Abstract
The hot corrosion behaviors of the aluminide coatings with different treatments on the surface of Ni-based alloy at high temperature were investigated. The aluminide coating modified by three different methods (pretreatment, Pt-modification and both), and then all the specimens were oxidized in lab air with NaCl salt at 1050 °C. During cycle oxidation, oxide scales on the surface of the aluminide coatings presented different morphologies, composition and corrosion behaviors. The oxide scale on the surface of the as-received aluminide coating without any treatments showed a poor dense and a bad adherence. However, the oxide scale on the surface of the aluminide coating with both Pt-modification and pre-oxidation exhibited the best hot corrosion resistance. In the NaCl corrosion environment, different treatments resulted in different corrosion mechanisms. Without any treatments, the oxide scale with NaCl at high temperature occurred internal active oxidation, which could promote the corrosion process and form the pores in the oxide scale. The internal active oxidation also presented in the oxide scale on the surface of the aluminide coating with pre-oxidation.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nickel based superalloys are widely used in gas turbines, aeroengines and other high temperature hot section components due to their excellent high temperature mechanical properties and oxidation resistance [1–4]. However, single nickel base alloy cannot meet the increasing service requirements, so scholars have done a lot of researches to improve the service performance of the Ni-based alloy, including optimization of alloy composition [5], surface modification [6, 7] and pretreatment methods [8]. As one of the important surface modification technologies, aluminide coating can effectively improve the corrosion resistance of the alloy [8, 9]. This is because aluminide coating can form a dense and continuous alumina layer on its surface. The high temperature mechanical properties of alumina are stable, which can inhibit the continuous oxidation of the coating and protect the alloy matrix. However, the surface roughness and active element content of the alloy seriously restrict the growth mechanism and bonding properties of the oxide layer. As we know, Pt can promote the alumina growth [10] and improve the adherence of alumina layer [8, 11], so that it is widely used in the aluminide coating as a modified element.
As mentioned before, the Ni-based alloy are widely used in the modern gas turbines, aircraft engines and some high temperature equipments. It means that this superalloy will face more complex service environment, such as marine environment, sulfur atmosphere, etc. During the service process, the salts would deposit on the surface of the Ni-based alloy at high temperature [12]. In the field of high-temperature corrosion, the researches on the high temperature oxidation and hot corrosion of Fe-based alloys and Ni-based alloys and MCrAlY coatings are more profound, the corrosion mechanism is clear and the theory is mature [13–18]. On this basis, the anti-corrosion measures proposed have been widely used in production and scientific researches [19, 20]. However, the research on the high temperature chlorination corrosion is relatively backward. Because most of the chlorine containing substances are poisonous, and the chlorides of many metals are volatile and have low melting and boiling points. The corrosion characteristics at high temperature are obviously different from that of metal materials in high temperature chloride environment. As far as we know, researchers have not put forward a widely accepted high temperature corrosion mechanism with chloride. In this study, the hot corrosion behaviour was investigated for the Pt-modified, pre-oxidized and pre-oxidized Pt-modified aluminide coating on the surface of the Ni-based alloy covered by NaCl at 1050 °C in air.
2. Experiments
The nominal chemical composition of the Ni-based alloy is 8.0 Co-15.7 Cr-3.2 Al-3.0 Ti-1.5 Ta-2.4 W-1.5 Mo-0.6 Nb, Ni-balance (wt.%). The samples for hot corrosion studies obtained by wire cutting that cut into circular with a diameter of 17 mm and a thickness of 3 mm. After polishing the surfaces, samples were washed with an ultrasonic cleaner. The composition of permeating agent was 76.0% Al2O3, 20.4% AlSi40 and 3.6% NH4Cl (wt.%). Then the samples with the agent were placed in the crucible in a tube furnace, where they were heat treated for 10 h at 1000 °C in Ar protection to form stable aluminide coating. Subsequently, a platinum film was sputtered on the surfaces of some samples by a plasma magnetron sputtering coater at a current of 120 mA for 120 s. Some samples were pre-oxidized at 1050 °C for 1 h in a muffle furnace. And some samples were sputtered with Pt and then pre-oxidized. The remaining samples were as-received coating. Before the hot corrosion process, the saturated NaCl solution was coated on the surface of the samples, then dried to remove water and then weighed. It can be coated and dried many times to ensure that the salt content on the surface of the sample reached 0.5–1.0 mg cm−2. After high temperature corrosion, we clear the surface and then weight again, then we can calculate the mass change of the samples. It is taken out and weighed every 24 h.
Before cross-section SEM analysis, the Ni coating layer would be sputtered on the surface of samples to protect the oxide scale. Then we mounted the sputtered samples by epoxy resin for the grinding and polishing. The elemental chemical composition, surface and cross-sectional microstructures of the oxide scale after hot corrosion were analyzed by scanning electron microscopy (SEM) coupled with energy-dispersive x-ray spectrometer (EDS) and electron probe microanalysis (EPMA). X-ray powder diffraction (XRD) was performed to investigate the phase composition of the oxide scale.
3. Results
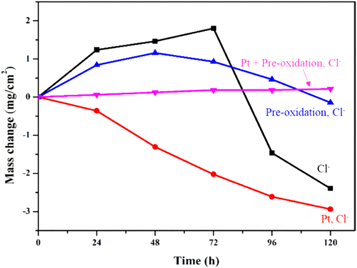
All the mass change curves of the samples covered by NaCl are shown in figure 1. The black curve indicates the aluminide coating without any pretreatment before hot corrosion. The red line shows that the sample had been modified with Pt before hot corrosion. The blue curve means the sample had pre-oxidation before hot corrosion. At the last, the pink line expresses the sample both had been modified with Pt and pre-oxidation.
Figure 1. The mass change of the studied samples with different pre-treatment oxidized at 1050 in air with NaCl salt.
Download figure:
Standard image High-resolution imageAs we can see, besides the red curve, the weight gains of the samples increase with time during the initial 48 h. This demonstrate that these samples formed oxide scales on the surface in the early stage of oxidation, but the growth rates of the oxide scales were different. Among them, the sample without treatment shows the highest oxidation rate and the sample with both Pt modification and pre-oxidation presents the lowest growth rate. The mass change of sample without treatment decreased rapidly after 72 h due to the oxide scale spallation. This is caused by the internal stress in the oxide scale, and the stress was generated at the interface during growth of the oxide scales. However, the weight of the sample with Pt-modification and pre-oxidation changes slowly and there is no obvious spallation of the oxides. The growth rate of the sample with pre-oxidation is between the two formers. But 48 h later, the weight of this sample decreases slowly. Finally, the sample with Pt modification indicates the different oxidation behavior. It seem like that the weight of this sample has not stopped losing during the whole oxidation process. Overall, the sample, which had pre-oxidation and Pt-modification has a higher oxidation resistance.
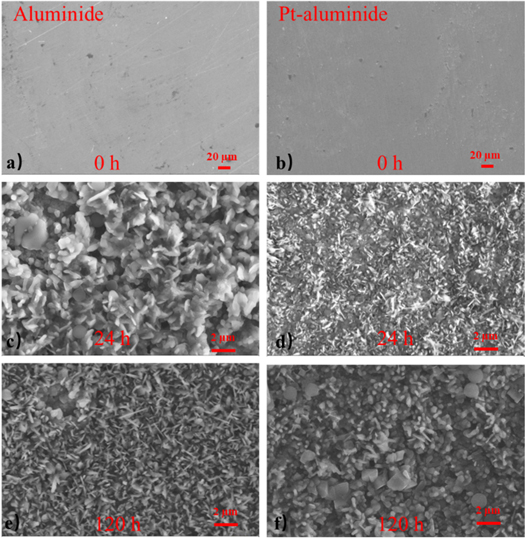
The surface image of the as-received samples show that all the samples contain a flat aluminide coating with and without Pt modification on the surface of the Ni-based alloy, as shown in figures 2(a) and (b). After 24 h oxidation, the surface morphologies of the two kinds of samples present different results in figures 2(c) and (d). The grain size of the oxides on the surface of the oxide scale clear lager than that sample with Pt-modification. The oxide grains on the surface of the oxide scale indicate that some new grains formed with whisker structure during longer oxidation process (120 h). However, the oxide grains on the surface of the sample with Pt-modification become lager in a bulk shape. Compared with the sample without modification, the content of the whisker oxide grains is obviously less.
Figure 2. The surface SEM results of the sample without any treatments (a) and Pt modification (b) and corresponding after different corrosion time, 24 h (c), (d) and120 h (e), (f).
Download figure:
Standard image High-resolution imageThe surface morphology of the samples after corrosion with NaCl for 120 h are observed by SEM and the results are shown in figure 3. The oxide scale morphologies of the samples with and without pre-oxidation present different compositions and structures. The oxide scale on the surface of the sample without pretreatment shows a loose structure with a large amount of pores. At the same time, there are obvious marks of spallation, as shown in figures 3(a) and (b). After pre-oxidation, there are some whisker alumina grains on the surface. However, the SEM results with large magnification shows that there are also many pores in the oxide scale that shown in figures 3(c) and (d). Compare with figures 3(b) and (d), the number of pores are reduced.
Figure 3. The surface SEM results of the sample with (a) and without (c) pre-oxidation after hot corrosion in NaCl for 120 h and corresponding magnification images (b) and (d).
Download figure:
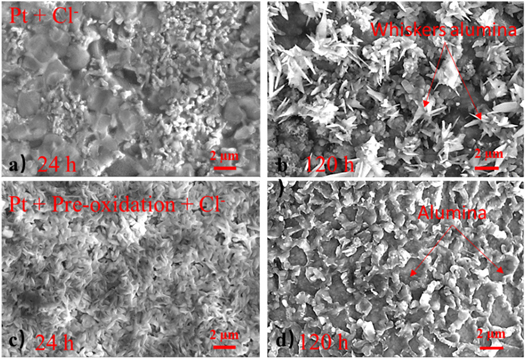
Standard image High-resolution imageThe SEM results of the surface morphology of the samples with Pt modification and pre-oxidation oxidized in NaCl environment are shown in figures 4(a) and (c) respectively. These two coatings seem to form a dense oxide layer on the surface of the coating after 24 h and 120 h oxidation. After 24 h of oxidation, the coating with Pt modification did not show pores on the coating surface, nor did it show obvious corrosion phenomena. At the same time, no whisker oxides form on the coating surface. However, a large amount of whisker oxide grains appear on the surface of the coating, and the bulk grains of alumina are continuously decreasing after the oxidation 120 h as shown in figure 4(b). Unlike the sample with Pt-modification, the alumina scale on the surface of the sample with Pt modification and pre-oxidation presents a dense and continues structure, as shown in figures 4(c) and (d). After 120 h oxidation, no whisker alumina is found on the surface of the aluminide coating, and there is no obvious pores and coating spallation. Moreover, from figure 4(d), it can be seen that the coating surface has obvious 'protrusions', from which it can be guessed that the alumina on the coating surface grows along the original alumina grain boundary.
Figure 4. The surface SEM results of the Pt-modification samples with (a) and without (c) pre-oxidation after hot corrosion in NaCl for 24 h and 120 h (b) and (d).
Download figure:
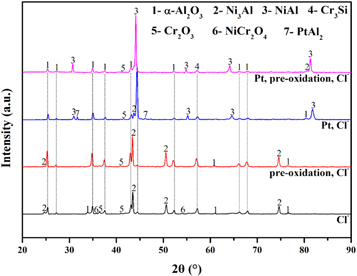
Standard image High-resolution imageX-ray diffraction is used to analyse the phase composition of coating after hot corrosion as shown in figure 5. The surface XRD results illustrate that the phase composition of the aluminide coating covered by NaCl after 120 h cyclic oxidation in air at 1050 °C. The presence of the main crystal phases of Al2O3, NiAl, Cr2O3 and Cr3Si was further confirmed using XRD. As shown in figure 5, after 120 h cyclic hot corrosion, Pt-doped coating exhibit a pattern of small PtAl2 phase. Compared with sample without Pt, the pattern of NiAl phase was higher and Ni3Al phase was lower for the sample after pre-oxidation Pt-modification coating, as well as the sample with Pt-modification. It clearly indicates that Pt can inhibit the transition from NiAl phase to Ni3Al phase.
Figure 5. The XRD results of the simple after hot corrosion in NaCl for 120 h.
Download figure:
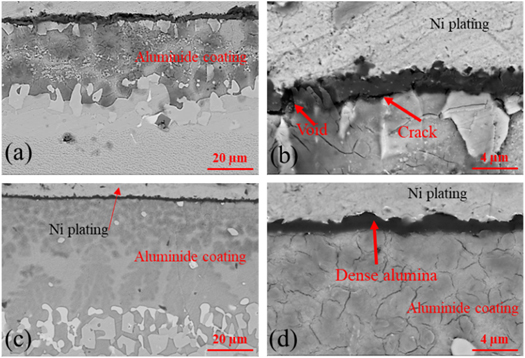
Standard image High-resolution imageThe SEM studies of the oxide scales on the as-received alumina coating in figure 6 show that the thickness of the oxide scale on aluminide coating without any treatment is relative uniform. But the oxide scale on the aluminide coating with Pt-modification presents an undulating structure with different thickness. The oxide scale on the surface of the alumina coating is not pure alumina, but mix with some oxides in it, as shown in the lager magnification (figures 6(b) and (d)).
Figure 6. The cross section SEM results of the sample without any treatments (a) and Pt modification (b) after oxidation in air for 120 h and corresponding magnification images (c) and (d).
Download figure:
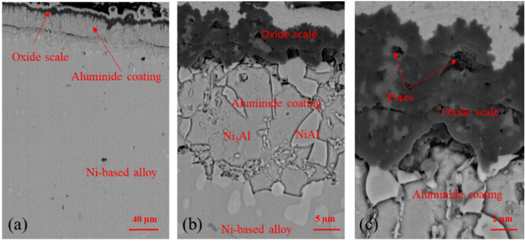
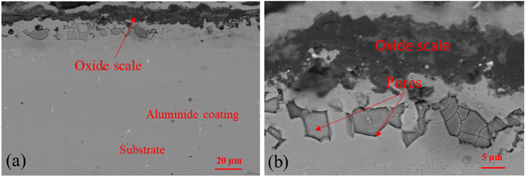
Standard image High-resolution imageThe cross sections of the aluminide coatings after corrosion with NaCl at 1050 °C for 120 h are shown in figure 7 with different magnification. Clearly, the oxide scale on the surface of the aluminide coating shows different thickness. There are many pores and other mixed oxides in this oxide scale. The aluminide coating mainly consists of two phases, Ni3Al and NiAl. As we know, during the high temperature corrosion, aluminide coating will form alumina scale on the surface, so that the some parts of NiAl phases will transform to the Ni3Al phases. It worth to mention, the pores also formed on the scale/coating interface. It means that the adherence of the oxide scale was not good. The SEM results of the cross section of the aluminide coating with pre-oxidation are shown in figure 8. The thickness of oxide layer fluctuates greatly that means oxide scale occurred spallation during oxidation, and this result fits the weight change. We also can find some small pores in the oxide scale, but the size is smaller than that in the oxide scale on the sample without any treatment.
Figure 7. Cross section SEM image with different magnification of the aluminide coating after oxidation with NaCl salt.
Download figure:
Standard image High-resolution imageFigure 8. Cross section SEM image with different magnification of the aluminide coating with pre-oxidation after oxidation with NaCl salt.
Download figure:
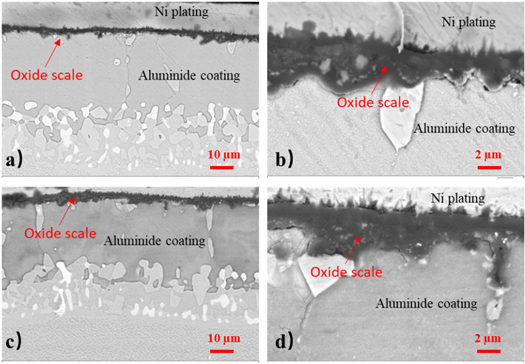
Standard image High-resolution imageFigure 9 shows the SEM cross section microstructures of the different magnifications of the Pt-modification at 1050 °C in air covered by NaCl after 120 h corrosion, as well as the sample with Pt-modification and pre-oxidation. The microstructures of the cross section show that some of holes and cracks appeared in the oxide scale of the Pt-modified aluminide coating after hot corrosion, and slight internal oxidation occurred. Combined with the Pt-modified aluminide coating, the Pt-modified and pre-oxidation aluminide coating covered by NaCl generat few pores and cracks at the interface, and a dense oxide scale is formed. The thickness of the oxides scale on the Pt-modified and Pt-modified pre-oxidation aluminide coating are about 2 μm and 1 μm, respectively.
Figure 9. Cross section SEM image of the Pt-modification aluminide coating with (a) and without (c) pre-oxidation and corresponding larger magnification image (b) and (d).
Download figure:
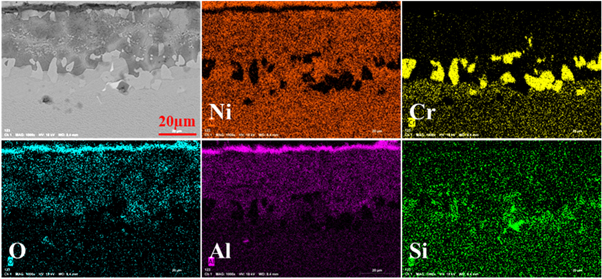
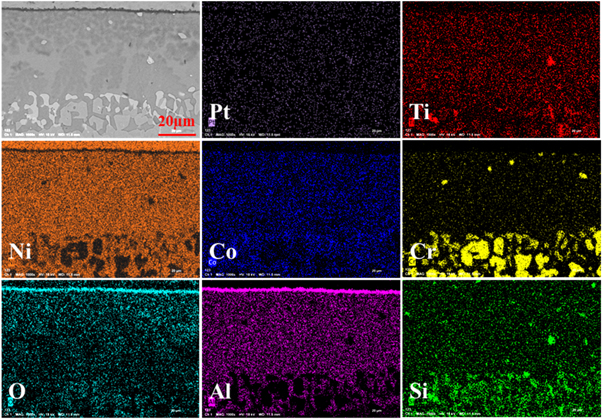
Standard image High-resolution imageFigure 10 is a cross-sectional EPMA result of the coating with Pt modification after 120 h corrosion in NaCl-salt environment. From the element diagram, it can be seen that the distribution of each element is uniform. The results show that the Cr element is enriched in the aluminide coating near the Ni-based substrate, and the oxide layer is the alumina scale again that can proved from the distribution of Al and O elements. There is a certain overlap between Cr and Si, which explains that there is really Cr3Si phase. At the same time, the matrix and the coating contain a large amount of Ni, indicating that Ni diffuses from the matrix into the coating and combines with Al to form a Ni-Al compound. This element diffusion can improve the interface bonding strength of the coating, from the element distribution of Al and Si. About the EPMA results of the sample with Pt-modification and pre-oxidation after 120 h corrosion is shown in figure 11. All the elements present a uniform distribution. Due to the concentration of Pt was quit low (maybe lower than 0.1%), the Pt cannot find in this sample. Just like the former results, there is also an alumina scale on the surface of the aluminide coating. The Cr3Si also presents in the aluminide coating near the Ni-base alloy substrate.
Figure 10. EPMA results of the aluminide coating with Pt modification after hot corrosion for 120 h (a) and the corresponding elemental distribution of Ni, Cr, O, Al, and Si.
Download figure:
Standard image High-resolution imageFigure 11. EPMA results of the aluminide coating with Pt modification and pre-oxidation after hot corrosion for 120 h (a) and the corresponding elemental distribution of Pt, Ti, Ni, Co, Cr, O, Al, and Si.
Download figure:
Standard image High-resolution image4. Discussion
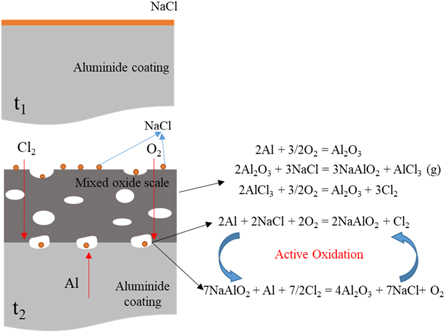
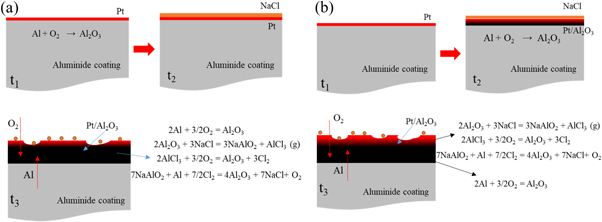
As we know, due to the alumina scale formed on the surface, the aluminide coating present a good high temperature corrosion resistance. But in the salt environment with NaCl, the alumina shows a different corrosion behavior with different pre-treatment in the air at 1050 °C. Based on the previous studies [21–24], the properties of the oxide layer, especially the bonding property, are affected by the element content in the coating and the oxidation environment. In this study, we focus on the corrosion behavior of the aluminide coating with NaCl salt. Different pre-treatments result in different corrosion behaviors, and the corrosion mechanisms are shown in figures 12–14. For the sample without any treatments, the alumina scale formed on the surface of the aluminide coating. At the same time the salt NaCl reacted with the aluminide coating and alumina scale formed mixed oxides on the coating surface, as shown in figure 12. During the hot corrosion process, the salt NaCl reacted with aluminide coating and formed mixed oxides just like blew [25]:
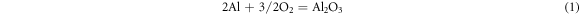
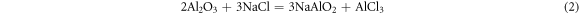
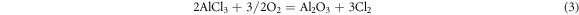
Figure 12. Schematic image of the sample without treatment oxidized in the NaCl environment at 1050 °C.
Download figure:
Standard image High-resolution imageFigure 13. Schematic image of the sample with pre-oxidation oxidized in the NaCl environment at 1050 °C.
Download figure:
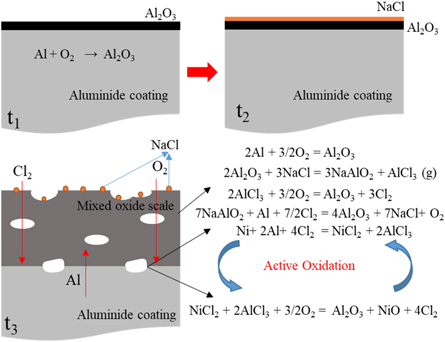
Standard image High-resolution imageFigure 14. Schematic image of the Pt-modification samples with (a) and without pre-oxidation (b) oxidized in the NaCl environment at 1050 °C.
Download figure:
Standard image High-resolution imageOn the scale/coating interface, the Cl2 will diffuses to the oxide/coating interface and results to [26]
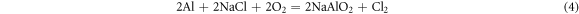
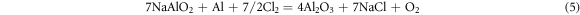
It means that during the hot corrosion, the alumina formed and then reacted with the NaCl salt to form the NaAlO2 and AlCl3 compounds on the surface. But at the 1050 °C,
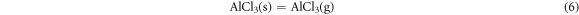
When the AlCl3 phases evaporate, they leaver holes on the surface of the oxide scale that fit the SEM results in figure 3(b). During the hot corrosion process, the oxide scale/coating interface initiates an active reaction, and chlorine gas acts as a catalyst to continuously promote the formation of alumina and pores at the interface. This also means that the adhesion of the oxide layer is poor and occurs spallation during hot corrosion.
After pre-oxidation, the alumina scale first formed on the surface of the aluminide coating. During the corrosion process, the salt NaCl reacted with alumina and formed many pores on the surface. The corrosion mechanism similar with the as-received coating, which shown in figure 13. In the outer part of the oxide scale, three are some reactions same with reaction (1)–(3). On the interface, the Cl2 will diffuse to the oxide/coating interface and result to
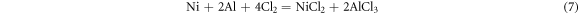
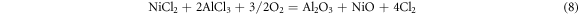
the chlorine also gas acts as a catalyst to continuously promote the formation of mixed oxides and pores at the interface. The oxide scale on the surface of the aluminide coating with pre-oxidation present poor adherence.
For the Pt-modification samples, the corrosion mechanism are different that shown in figure 14. In the out part of the alumina scale, there are no different reactions between the samples with and without Pt doping. After Pt doping, the alumina may grow fast on the aluminide coating, and prevent the Cl2 diffusing to the oxide scale/coating interface. It means that the Pt can inhibit the mixed oxides and pores formation on the scale/coating interface. On the alumina/coating interface, aluminum react with oxygen and form alumina. The alumina scale with Pt-modification present a better adherence than that on the sample without Pt. About the Cl2 diffusion characteristics and diffusion mechanism need further study.
Based on the previous research [27–29], the whisker alumina grains always formed at the initial stage and transform to the stable structure, α-Al2O3. However this process was depend on the corrosion temperature and time. Normally, these whisker alumina grains belong to the intermediate phases, θ-Al2O3, which formed on the surface of the aluminide coating at 900 °C–1000 °C. With the corrosion time increases, this kind of intermediate phase will transform to α-Al2O3 above 1000 °C. Based on the reaction (2) and (6), the alumina react with the NaCl salt and then form AlCl3 which can turn to a gaseous state at high temperature, and then the pores form. As we know, the formation of a large number of pores will weaken the strength of the oxide layer. At the same time, the coating contact area is increased, so that the corrosion rate is increased, as well as the growth rate of the oxide scale. The oxide scale on the aluminide coating with Pt-modification present a dense structure and the pores form on the outer part of the oxide scale, so that the mass gain of the sample with Pt in the figure 1 keeps decreasing.
5. Conclusions
The aluminide coating was produced on the surface of the Ni-based alloy and its hot corrosion behavior and mechanism were investigated at 1050 °C in air with NaCl salt. During corrosion process, an alumina scale formed on the surface of aluminide coating with different growth rate. The oxide scale of the sample without any treatments presented the highest growth rate. The oxide scale of the sample with Pt modification and pre-oxidation had the lowest growth rate. The pretreatments had an important effect on the hot corrosion mechanism of the aluminide coating on the Ni-based alloy. No matter pre oxidation or platinum modification, the alumina scale formed on the surface of the alumina layer, and then reacted with the NaCl salt to form the volatile substance AlCl3 at 1050 °C. For the peroxidation, the aluminum chloride formed and it resulted in the pores formation in the alumina scale, as well as the mixed oxides. The oxide scale occurred active oxidation and promoted the mixed oxides and pores formation on the oxide scale/coating interface. Compared to the sample with peroxidation, the sample with Pt-modification presented a better corrosion resistance. During the oxidation process, the alumina scale on the surface of the Pt-modification coating showed a dense structure without any pores. Pt can inhibit the mixed oxides and pores formation on the scale/coating interface in the aluminide coating.
Acknowledgments
The authors would like to acknowledge the Yunnan Province Science and Technology Major Project (Grant No. 2019ZE001) for funding this research.