy dispersive x-ray spectroscopy (EDS) together with the surface element identification, figure 3(b)–(e), corroborates the nickel ferrite formation with an approximated 2:1 iron to nickel ratio.
Figure 2. (a) to 2(j) TEM micrographs and sized particle distribution. (k) Displays the HRTEM and FFT analysis.
Download figure:
Standard image High-resolution imageFigure 3. (a) SEM image of the nickel ferrite sample. (b)–(e) Show the EDS and surface element identification.
Download figure:
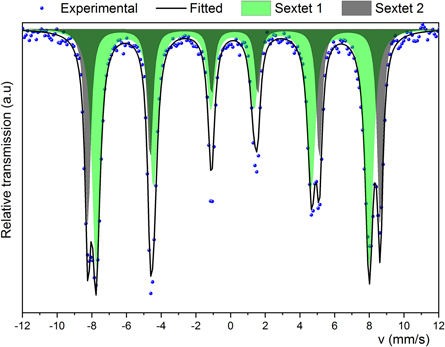
Standard image High-resolution imageThe Mössbauer spectrum, figure 4, shows two sextet contributions related to two different iron sites inside the crystal structure. Sextet 1 was fitted with an isomer shift ( ) between 0.23–0.24 mm s−1, and a hyperfine magnetic field (
) between 0.23–0.24 mm s−1, and a hyperfine magnetic field ( ) around 49 T; indicative of iron atoms in tetrahedral coordination. Sextet 2 was fitted with a
) around 49 T; indicative of iron atoms in tetrahedral coordination. Sextet 2 was fitted with a  value between 0.32–0.34 mm s−1 with
value between 0.32–0.34 mm s−1 with  around 52 T, associated with iron atoms in octahedral coordination [26, 29].
around 52 T, associated with iron atoms in octahedral coordination [26, 29].
Figure 4. Mössbauer spectrum of the nickel ferrite.
Download figure:
Standard image High-resolution imageUsually, Ni2+ cation migrates from the B site to the A site as a high-temperature effect followed by a rapid cooling, modifying the amount of divalent Ni cations in tetrahedral and octahedral sites, this is commonly known as the degree of inversion, denoted with the  symbol, making reference to the
symbol, making reference to the ![${\left[{{Ni}}_{1-\zeta }^{2+}{{Fe}}_{\zeta }^{3+}\right]}_{A}{\left[{{Ni}}_{\zeta }^{2+}{{Fe}}_{2-\zeta }^{3+}\right]}_{B}{O}_{4}^{-2}$](https://content.cld.iop.org/journals/2053-1591/10/7/076102/revision2/mrxace593ieqn29.gif) expression [30]. Estimation of
expression [30]. Estimation of  in the spinel like ferrites is commonly attained through the relation [29]
in the spinel like ferrites is commonly attained through the relation [29]  where
where  is the area ratio population of the Fe3+ cations in the octahedral (16d) and tetrahedral (8a) sites and
is the area ratio population of the Fe3+ cations in the octahedral (16d) and tetrahedral (8a) sites and  is the recoilless Mössbauer f-factors at room temperature; usually it is equal to
is the recoilless Mössbauer f-factors at room temperature; usually it is equal to 
However, this value is calculated for magnetite [29]. Salazar-Tamayo et al recently calculated a specific  for nickel ferrite [26]. The
for nickel ferrite [26]. The  value of the synthesized nickel ferrite is computed considering the
value of the synthesized nickel ferrite is computed considering the  value and
value and  and thus, it was estimated the stoichiometry and the magnetic moment per formula unit
and thus, it was estimated the stoichiometry and the magnetic moment per formula unit  see table 2. The stoichiometry determined by Mössbauer spectroscopy is in good correlation with that computed by XRD.
see table 2. The stoichiometry determined by Mössbauer spectroscopy is in good correlation with that computed by XRD.
Table 2. Mössbauer parameters of the nickel ferrite compound. The isomer shift is relative to  -iron.
-iron.
| Site |
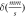
|
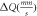
|
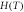
|
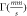
| % |
|---|---|---|---|---|---|
| Sextet 1 (8a) | 0.23(5) | 0.007(5) | 48.64(4) | 0.49(1) | 46(2) |
| Sextet 2 (16d) | 0.34(7) | −0.016(7) | 52.01(6) | 0.64(1) | 54(2) |
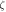
|
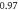
| ||||
| Stoichiometry |
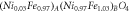
| ||||
| μ | 2.18 μB | ||||
The high value of the linewidth obtained for sextet 1 (tetrahedral 8a) and sextet 2 (octahedral 16d) after the fitting process could be understood in terms of a magnetic field distribution caused by the different particle size distribution, as evidenced by TEM and SEM (figures 2(a) and 3(a)). The plausible presence of another spinel magnetic phase overlapping the sextets linewidth contribution is ruled out, since EDS and XRD corroborate the purity of the NiFe2O4 phase.
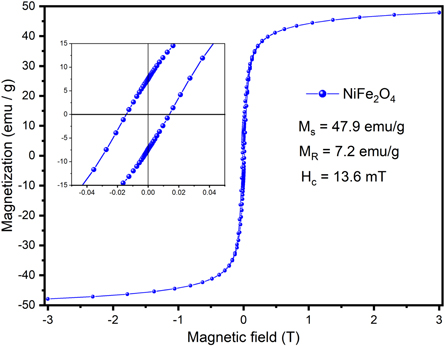
The behavior of the room temperature (RT) magnetization versus magnetic field M(H) of NiFe2O4 nanoparticles is shown in figure 5. The M(H) curve reveals a ferromagnetic contribution with a coercive field of  and a remanent magnetization of
and a remanent magnetization of  with a saturation magnetization
with a saturation magnetization  giving a reduced remanence magnetization
giving a reduced remanence magnetization  The low Hc field and MR values at small fields like 0.5 T, suggest that nickel ferrite nanoparticles are mainly single magnetic phase reaching saturation by a superparamagnetic contribution.
The low Hc field and MR values at small fields like 0.5 T, suggest that nickel ferrite nanoparticles are mainly single magnetic phase reaching saturation by a superparamagnetic contribution.
Figure 5. Magnetization (M) as a function of the magnetic field at room temperature.
Download figure:
Standard image High-resolution imageFigure 6 shows the UV–vis spectra measured from absorbance and DRS. Direct and indirect optical band gaps were obtained from Tauc and Kubelka-Munk approximations. The calculated direct band gaps measured were 2.01 eV and 2.37 eV while the indirect band gaps were 1.78 eV and 2.13 eV, which are energy gap values in the range of NiFe2O4 nanoparticles with sizes between 50 nm to 75 nm [11, 12, 14]. The measured band gaps in the rapid synthesized nickel ferrite suggest its potential appliance as a catalyst in the UV region [11, 12, 31].
Figure 6. UV–vis spectra of estimation of the band gap.
Download figure:
Standard image High-resolution image4. Conclusion
Many of the processes used in physics and chemistry to produce materials for industrial applications involve energy consumption and may produce hazardous waste that contributes to global pollution and warming. The alternative fast and scalable methodology for the synthesis of nickel ferrite proposed in the present work represents an effort to diminish these effects; although the synthesis is made at 1000 °C, the time is only 8 min. The performed characterization is an earlier setup for the development of studies in the future, focused on plausible applications, considering that a major effort must be made to develop the modified molten salts shock thermal treatment methodology.
Acknowledgments
This research was partially supported by UNAM-DGAPA-PAPIIT program IN112422. J León-Flores acknowledges the postdoctoral-scholarship granted by CONACYT. The authors acknowledge the technical support provided by S Tehuacanero-Cuapa and R Hernández-Reyes in TEM/SEM and A Tejeda in XRD. The authors acknowledge to the Laboratorio Universitario de Caracterización Espectroscópica LUCE_ICAT_UNAM and J Ocotlán Flores-Flores and V Maturano for the UV–vis DRS acquisition spectra.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).