Abstract
Every year a large amount of scrap can be produced from the Bi2Te3-based alloys when processing. A clean, new vacuum distillation method is proposed to purify Te from the scrap of Bi2Te3-based alloys. In this work, the saturated vapor pressure, distillation rate and separation coefficient of each metal element in Bi2Te3-based alloys are theoretically analyzed. The effects of distillation temperature and time on the volatilization and condensation behavior of elements are studied under the dynamic vacuum of 0.1 Pa. According to the research results, a multi-stage distillation method is proposed, and Te was purified from 41.53% to 98.88% while the total impurity content was reduced to 1.12% through this procedure. Moreover, Te of the scrap of Bi2Te3-based alloys can be separated and recovered effectively via this three-step vacuum distillation. The results lay the foundation for the industrial purification of Te and provide a low-cost, simple and green recycling process of thermoelectric scrap, which has important practical significance.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Bismuth telluride-based thermoelectric material is currently the thermoelectric material with the most complete system, the widest commercial application, and the best performance near room temperature [1]. The demand for thermoelectric devices in low-power generation and refrigeration fields such as MEMS (Micro-Electro-Mechanical System), IoT (Internet of things) wireless sensor modules, and smart wearable devices is increasing [2]. Most of these applications are near room temperature or low temperature, making the application prospects of Bi2Te3 based alloys broader in many thermoelectric material systems [3]. At present, commercial bismuth telluride-based thermoelectric materials are basically produced by uniaxial growth methods such as zone melting and the Bridgman method. However, the fracture toughness of Bi2Te3 based alloys is low, which leads to poor mechanical processing performance during the subsequent slicing and pelletizing [4]. It is easy to break or damage during the process for bismuth telluride-based thermoelectric materials, resulting in a large amount of scrap (fragments, waste particles and waste powder). Moreover, the thermoelectric legs in the failed devices also need to be recycled. Therefore, it reduces the material utilization rate to less than half and ultimately results in the production of approximately 48 tons of tellurium-containing scrap every year [5]. The main components of Bi2Te3 based thermoelectric materials, such as Te, Bi and Se, are low abundance elements, and their contents in the Earth are only 0.001, 0.02 and 0.05 ppm. At the same time, the price of Te is very expensive. The price of 4N Te is 10 times that of Cu [6]. It is worth mentioning that Bi, Te, Se are toxic elements in the waste, so it is necessary to recycle the scrap of Bi2Te3-based alloys in a greenway with obvious economic benefits [5]. The method of preparing high-purity tellurium is mainly divided into two categories: physical method and chemical method [6]. For chemical method, the oxygen pressure acid leaching method is represented by the Kaldo process [7]. The soda roasting process is to transfer tellurium from copper anode slime into soda residue, and then recover tellurium from the residue [8]. The solvent extraction method uses neutral extractant, nitrogen extractant, naphthenic acid and other extractants to separate and purify tellurium [9]. For physical method, zone melting is a method to change the precipitation and distribution of impurity elements in tellurium by using the difference of solubility of impurity elements in the solidified and molten state of Te [10]. As new technology in the field of metallurgy, vacuum metallurgy is a metallurgical process carried out at sub-atmospheric pressure [11]. This process is conducive to the volatilization of low-boiling substances with no metal oxidation and reduction reaction. Compared with traditional metallurgical methods, it has a high metal recovery rate. The advantages consist of low resource and energy consumption, no wastewater and waste gas generation, simple process flow, simple operation, etc [12]. Aiming at the disadvantages of a long process of existing treatment methods and severe environmental pollution, vacuum distillation was applied to separate and purify Te from a scrap of bismuth telluride-based alloys. The effect of distillation time and temperature was investigated, the optimal process parameters were determined, and a new method for the clean recovery of bismuth telluride scrap was provided.
Prasad [13] reported the purification of tellurium up to 5N by vacuum distillation, Ali [14] reported that 5N5 purity Te can be achieved by multiple distillation techniques, and Hageman [15] achieved the purification of tellurium to 6N. In their work, the raw material is almost 2N or more purity tellurium. However, the content of Te in Bi2Te3 based thermoelectric materials commonly used in industrial production is usually between 20% and 50% [16]. Therefore, it is necessary to study the recovery of Te from the scrap produced in the production process of Bi2Te3 based thermoelectric materials.
The multistage distillation method mentioned in this paper can extract tellurium from the waste materials of thermoelectric device manufacturers. The extracted tellurium can also be used to reprocess into new thermoelectric device raw materials, which can save a lot of production costs for these manufacturers. The application of vacuum metallurgy technology in the metallurgical process has made great development. The application of vacuum metallurgy technology can be easily achieved for metals that cannot or are difficult to produce by conventional metallurgical methods [17, 18]. At present, high-purity nickel, cadmium tin, zinc, lead, antimony, and lithium, etc. have been obtained through vacuum distillation methods, and have been produced by industrialization or small-scale experimentation [19]. Therefore, this paper mainly introduces the mechanism of vacuum distillation and its application in the purification of tellurium from bismuth telluride-based scrap. The effects of distillation temperature and time on the recovery of tellurium and other impurity elements were analyzed. Further, this study aimed to understand the behavior of Te and its influence on vacuum distillation.
2. Experimental section
2.1. Experimentation
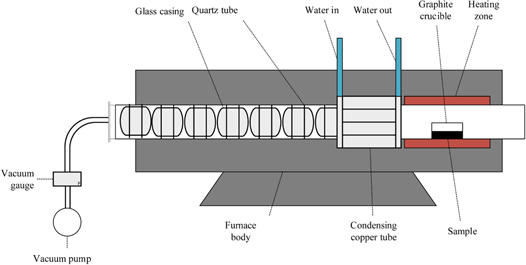
The initial materials were produced by Hubei Sagreon New Energy Co., Ltd, and the content of elements has been shown on table 1. In this research, we used a vacuum tube furnace developed by ourselves. As shown in figure.1, the entire vacuum device contains a thermocouple, heater, graphite crucible, condenser and glass casing. At first, 50 g of the n-type bismuth telluride-based scrap were washed with distilled water and absolute ethanol, dried in a vacuum drying oven at 60 °C and subsequently put into a high-purity graphite crucible. Then put the crucible in the quartz tube for the experiment at the conditions of 873 K–1073 K. A dynamic vacuum level of approximately 0.1 Pa in the distillation chamber was obtained by a mechanical vacuum pump. The heating rate was controlled at 5 K min−1, and the temperature was controlled by an automatic temperature control system when the target distillation temperature was reached. As the temperature inside the crucible reached the target temperature, the material started evaporating. After the end of distillation, as the temperature in the chamber dropped to room temperature, the condenser was cleaned off and weighed. The vacuum pump continued running during the cooling process, ensuring that the system was always in a vacuum state, thus preventing gas leakage and the oxidization of the metal. After the first vacuum distillation, take out the condensate and put it into a new crucible again. Repeat the above steps for the second and third vacuum distillation.
2.2. Characterization
The phases of the distillation products were examined by x-ray diffraction (XRD) in a Philips X'pert Pro diffractometer with Kα radiation (λ = 1.5418 Å). The fracture morphologies of distillation products were observed by field-emission scanning electron microscopy (FESEM, FEI/ Nova400 NanoSEM). The content of each element in the distillation product is analyzed by an x-ray fluorescence spectrometer (XRF/Panalytical Axios).
Figure 1. Schematic of the vacuum tube furnace.
Download figure:
Standard image High-resolution image3. Separation mechanism and influencing factors of vacuum distillation
The vacuum distillation method can effectively remove impurities with vapor pressure quite different from that of the matrix. The factors affecting the preparation of high-purity metals by the vacuum distillation method are as follows: the saturated vapor pressure, the separation coefficient, and the evaporation rate of the elements.
Table 1. The content of elements in n-type bismuth telluride-based scrap.
| n-type | |||
|---|---|---|---|
| Element | Atomic number | Mass percentage (wt. %) | Average mass percentage of EDS analysis (wt. %) |
| Bi | 2 | 53.04 | 50.34 |
| Te | 2.5 | 41.53 | 47.30 |
| Se | 0.5 | 5.43 | 2.360 |
3.1. Saturated vapor pressure of each element
The principle of vacuum distillation is to use the difference in saturated vapor pressure between elements to achieve the purpose of separating the subject metal and impurity components under a certain temperature and vacuum conditions. In a vacuum environment, elements with a higher saturated vapor pressure are preferentially volatilized into the gas phase, while other elements are not volatilized and remain in the residue, thereby achieving the separation of main elements and impurity elements. Under the same system pressure, the saturated vapor pressure of pure metal is only related to temperature. The saturated vapor pressure at different temperatures can be calculated by formula 1 [20]. The results are shown in table 2.
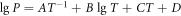
Table 2. The evaporation constant of each element A, B, C and D [16].
| A | B | C × 103 | D | |
|---|---|---|---|---|
| Bi | −10400 | −3.02 | — | 14.47 |
| Se | −4990 | — | — | 8.49 |
| Te | −7830 | −4.27 | — | 24.41 |
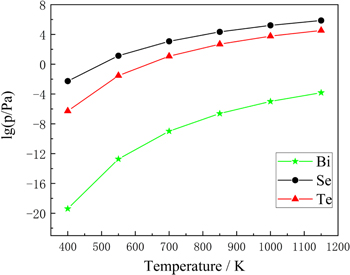
The required parameters A, B, C and D are evaporation constants. We calculated the saturated vapor pressure of various elements in the scrap of Bi2Te3-based alloys in the temperature range of 473 to 973 K, as shown in figure 2.
Figure 2. Saturated vapor pressure of elements in the scrap of Bi2Te3-based alloys.
Download figure:
Standard image High-resolution imageFigure 2 shows that the saturated vapor pressure of each component in the scrap of Bi2Te3-based alloys increases with temperature. At the same temperature, the saturated vapor pressure of Te is higher than that of other components (especially Bi). In the vacuum distillation process, Se and Te are easier to volatilize into the tube, while Bi tends to stay in the residue. Therefore, this experiment can separate Bi, Te and Se through vacuum distillation, and Te can evaporate into the vapor phase and candense to achieve separation [21].
In the range of 473 K–1173 K, the saturated vapor pressure of Te and Se is always higher than that of Bi. After comprehensively considering the volatilization rate of Bi, Se Te elements and the temperature selection of Hageman's work [15], we choose 873 K–1073 K as the temperature range of our experiment.
3.2. Separation coefficient between elements
For dilute liquid alloy solutions, the 'Separation Coefficient β' at the distillation temperature is calculated by formula 2 [16]:
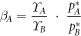
In the formula,  and
and  are the saturated vapor pressures of the pure substances of the elements A and B;
are the saturated vapor pressures of the pure substances of the elements A and B;  and
and  are the activity coefficients of A and B. If
are the activity coefficients of A and B. If  ≫ 1 or ≪ 1, the two components A-B can be separated well. We calculated the separation coefficients of Te-Se and Te-Bi at 873 K, 973 K, and 1073 K respectively, as shown in table 3.
≫ 1 or ≪ 1, the two components A-B can be separated well. We calculated the separation coefficients of Te-Se and Te-Bi at 873 K, 973 K, and 1073 K respectively, as shown in table 3.
Table 3. Separation coefficients of Te-Se and Te-Bi at different temperature.
| Temperature | Te-Se | Te-Bi |
|---|---|---|
| 873 K | 25.88 | 1.03 × 10–9 |
| 973 K | 19.04 | 2.38 × 10–9 |
| 1073 K | 15.46 | 4.73 × 10–9 |
It can be seen from table 3 that the Te-Se separation coefficient is about 20 while the separation coefficient of Te-Bi is 9 orders of magnitude lower than that of 1 at 873–1073 K. It means that Bi is remained in the liquid phase while Te and Se are concentrated into the vapor phase. Therefore, Bi can be collected in residue.
3.3. The evaporation rate of elements
According to the Langmuir evaporation rate formula, the evaporation rate of component A in the melt is expressed as formula 3 [16]:
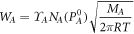
Where  is the evaporation rate
is the evaporation rate  of metal A at temperature T;
of metal A at temperature T;  is the activity coefficient of the component in the melt; NA
is the concentration of component A in the melt; PA
is the saturated vapor pressure of component A at temperature T; P1 is the system space vapor partial pressure;
is the activity coefficient of the component in the melt; NA
is the concentration of component A in the melt; PA
is the saturated vapor pressure of component A at temperature T; P1 is the system space vapor partial pressure;  is the atomic weight of component A; R is the universal constant of gas, R = 8.314
is the atomic weight of component A; R is the universal constant of gas, R = 8.314  We calculated the evaporation rate of elements in the scrap of Bi2Te3-based alloys at 873 K, 973 K, and 1073 K respectively, as shown in table 4.
We calculated the evaporation rate of elements in the scrap of Bi2Te3-based alloys at 873 K, 973 K, and 1073 K respectively, as shown in table 4.
Table 4. Evaporation rate of elements in the scrap of Bi2Te3-based alloys.
| Temperature | Te | Bi | Se |
|---|---|---|---|
| 873 K | 459.43 | 3.65 × 10–7 | 14767.58 |
| 973 K | 2287.47 | 4.17 × 10–6 | 54102.02 |
| 1073 K | 8066.26 | 2.93 × 10–5 | 154855.31 |
It can be seen from table 4 that the evaporation rate is affected remarkably by the change of distillation temperature. The evaporation rate can increase even ten times with the temperature increment of 100 K. It is concluded that Te and Se can volatilize quickly and concentrate into vapor phase than Bi. Also it can be predicted that Se and Te cannot be separated well by vacuum distillation technology. The evaporation rate of Bi is less than 9 orders of magnitude and above of Te and Se. In the vacuum distillation process, Bi will only evaporate a small amount, which is easier to separate.
4. Results and discussion
4.1. Phase and microstructure
The influence of distillation temperature on Te, Se, Bi in the scrap of Bi2Te3-based alloys was studied. The sample was prepared at a temperature of 873 K to 1073 K, a pressure of 0.1 Pa, a heating rate of 10 °C min−1 and maintained for 120 min. The XRD patterns of volatile components at different temperatures (873 K, 973 K, 1073 K) is shown in figure 3. It can be seen that the observed phases are Te and Se, while no obvious characteristic diffraction peaks of Bi are detected, presumably due to the small vapor pressure and low evaporation rate of Bi. It indicates a vacuum distillation is effective for separating Te and Bi from the scrap of Bi2Te3-based alloys. However, the presence of Se phase can be detected in XRD patterns at different temperatures. Since the saturated vapor pressures of Te and Se are close, In vacuum distillation, both elements volatilize and condense in vacuum distillation, in conformity with other's [22] observation.
Figure 3. XRD patterns of volatile components at 873 K (a); 973 K (b); 1073 K (c).
Download figure:
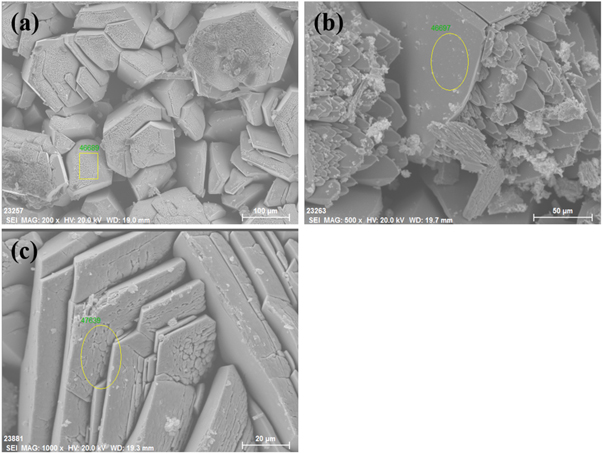
Standard image High-resolution imageThe SEM of condensate and resident under the conditions of a temperature of 873 K, a pressure of 0.1 Pa, and a vacuum distillation time of 1h is shown in figure 4. From figure 4(a), it can be clearly seen the 200 micron-sized polygonal tellurium crystals, and a little flocculent substance is also found on the surface of tellurium crystal, which is consistent with the SEM of tellurium deposit at high temperature in [23]. Figure 4(b) shows that the surface of the residue is relatively flat with many pores on the surface. The pores may be caused by the volatilization of tellurium and selenium during distillation [24].
Figure 4. Scanning electron micrograph of condensate (a) and resident (b).
Download figure:
Standard image High-resolution image4.2. Single distillation
The effect of distillation temperature on the volatilization of Se and Te was investigated under pressures of 0.1 Pa at a constant distillation time of 120 min. The highest recovery rate can be obtained only by volatilizing Te and leaving other elements in the residue. The recovery efficiencies of Te can be calculated as formula 4:
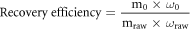
where mraw is the mass of raw material(g); ωraw is the content of Se, Te and Bi in raw material(%); m0 is the mass of condensate(g); ω0 is the content of Se, Te and Bi in condensate(%).
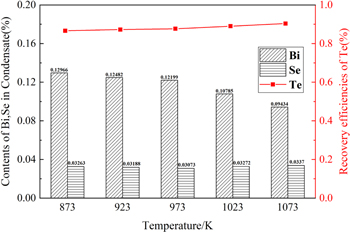
As shown in figure 5, the recovery rate of Te increases from 86.62% to 90.34% with the temperature increased from 873 K to 1073 K. It is important to note that with a single distillation of 873 K the recovery rate of Te can be easily purified from the initial scrap at 47.30% to 86.62%. When the temperature rises, the recovery rate does not increase significantly, suggesting that after the distillation at 1073 K for 2 h, the Te content has become saturated, and more distillation time has no obvious effect on increasing the Te recovery rate. However, the Se content in the condensate is always maintained at 3% and does not change too much with the increase of the distillation temperature. It may due to the very little difference of the saturated vapor pressure between Se and Te. In the vacuum distillation process, both Se and Te are easy to volatilize and condense the same time [25].
Figure 5. Contents of Bi, Se in condensate and recovery of Te at different temperatures.
Download figure:
Standard image High-resolution imageFigure 6 displays the EDS diagram of condensate at different temperatures. As shown in table 5, three regions were selected for EDS analysis. It can be seen that after two hours of vacuum distillation, the content of Bi is always zero, proving that Bi hardly evaporates and condenses during the distillation. Another phenomenon that cannot be ignored is that the content of selenium is still around 4%–10% at different temperatures, the result is also consistent with our previous studies. The results indicate that it is difficult to remove selenium from the scrap of Bi2Te3-based alloys at a distillation temperature of 873 K–1073 K. The findings from Guo's work [26] seem consistent with our study. For example, after a distillation temperature of 923 K maintained for 120 min, the recovery efficiency of Se and Te was 98.09% and 97.82%, respectively, which suggests Se is easier to collect than Te during distillation.
Figure 6. EDS diagram of condensate at different temperatures.
Download figure:
Standard image High-resolution imageTable 5. The content of various elements in the condensate at different temperatures.
| 873 K | 973 K | 1073 K | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | 1# | 2# | 3# | Avg | 1# | 2# | 3# | Avg | 1# | 2# | 3# | Avg |
| Bi | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Te | 95.71 | 93.53 | 91.92 | 93.72 | 92.23 | 93.66 | 91.86 | 92.58 | 90.00 | 92.47 | 89.6 | 90.69 |
| Se | 4.29 | 6.47 | 8.08 | 6.t328 | 7.77 | 6.34 | 8.14 | 7.42 | 10.00 | 7.53 | 10.4 | 9.31 |
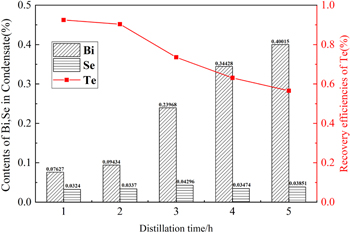
Figure 7 shows the contents of the scrap of different distillation times under the condition of the distillation temperature of 1073 K and the pressure of 0.1 pa. It can be seen from figure 7 that the longer the distillation time, the lower the Te purity in the condensate, and the higher the impurity content in Te. With the extension of the distillation time to 3 h, we can see that there is an obvious increase in the amount of Bi in the condensate, indicating Te and Se have been completely evaporated in the raw material, and further extension of distillation time will lead to the evaporation and condensation of Bi. It can also be seen that no matter how the distillation time is prolonged, the content of Se is always between 3% and 4% [27].
Figure 7. Contents of Bi, Se in condensate and recovery of Te at different distillation time.
Download figure:
Standard image High-resolution image4.3. Multi-stage distillation
After that, the recovery of Te from the scrap of Bi2Te3-based alloys is achieved through a multi-stage distillation. The method is to first distill at 1073 K for 2 h, then distill the condensate again at 873 K for 2 h, and finally distill the condensate at 723 K for 2 h.
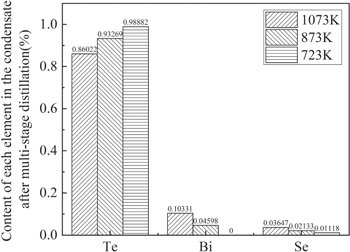
As shown in figure 8, after the multi-stage distillation, the purity of Te increased from 41.53% of the raw material to 98.88%. And Bi was not detected in the condensate after the third distillation. This is probably due to the temperature of 723 K is relatively low and it's difficult for Bi to volatilize and condense during distillation. The volatiles are a mixture of Te and Se, while Bi is collected in the residue. The recovery efficiency is conducive to further purification of Te. This means that Te scrap of Bi2Te3-based alloys can be purified and separated by multistage vacuum distillation, and then applied to production again.
Figure 8. Contents of Te, Bi and Se.
Download figure:
Standard image High-resolution image5. Conclusion
In this work, the influence of distillation temperature on the purification of Te from the scrap of Bi2Te3-based alloys was investigated by using vacuum distillation at 873 K–1073 K, and the influence of distillation time on the purification of Te was investigated at 1073 K. The results show that the Te content has become saturated when distilled at 873 K for 2 h, and the effect of continuing to extend the time to increase the Te recovery rate is not obvious. After distilling at a distillation temperature of 1073 K for 2 h, the Te distillation has become saturated, and further extension of the time would allow the Bi element to continue to volatilize and condense. In addition, according to the optimal purification process determined by experiments, a multi-stage vacuum distillation method was proposed to purify Te. After three consecutive vacuum distillation purifications, the purity of Te in the final condensate reached 98%. It also enriches the application of separation coefficient theory in vacuum. Compared with the traditional purification method, the vacuum distillation method has a short process, no pollution, and a high recovery rate. The condensate after vacuum distillation can be directly used in production, which can reduce the cost loss caused by poor mechanical performance, and has a great impact on production.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51674181).
Data availability statement
The data generated and/or analyzed during the current study are not publicly available for legal/ethical reasons but are available from the corresponding author on reasonable request.
Conflict of interest
The authors declare that they have no conflict of interest.