Abstract
Photocatalysis is a promising technique for the production of hydrogen gas (H2), which has high energy capacity. Investigations into raising the quantum yield of H2 have considered the process itself and the compositions of the photocatalysts used. In particular, sensitization of semiconductors or their doping with metals has had a remarkable impact on the alternative energy industry. Dyes have great absorbance power under visible wavelengths, which overcomes a key limitation of TiO2 as a semiconductor. In this research, cyanine derivatives connected with Ag/TiO2 were characterized by XRD, SEM-EDX, TEM, and optical spectroscopy. The nanomaterials (48–88 nm) that were prepared had high crystallinity, and they were shifted to a region of sunlight radiation rich in photons, thereby enhancing the production of hydrogen. The improvement was more than by three-fold after 6 h. In addition, the combined light and ultrasound radiation yielded spectacular results, around six-fold of what was accomplished in the presence of light alone. Sonophotocatalysis has proven to be good for ensuring that particles do not aggregate during radiation and thus continue to produce abundant reactive oxidative species.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Photocatalysis decomposes water pollutants [1, 2] and water into their elements [3]. Concerning the decomposition of water, this technique overcomes a thermodynamically unfavourable process (ΔG° = 228.71 kJ mol−1) and allows solar energy to be converted easily into chemical energy, a desirable conversion that is of great interest in the energy sector. The most important component in photocatalysis is the semiconductor, and countless investigations of semiconductor materials have been conducted in efforts to achieve the maximum yield of hydrogen gas and provide a clean, alternative source that provides high energy capacity. TiO2 has attracted significant attention as a semiconductor, but it has the disadvantage of only being active under UV, which constitutes only 4% of sunlight [4]. In addition, the lifetimes of its photogenerated electrons and positive holes are short. As a result, metal loading of TiO2 is seen as vitally important to promote the quantum yield of hydrogen by providing a sink for the excited electrons, and the metals that are used include Pd, Pt, and Ag [5–7]. Noble metals, such as silver, can tailor the electronic structure of semiconductors by altering the band gap energy positively in the photocatalytic production of hydrogen. The extent of change depends on various factors, such as the precursors and the preparation method, and it was proved that the photocatalytic production of hydrogen over TiO2, whether as commercial powder (P25) or prepared from another Ti precursor, is promoted significantly by silver doping [8, 9]. In particular, silver, which was used in this research, has been investigated in numerous studies because of its low toxicity and high activity [10]. Nevertheless, such studies have not yet solved the need of the clean fuel (H2) industry for highly-efficient, economically-feasible materials. In parallel research, many organic compounds have been investigated for applications in the production of hydrogen. Such compounds should absorb visible light efficiently in order to maximize the benefit provided by sunlight [11]. Sensitization of semiconductors is a promising strategy to obtain large-scale absorption in the visible region, thereby enhancing the photocatalytic reactions of semiconductors [12]. In particular, very encouraging results have been achieved by using dye-sensitized TiO2 for the photocatalytic production of hydrogen [13–15]. The composition of the dye influences the ease with which the photogenerated electrons make the transition from the excited level of the dye to the semiconductor [16, 17] since the excited energy level of the dye molecule usually is higher than the conduction band (CB) of TiO2 [18]. For example, Eosin Y adsorbed on CuO/TiO2 demonstrated a significant enhancement in the rate of hydrogen production (∼5.1%) compared to that before sensitizing [19]. Additional improvement might be achieved by modifying the structures of some dyes, potentially enhancing the absorption of visible light or the quantum yield and overcoming of the agglomeration of particles through sensitization. The latter process usually occurs because of the existence of different numbers of locations and linkages in such chromophores. Cyanine is a low-cost, high-stability photosensitizer, and many of its derivatives have been prepared in many studies by controlling of their linkages function, resulting in dyes that bind well with TiO2. The heptamethine cyanine dyes, which absorb in the near infrared, have been applied, and the results have been published previously in the context of a TiO2 photocatalyst doped with Cu (cyanine-sensitized Cu/TiO2) [20]. The activity of these chromophoric compounds results from their high photosensitization under UV–vis radiation; this promotes resonance, which in turn increases the electron currents. In such compounds, excitation occurs from the ground state to the excited state and then to the triplet excited state by intersystem crossing. Then, charge transfers from the triplet excited state lead to the generation of singlet oxygen species, which is responsible for the effectiveness of the dyes in sensitizing photocatalysts. Cyanine has been studied extensively in the field of photography, but it has had very limited use as a photosensitiser in the hydrogen energy sector.
Meanwhile, the conversion rate also may be enhanced through alteration of the photoreactor by synergizing light radiation with ultrasound in several reactions [21–23]. Ultrasound radiation has been shown to have a clear effect in maintaining the particle size of the photocatalyst and boosting the formation of photogenerated charge carriers.
In this study, the effect of dye-sensitizing with different derivatives of cyanine was investigated on silver loaded on TiO2 prepared by a simple method. The effect of the structure of these derivatives on the photocatalytic production of hydrogen also was investigated. In addition, sonophotocatalysis in the presence of methanol as a sacrificial reagent was proven to have a positive effect on the production of hydrogen through the oxidation of water by reactive oxygen species.
2. Experimental
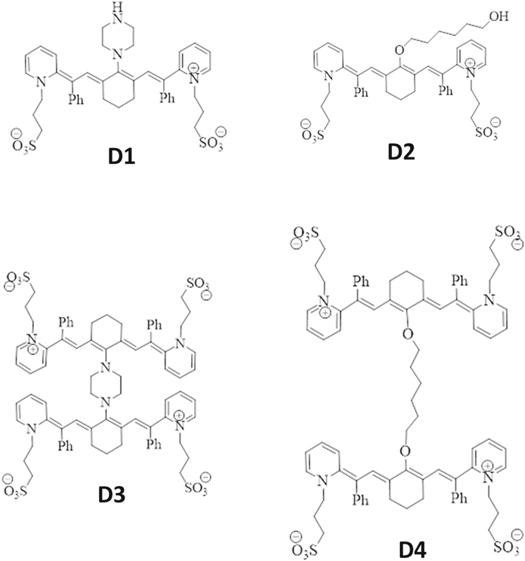
All chemicals in this work were used without additional purification. The four derivatives of cyanine dyes (D), identified as D1, D2, D3, and D4, were prepared as described in a previously published work [20]. Figure 1 shows the structures of these derivatives, which determined in the previous work by elemental, melting point, and infrared analyses.
Figure 1. Molecular structures of the cyanine derivatives D1, D2, D3, and D4.
Download figure:
Standard image High-resolution image2.1. Synthesis of the dyes sensitized Ag/TiO2
For the synthesis of 1% Ag/TiO2, an appropriate amount of AgNO3 solution was added gradually to TiO2 powder (Degussa P25) according to the witness-impregnation method. After calcination of 1% Ag/TiO2 at 500 °C (two hours), 0.5 g of this sample was incubated with each cyanine derivative solution for 24 h with stirring and in a dark place to ensure the complete adsorption of the dye on the surface of the Ag-loaded TiO2. This mixture was centrifuged, washed with ethanol, washed with deionized water several times, and dried overnight.
2.2. Characterization
All of the dye-sensitized Ag/TiO2 samples that were prepared were characterized by powder x-ray diffraction (XRD) (Philips, CuKα at 40 kV and 30 mA), and particle size was calculated from these data using the Scherrer equation. Scanning Electron Microscope (SEM) analysis (30 kV and 7500 × 10,000 and 15,000 magnification of the original specimen size) and Transmission Electron microscope analysis (TEM) (JEOL JEM 1400) were used to analyze the morphology of the nanostructure materials. Also, the confirmation of the elemental analysis (C, Ag, Ti, and O) and their uniform distribution of sensitized photocatalysts were investigated by Energy Dispersive x-ray spectrometry (EDX) (Philips XL 30CP, USA). The band gaps of the catalysts that were prepared were calculated from the data of UV-visible spectrometry (JascoV-770) in the spectral range of 200–1000 nm).
2.3. Study of the sonoactivity and photoactivity of dye sensitized Ag/TiO2
The photocatalytic and sonophotocatalytic activities of the samples were assayed by measuring the hydrogen evolved over an interval from a mixture of a 0.1 g sample dispersed in 100 ml of water and 0.2 ml of methanol irradiated by a 500 W Xe lamp. Gas chromatography (Agilent GC 78900A) was used to detect the hydrogen gas precisely with a thermal conductivity detector (TCD) using argon as a carrier gas. In the sonophotocatalytic evaluation, a Ti probe was immersed in the photoreactor mixture to transfer the ultrasound waves from the generator. This source of irradiation was accompanied simultaneously by photoirradiation.
3. Results and discussion
3.1. Characterization
3.1.1. XRD analysis
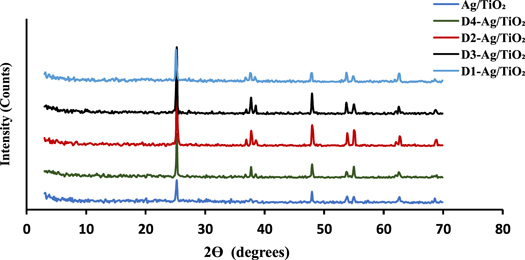
Figure 2 shows the XRD patterns of all of the samples. Sensitizing of Ag/TiO2 with different derivatives of cyanine did not change the positions of the peak, indicating no change in the composition of Ag/TiO2. In addition, the sharp peaks evident in all of the patterns were clear signs of crystallinity, and they were identical to the standard XRD of TiO2 (JCPDS NO. 21-1272). Because of the small quantities and the high dispersion (as discussed later in the EDX section) of incorporated silver and cyanine derivatives, either there were no corresponding peaks in the XRD patterns or they may have been under the detection limit of XRD analysis [24, 25].
Figure 2. XRD patterns of Ag/TiO2 and dye-sensitized Ag/TiO2.
Download figure:
Standard image High-resolution imageXRD patterns are a good way to determine the particle sizes of crystalline materials through the Scherrer equation. Table 1 shows that all samples, pure and dye-sensitized, exhibited nanostructures; however, the cyanine derivative compounds resulted in larger crystalline size due to their large organic structures. Dye sensitization increased the agglomeration of the particles depending on the composition, structure, and geometry of these dyes [26, 27]. These derivatives have different binding positions have a role in the agglomeration process. D1-Ag/TiO2 had the smallest particle sizes, while the size of others were close. had approximate sizes. This can be interpreted based on the extent of the anchoring modes of the adsorption of dye molecules on surface of the TiO2. For example, the existence of a hydrocarbon chain in the D4 dye restricted the agglomeration process in spite of the polydentate binding positions, resulting in particles sizes smaller than D2 and D3 [28].
Table 1. Crystalline sizes of the Ag/TiO2 photocatalysts that were prepared based on XRD data.
| Prepared photocatalyst | Crystalline size, nm |
|---|---|
| Ag/TiO2 | 48.4 |
| D1-Ag/TiO2 | 66.9 |
| D2-Ag/TiO2 | 87.9 |
| D3-Ag/TiO2 | 86.2 |
| D4-Ag/TiO2 | 81.8 |
3.1.2. Topographical analysis of the sensitized Ag/TiO2
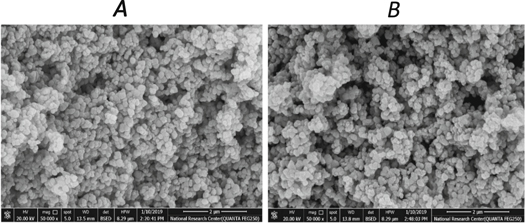
SEM analysis of the photocatalysts before and after sensitizing demonstrated that the nanostructure and the sensitization of Ag/TiO2 did not alter its morphology (figure 3). The agglomeration of the particles might be seen slightly in the case of the sensitized photocatalyst (B). This process, which occurred during the sensitization, gave a larger range of irregular spherical nanostructure (84.03–149.3 nm), while Ag/TiO2 had 90.05–115.9 nm [29]. The inability to notice an obvious difference before and after sensitisation could be due to the small quantity dopants, whether silver or the dyes.
Figure 3. SEM images of Ag/TiO2: (A) before sensitizing by D4 and (B) after sensitizing by D4.
Download figure:
Standard image High-resolution imageElemental analysis with EDS mapping spectra proved the presence of Ti, O, Ag, and carbon as the main elements (figure 4(A)), with the metal and dye demonstrating homogeneous distributions on the surface of the TiO2 (figure 4(B)). These results support the successful preparation of these photocatalysts.
Figure 4. (A) Elemental analysis of sensitized D4-Ag/TiO2; (B) EDX mapping of sensitized D4-Ag/TiO2.
Download figure:
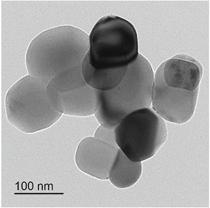
Standard image High-resolution imageThe TEM image in figure 5 illustrates the near-spherical nanostructure of the D4-Ag/TiO2 crystals; the dark spots may be silver particles, which have a high electronic density. There was no considerable difference in the shape of structure before and after sensitizing with low-electronic density organic compounds. In addition, the uniform distribution of silver over TiO2 through the electronic density confirmed the EDS mapping spectrum. Therefore, we can say that there was no silver cluster on the surface [27]. As measured by N2 adsorption, the Brunauer–Emmett–Teller (BET) surface areas of Ag/TiO2 and D4-Ag/TiO2 were 16.7 and 17.7 m2 g−1, respectively. The slight difference in these values may be among the aspects by which sensitizing can enhance the photoactivity of a photocatalyst without changing its other good properties.
Figure 5. TEM image of D4-Ag/TiO2.
Download figure:
Standard image High-resolution image3.1.3. Optical properties
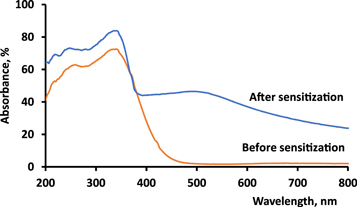
Clear enhancement of the optical properties of Ag/TiO2 by sensitizing with the derivative D4 is illustrated in figure 6. Loading of silver is known to shift the absorbance of TiO2 to the visible region, and this shift is the main goal in the photocatalysis field. However, this red shift is still shallow up to about 450 nm, as indicated elsewhere [30, 31]. In contrast, the cyanine derivatives with highly conjugated double bonds significantly promoted the optical activity of the photocatalysts throughout the visible region, increasing it by 40%. In addition, the optical spectrum of the sensitized photocatalysts confirmed the existence of the adsorbed dye compared to Ag/TiO2 alone. The broad band of sensitised sample indicated the uninform distribution of the dye on the surface of the TiO2. The band gap energy of the samples were calculated using the Tauc equation, which represents the relationship between (αhν)^2 and hν (eV) by considering that these samples had a direct band gap [32]. The estimated Eg values of Ag/TiO2 and D4-Ag/TiO2 were 2.65 and 2.68 eV, respectively.
Figure 6. Optical spectra of Ag/TiO2 and D4-Ag/TiO2.
Download figure:
Standard image High-resolution image3.2. Hydrogen production evaluation with dye-sensitized Ag/TiO2
3.2.1. Photocatalytic activity study
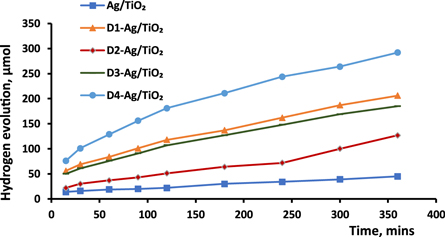
The effect of cyanine dyes on the activity of Ag/TiO2 was clearly confirmed, regardless of the specific cyanine derivative used; this is illustrated in figure 7. As in the case of Cu/TiO2 in our previous work [20], the efficiency of hydrogen evolution was elevated by the dyes, with the order of activity as follows: D4 > D1 > D3 > D2. The activity of D4-Ag/TiO2 (5.98 μmol h−1.g) was greater than that of Ag/TiO2 (0.88 μmol h−1.g). Two conclusions can be reached from this order. First, conjugation with a photocatalyst increases passing between the two nitrogen atoms of pyridine and pyridinium rings that are connected by a methine bridge, causing bathochromic shift, and increasing photosensitization. Proceeding from this principle, these compounds were used as a positive aid in photocatalysis using TiO2 doped with silver [33].
Figure 7. Photocatalytic hydrogen production over Ag/TiO2 before and after sensitization by cyanine derivatives.
Download figure:
Standard image High-resolution imageSecond, the greater effect of D1 relative to D3 and D2 might be ascribed to its having the smallest particle size, as evidenced in the XRD data, despite the fact that D3 and D2 have higher conjugation systems. When considering dyes other than D1, there is a reasonable order (D4 > D3 > D2) consistent with increasing particle size and decreasing delocalization of the expanded π-system. In addition, it was known that cyanine molecules have an ability to aggregate strongly between their molecules in several ways since the chelating sites of these dyes have a significant role in this phenomenon [34, 35]. D1-Ag/TiO2 has a smallest particle size, which is attributed to the two linkage positions with TiO2, so the probability of aggregation is low, unlike D2-Ag/TiO2, which has the largest particle size because of the –OH function group. Therefore, this aggregation impacts on the particle size and is consistent with the results of the photocatalytic activity of these sensitised samples.
Another finding from this study is that the yield of hydrogen gas over Ag/TiO2 (after 6 h) was notably higher, i.e., 3.5 to 5 times higher, than that over Cu/TiO2 before and after D4 sensitizing, respectively. Connecting a metal with a semiconductor results in a Schottky barrier, which plays a role in trapping excited electrons. A higher barrier makes the metal act as an electron sink, diminishing the recombination of photogenerated charge carriers. The elevated performance of Ag/TiO2 suggests that the difference of work functions (Schottky barrier) between silver and TiO2 might be higher than that between copper and TiO2. Notably, size, shape, kind of metals, and preparation method are among the factors considered important in the state of the Schottky barrier and, in turn, on the photocatalytic reactions [36, 37].
3.2.2. Sonophotocatalytic activity study
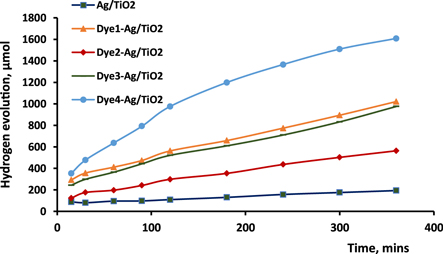
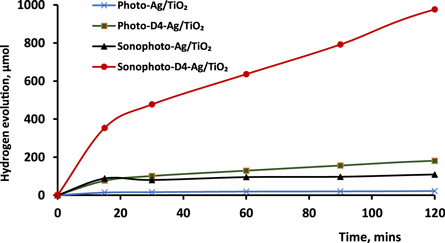
In terms of sonophotocatalytic activity, the photocatalysts ranked in an order similar to that for photocatalysis, as shown in figure 8. The highest rate of hydrogen production was obtained again over D4-Ag/TiO2. Overall, a considerable synergistic effect on the rate of hydrogen evolution was apparent in both processes (figure 9), with the evolution of hydrogen gas being increased by around a factor of six. The hybrid action of light (Xe lamp irradiation) and ultrasound may boost the generation of reactive radical species, which are the main pillars in advanced oxidation processes (AOP), such as hydrogen production [38], as explained in the mechanism section.
Figure 8. Sonophotocatalytic hydrogen production over Ag/TiO2 before and after sensitization by cyanine derivatives.
Download figure:
Standard image High-resolution imageFigure 9. Comparison between the photocatalytic and sonophotocatalytic hydrogen production activities of Ag/TiO2 before and after sensitization.
Download figure:
Standard image High-resolution image3.2.3. Reusability of the sensitised photocatalyst
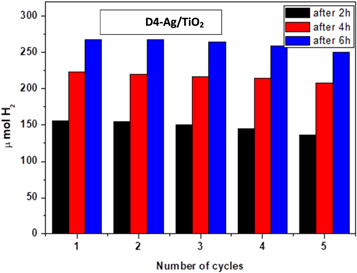
The photostability of the D4-Ag/TiO2 as a model of the rest of the prepared photocatalysts was tested through 5 cycles. Figure 10 proved the high stability of such catalyst, since the decrease in the amount of hydrogen released was very low at each use whether after 2, 4, or 6 h. The reason of a slight decrease in photocatalytic rate after the six cycles may attributed to the consumption of sacrificial reagent. Cyanine derivatives as well as Ag/TiO2 were characterised by their high thermal stability and photostability [31].
Figure 10. Reusability test of D4-Ag/TiO2 after 5 cycles.
Download figure:
Standard image High-resolution image3.2.4. The suggested mechanism of enhancement
The photocatalytic production of a meaningful quantity of hydrogen is plagued by many obstacles, including the absorption limitation, migration of photogenerated charge carriers to the surface, recombination or trapping of these carriers, the back reaction of element split from water splitting, and more. Dye sensitization of Ag-doped TiO2 nanostructured particles is valuable in to overcoming these obstacles in order to provide a high quantum yield of hydrogen energy. Because the migration of charges from the bulk to the surface must be smooth throughout the reaction, ultrasound waves are further beneficial for inhibiting the aggregation of the particles.
It is characteristic of dyes to have high absorption coefficients, which achieve the task of harvesting most of the light rather than leaving that to the semiconductors. Accordingly, the suggested mechanism by which dyes enhance these photocatalysts can be summarized in steps represented by the following equations [39–42]:
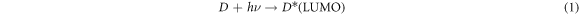
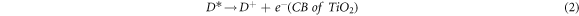
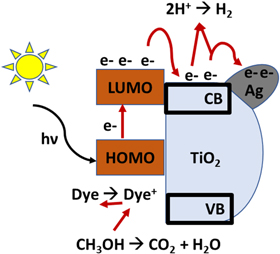
Electrons are excited from the ground level (HOMO) of the dye molecule to the excited level (LUMO), which was higher than the CB of TiO2. Then, these photogenerated electrons transfer from the excited level of dye to the CB of TiO2 easily. Additionally, conjugated system for these dyes might inhibit the charges recombination between dyes molecules and the support.
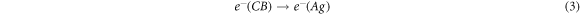
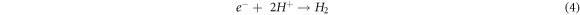
Generally, metals act as active centres for hydrogen production since it was found the long lifetime of the photogerated electrons increases in the presence of metal [37]. So, the electrons prefer to transfer to the loaded metal particles; according to EDX mapping, these are distributed uniformly on the surface of the TiO2.
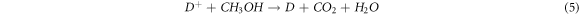
Methanol as an electron donor was used to ensure the continuous hydrogen production process. According to that, oxidization of the methanol molecules by D+ obstructs the back reaction (recombination between the oxidized dye with photoexcited electrons), therefore potentially enhancing the rate of hydrogen production. These steps are presented more clearly in figure 11.
Figure 11. One of the suggested mechanisms of hydrogen production over dye-sensitised, metal-doped TiO2.
Download figure:
Standard image High-resolution image4. Conclusion
Photocatalytic hydrogen production was enhanced by using different derivatives of cyanine to fabricate new dye-sensitized Ag/TiO2. These compounds exhibited greater efficiency than Ag/TiO2 alone, with up to around three-fold improvement. Moreover, coupling of ultrasound waves with light radiation gave a further promising effect on the rate of hydrogen production, especially for D4-Ag/TiO2, which increased yield over that from photocatalysis alone by six-fold. These improvements were obtained using small quantities of dyes and without changing the morphology and composition of Ag/TiO2, yet still harvested more light energy efficiently.
Acknowledgments
Great thanks to the Deanship of Scientific Research for supporting this work financially (Grant number: 15SCI-3-3-0024).