Abstract
Designing new anode materials with high performance is vital for the development of full-cell potassium-ion batteries (KIBs). Although boron-doped graphene (BDG) anodes have been widely studied for lithium- and sodium-ion batteries, there are few works considering BDG anodes with controllable doping concentration applied for KIBS. Herein, by first-principle calculations, we propose a novel BDG with controllable doping concentration as a promising anode material for KIBs. As a result, the chemisorption ability of the proposed BDG (BC20) for K is greatly enhanced in comparison with the pristine graphene, since the B dopants introduce electron-deficiency. Besides, the diffusion energy barrier of K on the surface of BC20 is as low as 0.19 eV, indicating high-rate performance. Noticeably, the maximum K storage capacity is 854 mAh g−1 with a low open circuit voltage (OCV) of 0.29 V. Moreover, the chemical window of OCV is in a low range without large variation, which is favorable for providing a large operating voltage. The results suggest that the presented BC20 is not only a promising anode candidate for KIBs; but also opens an avenue for designing novel BDG structures with controllable doping concentration applied to energy storage.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
With the growing demand of energy storage systems, the potassium-ion battery (KIB) has been emerging as one of the most promising alternatives [1] due to the low cost, safety, relatively high energy density, and high-rate insertion/extraction compared with lithium- and sodium-ion batteries (LIBs and SIBs) [2]. In comparison with Li resources (0.0017 weight % (wt%)), K is abundant in the Earth's crust (1.5 wt%) which benefits for developing next-generation non-Li-based storage systems with low cost [3, 4]. Although SIB has attracted much attention because of sodium's high abundance, its high standard reduction potential (−2.71 V versus standard hydrogen electrode (SHE)) results in the relatively low energy density greatly limiting the practical applications [5]. Given this issue, KIB has a close standard reduction potential (−2.93 V versus SHE) to Li (−3.04 V versus SHE) indicating its competitive performance in higher energy density compared with SIB [6]. Besides, K+ has the smallest Stokes' radius (3.6 Å) compared with Na+ (4.6 Å) and Li+ (4.8 Å) in propylene carbonate solvents [7], suggesting it possesses the highest ion conductivity and mobility [6, 8]. However, shedding light on the development of full cell KIBs, designing new anode materials with high performance and low cost for KIBs is a crucial challenge.
Recently, there have been various types of materials studied as the anode candidates for KIBs including the carbon-based materials [9, 10], MXenes [11, 12], metal oxides [13, 14], transition metal dichalcogenides [15, 16]. Among these materials, the carbon-based materials, including graphite [17], reduced graphene oxide [18], and amorphous carbon [19], attract extensive interest due to their low cost, non-toxic, and scalable synthetic process [20, 21]. Besides, the low-dimensional carbon-based materials play a critical role in addressing the challenges of poor cycling stability, huge volume change, and limited storage capacity due to their unique structural and electronic properties [5]. Particularly, graphene anode has been studied as a promising candidate for KIBs since it has the advantage of slight surface deformation, high electronic conductivity, and large surface area [22]. However, the adsorption of metal ions on pristine graphene still needs to be improved, which becomes a key factor in increasing the capacity of KIB. In order to enhance the electrochemical performance of graphene anodes for KIBs, several methods including introducing point defects [9], edge modification [23], and doping [24] have been applied. Share et al [25] reported that the N-doped graphene anodes effectively improve the adsorption ability and storage capacity of metal ions for KIBs. Therefore, doping has become a reliable and viable method to improve the electrochemical performance of graphene anodes for KIBs. There are many elements that can be used as dopants in graphene anodes, including N, O, S, and P [26]. By contrast, the B doping is more attractive because of the similar atomic size of B and C atoms and the electron-deficiency induced by B dopants, which is favorable for the charge transfer and adsorption of metal ions [27]. Although the boron-doped graphene (BDG) anode materials with large capacity and high stability have been widely studied for LIBs and SIBs both in experiments [28–30] and theories [31, 32], only a few works have considered the BDG with a controllable doping concentration applied for the KIBs anodes. Gong et al reported that the BDG anode with a 12.5% doping concentration (B4C28) had a capacity of 546 mAh g−1 based on first-principle calculations [33]. In addition to the random doping [33], the controllable design of a BDG anode with large capacity for KIBs is rarely investigated. However, the bottom-up synthesis of graphene-type nanostructures in organic-conjugated chemistry stimulates us to provide the possible solution [34].
In this work, by first-principle calculations, we propose a novel BDG (BC20) anode for KIB with highly controllable doping concentration suggested by the on-surface chemical reactions [35–39]. As an anode for KIB, its structural stability, electronic structures, adsorption and diffusion ability, theoretical capacities and open circuit voltages (OCVs) have been investigated. As a result, BC20 is thermodynamically stable at ambient condition and even high temperature. The theoretical capacity of BC20 is 3.13, 4.88, 1.38, and 1.56 times greater than graphite [40], carbon nanocage [41], carbon nanocage [42], and B4C28 [33], respectively. According to all the findings, BC20 is a promising candidate for serving as an anode for KIBs.
2. Computational methods
All the first-principle calculations were performed by the Vienna ab initio simulation package (VASP) based on the density-functional theory (DFT) [43]. The Perdew–Burke–Ernzerhof (PBE) functional of the generalized gradient approximation (GGA) is used to describe the exchange–correlation interaction [44]. Geometric structures were fully optimized until reaching the convergence criteria of 0.01 eV/ Å and 10–5 eV for force and energy, respectively, where a cutoff energy of 550 eV was employed for the plane wave expansion. A 3 × 3 × 1 k-point mesh via Γ-centered Monkhorst-Pack method [45] was used for geometry optimization, where a 7 × 7 × 1 k-point mesh was used for electronic structure calculations. A vacuum region of 20 Å was used to avoid the interaction between the periodic images in the z-direction. The van der Waals (vdW) interaction between K atoms and the BC20 monolayer was included by introducing the DFT-D3 method within Grimme scheme [46]. For the phonon spectrum calculation, the convergence criteria for force and energy are 0.001 eV/ Å and 10–8 eV respectively. The phonon spectrum was calculated using a 2 × 2 supercell with Phonopy code [47]. Ab initio molecular dynamics (AIMD) simulations with an NVT ensemble at 300 K, 650 K, and 1000 K was conducted to investigate the thermal stability of the BC20 monolayer structure. Each MD simulation lasted for 5 ps with a time step of 2 fs. The climbing image nudged-elastic-band method (CI-NEB) [48] was used to obtain the minimum energy pathway in the diffusion energy barrier calculations. All the images were fully optimized until reaching the convergence criteria of 10–4 eV and 0.015 eV/ Å for energy and force, respectively.
The formation energy of monolayer BDG was calculated based on the formula:
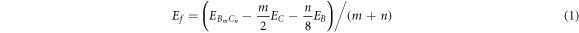
where m and n are the number of B and C atoms in the unit cell, respectively; 
 and
and  are the total energy of BDGs, graphene, and α-sheet of borophene [49], respectively.
are the total energy of BDGs, graphene, and α-sheet of borophene [49], respectively.
The adsorption energy of K atoms on BC20 monolayer was calculated by the formula below:

where the  is the total energy of the whole structure,
is the total energy of the whole structure,  is the energy of the BC20 supercell, n is the number of adatoms, and
is the energy of the BC20 supercell, n is the number of adatoms, and  represents the energy of a single K atom in the bulk.
represents the energy of a single K atom in the bulk.
The charge density difference was calculated based on the formula:
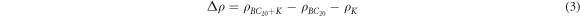
where the 
 and
and  are the charge densities of the whole adsorption system, pure BC20 monolayer and the single K atom, respectively. Furtherly, the Bader charge integration [50, 51] was used to quantitatively evaluate the charge transfer between K atoms and the BDG monolayer.
are the charge densities of the whole adsorption system, pure BC20 monolayer and the single K atom, respectively. Furtherly, the Bader charge integration [50, 51] was used to quantitatively evaluate the charge transfer between K atoms and the BDG monolayer.
Finally, the theoretical capacity [52] and OCV [53, 54] were calculated based on the following two equations respectively
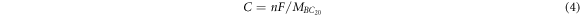
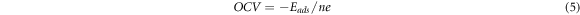
where C represents capacity, n is the number of electrons during intercalation that adsorption atoms could afford (n = 1 for K), F is the Faraday constant (F = 26.8 Ah mol)−1, and the  is the molecular mass of BC20.
is the molecular mass of BC20.
3. Results and discussion
3.1. Crystal structure and stability
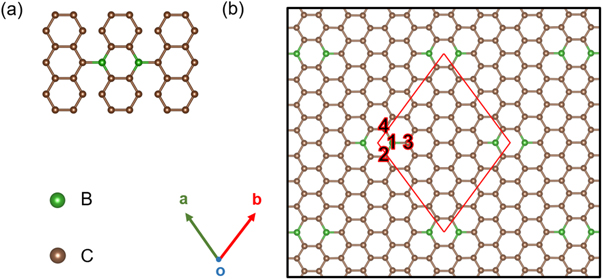
We first examine the crystal structure and stability of proposed BDG in detail. The possible realization of the highly controllable BDG is suggested by the potential on-surface chemical reactions of Ullman polymerization and thermal cyclodehydrogenation [35] with the building block in figure 1(a). Consequently, there are two BDG structures with same formula formed with controllable doping concentration according to the symmetry analysis of the precursor and the on-surface reactions on different C atoms. The optimized atomic structure of BC20 is shown in figure 1(b). The unit cell of optimized BC20 consists of 40 C and 2 B atoms, leading to the corresponding stoichiometry. The lattice parameters of the unit cell indicated by red line, are a = b = 10.85 Å, and the space group is Cmm. The bonds length of B1-C2, B1-C3, and B1-C4 shown in figure 1 are 1.52 Å, 1.50 Å, and 1.52 Å, respectively, indicating the strong covalency. While the other isomer of BC20 is shown in figure S1 (available online at stacks.iop.org/MRX/9/065604/mmedia) (supporting information). It is evident that the BDG structure has fixed doping concentration, where the B atoms are uniformly distributed in the covalent system. Next, the energy stability of the two types of BDG is evaluated by equation (1). Table S1 compares the total energy and the formation energy among these two and other reported BDGs [55, 56]. Clearly, BC20 has both the lowest total energy and formation energy, indicating its high energy stability.
Figure 1. (a) The building block of triarylborane precursor. (b) Top view of BC20 monolayer structure. The unit cell is shown by red solid lines. C and B atoms are represented by brown and green balls, respectively.
Download figure:
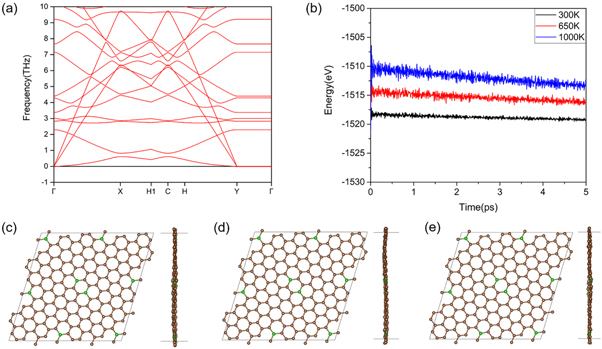
Standard image High-resolution imageIn addition, the dynamic and thermodynamic stability of BC20 monolayer is further explored by the phono spectrum calculation and AIMD simulations. In the calculation, a 2 × 2 × 1 supercell with 168 atoms is used. As shown in figure 2(a), evidently, there are no imaginary frequencies observed in the phonon spectrum, revealing the dynamic stability of BC20. Moreover, the AIMD simulations last for 5ps at 300 K, 650 K and 1000 K respectively. Figures 2(c)–(e) shows that there is no structural decomposition at room temperature and even high temperature, confirming the thermal stability of BC20. Although the energy fluctuation of 1000 K is relative larger, the superb structural integrity remains. All the evidence suggests that the synthesis of BC20 with highly controllable doping is possible. Therefore, the stable BDG will be adopted in the following investigation.
Figure 2. (a) Phonon dispersions for BC20. (b) The results of AIMD simulations for BC20. Top and Side views of final snapshot after AIMD simulation for 5 ps at (c) 300 K, (d) 650 K and (e) 1000 K.
Download figure:
Standard image High-resolution image3.2. Adsorption and electronic structures
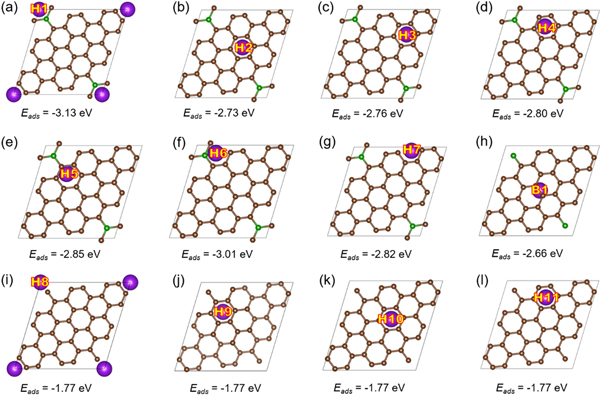
Here, we first study the stable adsorption of potassium ions that is fundamentally required for anode materials for KIBs. For comparison, the adsorption performance of different alkali metals (Li, Na, K and Mg) on BC20 is calculated as shown in figure S2. It is evident that K has the highest adsorption energy among those, indicating BC20 is suitable to be an anode material with high stability for KIBs. Then we calculate the adsorption energy of single K atom on BC20 considering all the hollow, bridge, and top sites based on the symmetry analysis. After optimization, as shown in figure 3, the results confirm that all the other bridge and top sites are converged into hollow sites (figures 3(a)–(h)) except one bridge site (figure 3(h)). To clearly show the convergence, figure S3 illustrates the initial (bridge and top sites) and final optimized structures (hollow sites). Therefore, there are 8 stable sites in total for K adsorption on BC20. The adsorption energy calculated by equation (2) are shown in figure 3 underneath each configuration. For comparison, four other favorable adsorption sites for K on pristine graphene are considered, as shown in figures 3(i)–(l).
Figure 3. The optimized configurations of single K adsorbed on the sites (a-h) of BC20 and (i-l) of pristine graphene. The corresponding adsorption energy is underneath each configuration, and K atom is represented by purple ball.
Download figure:
Standard image High-resolution imageAs a result, the calculated adsorption energy and adsorption height of K on BC20 and pristine graphene with different sites are summarized in table S2. Obviously, the hollow site of hexagonal B-C ring is the most favorable for K adsorption on BC20. B has one less valence electron than C, so that the dopants can gain electrons from π bonds of graphene to form p-type structure [57]. Similar results have been revealed on the adsorption of Li on pristine graphene and BDG [52]. Comparing BC20 with pristine graphene, it is clear that the adsorption ability of BC20 is greatly enhanced after doping due to the introduction of electron-deficiency by the B dopants. Moreover, it is noted that the adsorption height of K adatom on different sites is almost identical, indicating the slight deformation during the diffusion of K atom on BC20 monolayer. Besides, the adsorption energy of K adatom on B4C28 [33] is calculated, which is compared with BC20, graphdiyne [42], and Ti2CS2 [12] as shown in table S3. All the results reveal that the proposed BDG has multiple stable adsorption sites, which is beneficial to highly enhance the capacity as a KIB anode.
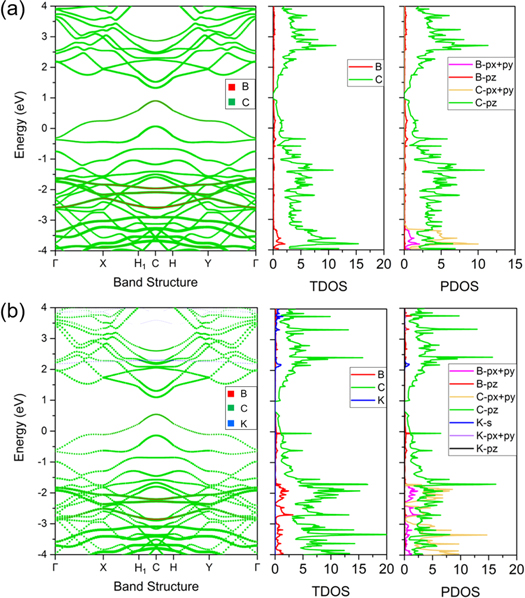
In order to understand the electronic properties of the pristine BC20 and adsorbed systems, the projected band structures, total density of states (TDOS), and projected density of states (PDOS) are investigated next. As shown in figure 4(a), the proposed BDG has metallic properties and is generally preferred for an electrode. Interestingly, there are two parabolic bands across the Fermi level instead of linear dispersion occurring in graphene monolayer, potentially contributing to fine electronic conduction. The states around Fermi level are mainly dominated by the C atoms shown in the TDOS, originating from the pz orbitals of both C and B atoms which is further confirmed by the PDOS. It is also noted the Fermi level of BC20 shifts downward due to the electron-deficiency of B dopants comparing with graphene case. Figure 4(b) shows the results of the adsorbed system, it is clear that the metallicity is preserved after K adsorption. Moreover, the intrinsic band structure is rarely affected, except that the Fermi level shifts upward leading to single parabolic band crossing. Besides, the K adatom donates electrons to the states above the Fermi level, and more hybridization occurs between the pz orbitals of C and B atoms. After all, as an electron deficient system, BC20 has lots of empty states to accommodate electrons from K adatoms, which is beneficial to be applied as an anode for KIB.
Figure 4. Projected band structures, TDOS, and PDOS of (a) BC20 and (b) K-BC20 system. The Fermi level is set as 0 eV.
Download figure:
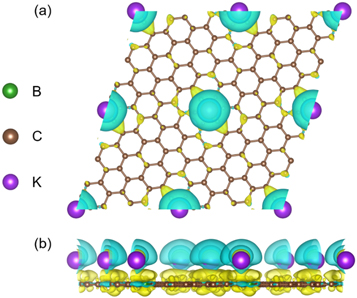
Standard image High-resolution imageTo further understand the adsorption performance, figure 5 shows the charge density difference calculated by equation (3). The yellow and cyan-blue color represent the electron accumulation and depletion, respectively. Evidently, the electrons are transferring from K adatom to the BC20 monolayer. The electrons accumulate mostly around the hexagonal B-C ring indicating a strong chemisorption between K adatoms and the substrate. Furthermore, to explore the charge transfer behavior quantitatively, the Bader charge analysis is performed. As a result, the transfer of valence electrons for K adatom and its nearest six atoms labeled in figure S4 are shown in table S4. Obviously, the K adatom loses 0.87 e− and two B atoms lose 1.85 e−, while another four C atoms obtain 0.69 e−, 0.69 e−, 0.60 e−, 0.60 e−, respectively. Therefore, there are significant charge transfer from K adatom to BDG, further confirming the strong chemisorption between K adatom and BC20. The strong chemisorption facilitates the high loading of K, possibly increasing the capacity of BC20 as an anode for KIB.
Figure 5. The charge density difference of K-BC20 system shown by a 2 × 2 supercell. The yellow and cyan-blue color represent electron accumulation and depletion, respectively (the isosurface value is 0.001 e−/bohr3).
Download figure:
Standard image High-resolution image3.3. Diffusion, capacity, and OCV
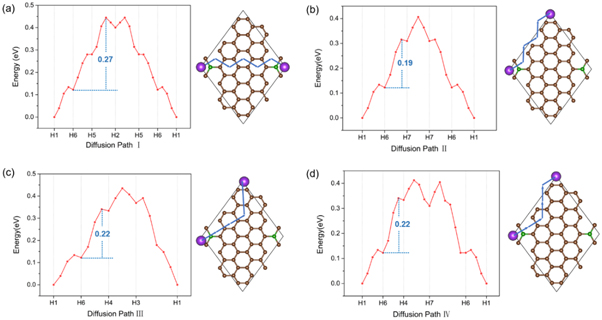
On the other hand, in spite of above discussion about the adsorption and electronic properties, the anode materials for KIBs also need a suitable diffusion path for the transport of ions that pertain to the charge and discharge performance of KIBs. There are four potential diffusion paths taken into consideration in the CI-NEB calculation based on the adsorption performance and structural symmetry. As a result, figure 6 illustrates the four long diffusion paths of K on BC20. The H1 site is set as the initial and final position during each diffusion path due to its lowest adsorption energy. Every long path comprises several sub-paths considering the diffusion for K from one stable adsorption site to another adjacent one, displayed by the blue arrows in the insets of figure 6. Evidently, path II has the lowest energy barrier of 0.19 eV, which is the most favorable for K diffusion on the surface of BC20. Although other three paths have slightly larger activation energy barriers, the comparable values still confirm the small energy cost for each diffusion step.
Figure 6. (a)–(d) Four diffusion paths of K on BC20 monolayer, where the sub-paths are denoted by blue arrows shown in each atomic configuration. The values marked in blue color denote the diffusion barriers.
Download figure:
Standard image High-resolution imageAlso, interestingly, K prefers to migrate along the edge of unit cell without trapping at any specific sites. It is noted that the diffusion barrier of K on proposed BDG is lower than the case of Li on BDG [52]. Meanwhile, in comparison with K on other anode materials [37, 58, 59], as summarized in table 1, this value is much smaller, revealing the competitive advantages as a high rate KIB anode.
Table 1. The diffusion energy barriers of K adatom on several substrates and Li adatom on BDG from previous study.
| System | Diffusion Barrier (eV) |
|---|---|
| BC20 | 0.19 |
| MoN2 [37] | 0.49 |
| YN2 [58] | 0.27 |
| V5S8 [59] | 0.45 |
| Li on BDG [52] | 0.40 |
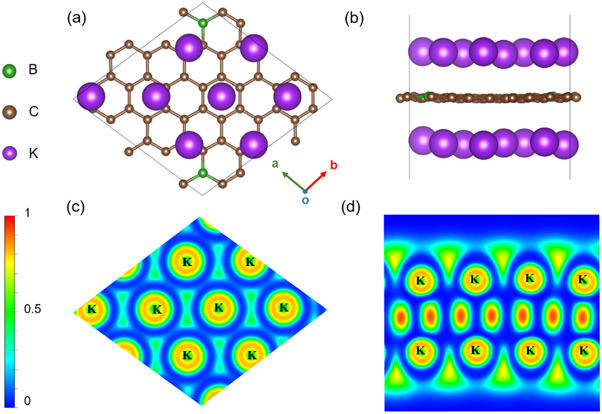
Having discussed the diffusion properties of K on the proposed BDG, we will finally investigate the theoretical capacity and OCV of BC20. It is well known that a full adsorption layer of K on most anode materials is not easy because of the large atomic radius of K, which greatly hinders the improvement in capacity [5]. Therefore, it is essential for BC20 to have large theoretical capacity as an anode material for KIBs. As a result, the full stable adsorption layers of K on both two sides of BC20 monolayer are achieved as illustrated in figures 7(a)–(b). Clearly, the maximum adsorption of K gives rise to a stoichiometry as B2C40K16. With the increasing in the concentration of K adatoms, the adsorption energy is gradually decreasing, and the adsorption energy of each potassiated step is clearly shown in figure S5. Interestingly, the K adsorption layers uniformly construct a triangular lattice, and some K adatoms move to the bridge or top sites, due to the interaction between them. Moreover, it is noted that the structural integrity of BC20 monolayer remains without any deformation, where the interlayer distance between BC20 monolayer and K adsorption layer is about 2.9 Å. Figure S6 shows the AIMD simulation results of the initial (B2C40K1) and final (B2C40K16) potassiated systems. Obviously, the substrate retains the structural integrity without large deformation, confirming the thermal stability of BC20. As shown in figure S7, B2C40K16 maintains metallic properties according to the band structure and DOS.
Figure 7. (a) The top and (b) side view of B2C40K16 structure. Electron localization functions (ELF) of B2C40K16 on (c) (0 0 1) plane and (d) (−1 1 0) plane.
Download figure:
Standard image High-resolution imageGiven such high K loading, the electron localization function (ELF) of B2C40K16 is shown in figures 7(c)–(d) to examine the possibility of K clusters forming, that may lead to dendrite growth being detrimental to safety and cycling [60]. Obviously, there is no electron localization between the K adatoms, confirming no K clustering occurred on the BC20 substrate. Therefore, such stable and large K adsorption is of great benefit to improving the capacity of BC20 anode for KIB.
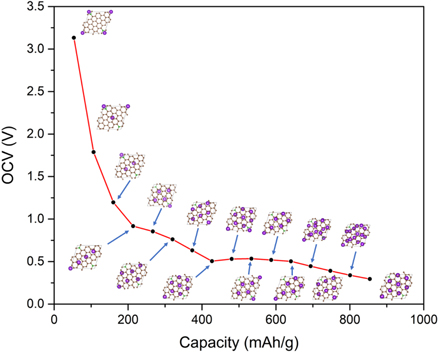
After examining the maximum adsorption, the OCV and theoretical capacity of BC20 are calculated by exploring the potassiation process in sequential steps. The most favorable adsorption site H1 is firstly taken by K atom, and then the subsequent adsorption is carried out until the full coverage is obtained on both sides symmetrically. Figure 8 shows the OCV and corresponding capacity of each step. Consequently, the obtained OCVs decrease monotonically with the increasing on specific capacity. Meanwhile, the chemical window of OCV is in a low range, that is greatly desired for the half-cell reaction with related electrolytes [61, 62]. Besides, a broad and steady plateau region is found without great oscillations below 0.6 V in the OCVs versus specific capacities profile, which is required for providing a large operating voltage. Noticeably, the maximum theoretical capacity for the fully adsorbed structure is as high as 854 mAh g−1 with a low OCV of 0.29 V. By contrast, the maximum theoretical capacity of BC20 monolayer is much larger than other BDG [33] and carbon based anodes [40–42], as illustrated in table 2.
Figure 8. OCVs versus capacities profile with sequential potassiation process. The insets are the top view of adsorbed structures for each step, where the corresponding side view is shown in figure S8.
Download figure:
Standard image High-resolution imageTable 2. The theoretical capacities of different types of anode materials.
| System | Theoretical capacity (mAh g)−1 |
|---|---|
| BC20 | 854 |
| Graphite [40] | 273 |
| Carbon nanocage [41] | 175 |
| Graphdiyne [42] | 620 |
| B4C28 [33] | 546 |
To better understand the origin of capacity enhancement, the image chare model [63] is used to analyze the difference between BC20 and B4C28. Generally, the weak binding between the metal atoms and substrate is beneficial to enhance the capacity. The binding energy pertaining to capacity is described as E-b = Eion + Ecp, where E-b is the energy change when a K atom moves from the vacuum to the binding site of the substrate; Eion is the energy change from ionization of K by transferring its charge to the substrate; and Ecp is an energy decrease by the coupling of the cation to the substrate (negatively charged). Furtherly, E-b can be expressed as [64]:
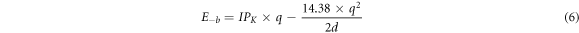
where  is the ionization potential of K atom (∼4.3 eV); q is the amount of charge lost from K atom; and d is the distance between K and the substrate. Therefore, the capacity is mainly dominated by the charge transfer of K atom and the distance between K and substrate. After optimization of two highest loading structures (B2C40K16 and B4C28K8), we found that the distances between K adatoms and substrates are quite different (∼2.95 Å of BC20 and ∼2.69 Å of B4C28). The value of q is obtained from the Bader charge analysis. The results show that K adsorption layers lost 0.29 e− charge per K adatom to BC20 for B2C40K16. As for K8B4C28, the value of q is 0.46 e−. Finally, according to equation (6), the E-b of B2C40 and B4C28 are 1.02 eV and 1.38 eV, respectively. The weaker E-b indicates that the capacity of BC20 is quite larger than B4C28. Overall, the high capacity with low OCV indicates the proposed BDG structure is a promising anode material as applied in KIBs.
is the ionization potential of K atom (∼4.3 eV); q is the amount of charge lost from K atom; and d is the distance between K and the substrate. Therefore, the capacity is mainly dominated by the charge transfer of K atom and the distance between K and substrate. After optimization of two highest loading structures (B2C40K16 and B4C28K8), we found that the distances between K adatoms and substrates are quite different (∼2.95 Å of BC20 and ∼2.69 Å of B4C28). The value of q is obtained from the Bader charge analysis. The results show that K adsorption layers lost 0.29 e− charge per K adatom to BC20 for B2C40K16. As for K8B4C28, the value of q is 0.46 e−. Finally, according to equation (6), the E-b of B2C40 and B4C28 are 1.02 eV and 1.38 eV, respectively. The weaker E-b indicates that the capacity of BC20 is quite larger than B4C28. Overall, the high capacity with low OCV indicates the proposed BDG structure is a promising anode material as applied in KIBs.
4. Conclusion
In summary, we proposed a highly controllable BDG (BC20) as the potential anode material for KIBs, and the high stability at the ambient condition is confirmed. The realization of controllable B doping is suggested by the on-surface chemical reactions with pertinent building block. The newly designed structure possesses metallic feature of parabolic bands crossing the Fermi level, indicating a fine conductivity. Compared with pristine graphene, the adsorption capacity of K is greatly improved because of the introduction of electron-deficiency by the B dopants. The structural integrity is preserved during the adsorption process, which is beneficial to a good cyclability. The strong adsorption results in a fully covered configuration with a stoichiometry as B2C40K16. Besides, the low diffusion energy barrier is 0.19 eV, suggesting the fast charging/discharge process. Moreover, the maximum capacity of BC20 is as high as 854 mAh g−1 with a low potassiation voltage of 0.29 V. This value is much larger than other BDG and carbon related anodes. Besides, the value of OCVs in a low range with a steady plateau region without rapid change, which is important to avoid the safety issues caused by the formation of dendritic deposits. Our results open an avenue for the design of both highly controllable BDG structures and novel anodes with large capacity for KIBs.
Acknowledgments
We thank for the funding supports from the National Natural Science Foundation of China (11904047) and China Postdoctoral Science Foundation (2019M663460).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).