Abstract
Aluminum is widely used as the discharge chamber structures of excimer lasers which are important high-power UV-band lasers. Corrosion behavior of aluminum in fluoride-containing discharge condition greatly affects the laser output by consuming fluoride medium and generating gases. This paper characterizes the structure, morphology and element distribution of the corrosion layer by scanning electron microscope analysis, electron probe micro analysis, and glow discharge mass spectrometry analysis. The aluminum was fluorinated mainly by pitting corrosion and did not generate a dense passivation layer. Elements aggregated at the surface with different degrees: the elements of IA, IIA and VIB groups within 2.4 μm from the surface aggregated over 10–1000 times, but Fe, Ni and Si, C elements aggregated only a few times. The serious element diffusion zone was about 5 μm, and some elements diffused tens of microns. Considering the stability to fluorine medium and the ability to improve thermodynamic stability, the Ni element should be a good choice for anticorrosive layer. The aggregation of elements like Si greatly increases the possibility to form harmful gases, and their content should be strictly controlled. The physical models of passivation, operation, and air exposure of the excimer laser are established. The passivation effect largely depends on the surface content and state of harmful elements, but the long-term chemical stability in the operation is related to the surface quality, internal content of harmful elements and diffusion rate.
Export citation and abstract BibTeX RIS
1. Introduction
Aluminum is an important structure material given its the excellent comprehensive performance and reasonable manufacturing cost [1, 2]. It is widely used for the discharge chamber wall and internal structures of the fluoride excimer lasers, which are important high-power UV-band lasers used in semiconductor, automotive, medical, scientific research and national defense [3, 4]. However, the corrosive working media in the chamber—fluoride (in molecular and F− forms [5]) will corrode aluminum, and its surface contamination reduces the effective fluoride [6, 7] and generate gases [8] that damage the output energy and stability of the laser [9–11].
Passivation by gases with high fluorine content is used to generate clean and stable fluorinated layer before the laser operation [12]. However, the aluminum is an active material with remarkable tendency to react with environment. It is easily covered with a thin oxide layer [13, 14] during manufacture process, and exposed to the air again for the component replacement of the discharge chamber. According to the research on metal with the fluoride medium, the corrosion generally includes fluorination of elements and penetration of free fluoride [15, 16]. The material is passivated by generating fluoride layer [17, 18] and the F–O substitution reaction occurs during air exposure [19]. Trace elements, which are inevitable [20, 21], play an important role in the corrosion [22, 23]. Al and Cr elements generate a protective layer in passivation condition and are considered to improve the corrosion resistance [24–26]. Ni element improves the thermodynamic stability of materials [22] to increase the corrosion resistance. Si and C elements are commonly used to improve the deoxidization of materials. However, in the discharge chamber, elements like Si, C and H fluorinate to generate harmful gases SiF4, CF4 and HF which reduce the output energy of ArF excimer laser by 10% when the content reaches 10 ppm level [27]. Other chemical active elements consume the effective fluorine. Researchers and manufacturers proposed heat treatment and coating process to reduce corrosion by experimental comparison [28], but the corrosion behavior, its influence factors, and the effect of the trace elements, still lack systematic research and quantitative analysis. The corrosion behavior of aluminum requires further study.
This paper studied the corrosion behavior of trace elements containing aluminum in the high-pressure fluorine-containing discharge chamber of excimer laser. The structure transformation and its influence factors were analyzed, the distribution law of the main and trace elements was revealed, the physical models of passivation, operation, and air exposure of the excimer laser were established. It will provide theoretical and data support for material selection, anti-corrosion, element control of aluminum used in corrosive environment.
2. Experiment
Commercial purity aluminum was used. The ω(Al) is ≥99.50%, the ω(Si) and ω(Fe) was ≤0.1% and ≤0.2%. The mass percentage content of other impurity elements was below 0.05%, respectively. The sample was cut into 20 × 20 × 2.5 mm3 and embedded into the structures in a self-developed ArF excimer laser with copper electrodes before passivation. The upper surface of the sample was exposed to the chamber environment. The passivating pressure was 0.1 MPa, with 0.4% F2 as passivating gas and He as buffer gas. After two days of passivation, the aluminum worked in the chamber for another six months. The working pressure was 0.4 MPa, with 0.1% F2 and Ar as working gas, and Ne as buffer gas.
To limit contamination, the samples used for surface study was analyzed directly without cutting or cleaning. The sample used for sub-surface analysis was carried on a 30 min water ultrasonic cleaning to destroy the fluorite surface layer. Scanning electron microscope analysis (SEM, Hitachi, SU8010, Japan) was used to characterize the morphology. Electron probe micro analysis (EPMA, Shimadzu, 1720, Japan) was used for the surface composition study. To reduce the analysis deviation caused by micro-distribution unevenness, mapping analysis was used. glow discharge mass spectrometry analysis (GDMS, Thermo Fisher, Element GD, USA) with Ar+ ion sputtering was used to study the element distribution in depth, with an 8 mm scanning diameter.
3. Result and discussion
3.1. Morphology and structure
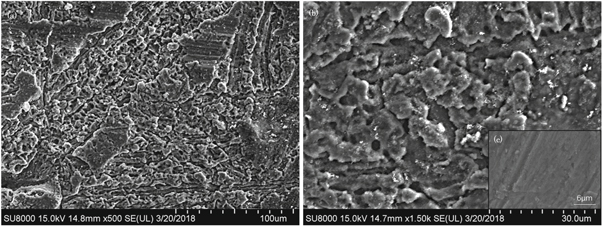
Figure 1 shows the typical morphologies of the corroded aluminum. The aluminum was fluorinated mainly by pitting corrosion and did not generate a dense passivation layer. It owned two different features, the dense zone and the loose zone with 2–4 μm pores or grooves (figure 1(b)). Compared with the morphology of only some scratches caused by grinding before corrosion(figure 1(c)), the sample was severely corroded.
Figure 1. Surface morphologies of the corroded aluminum (a), its loose zone (b) and the morphology before corrosion (c).
Download figure:
Standard image High-resolution imageThe corrosion was apparently uneven. In the dense zone, the average ω(Al: F: Cu) was 87.22: 9.13: 2.92. Na, K, Ca elements were non-uniformly distributed with the average mass content as 0.20%, 0.17% and 0.36%. In the loose zone, the average ω(Al: F: Cu) was 81.23: 14.46: 3.18, and the impurity elements were non-uniformly distributed. The fluorination degree of the loose zone was higher. The corrosion increased the surface area of the loose zone, which in turn intensified the fluoride and F–O alternative reaction.
Some 1–2 μm particles with the average ω(Al: F: Cu: Zn) = 9.16: 22.30: 58.13: 10.41 were found on the surface of the aluminum. The sputtered particles from the electrode could not be completely removed by sampling, and the detected content of Cu, Zn and F elements might be higher than the real value.
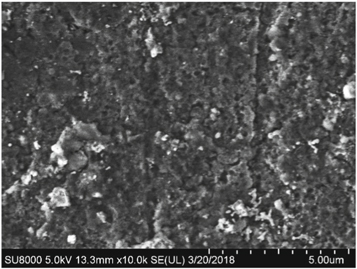
Figure 2 shows the sub-surface morphology of the sample whose surface has been destroyed by ultrasonic cleaning. Compared to the surface layer, the number of pores decreased obviously and the pore size decreased to 0.5–1 μm. The average ω(Al: F: Cu: Na: K: Ca) was 91.08: 5.90: 2.24: 0.20: 0.49: 0.10. The content of F and impurity elements on the sub-surface layer was low, and the influence of fluorination corrosion on this layer was reduced.
Figure 2. Sub-surface morphology of the corroded aluminum.
Download figure:
Standard image High-resolution image3.2. Trace elements distribution
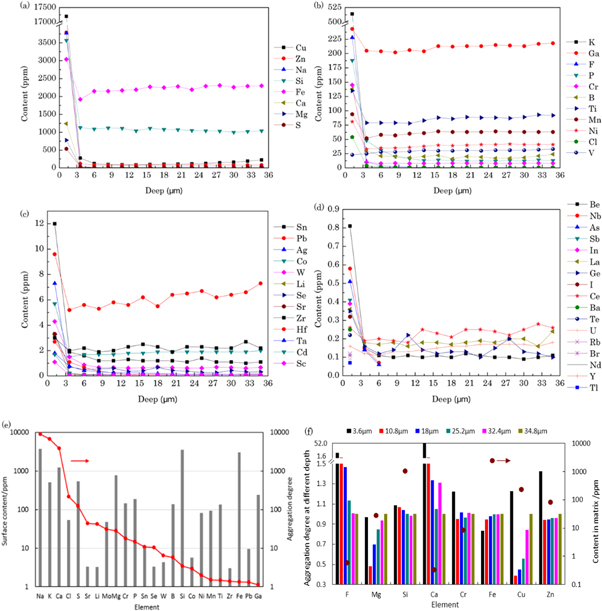
Figure 3 shows the trace elements distribution of aluminum. Almost all elements detected show a tendency to aggregate near the surface. The content of F element was decreasing from the surface to the depth of 34.8 μm, conformed to the characteristics of external diffusion. The content of Cu, Zn, Na, Si, Fe and Ca elements within 2.4 μm from the surface were over 1000 ppm. Most elements did not reach such high content, but also highly aggregated. The chemically active metal elements of the IA, IIA and VIB groups were representative. The alkali metal and alkaline earth metal elements Na, K, Ca, Mg within 2.4 μm from the surface were aggregated two to three orders of magnitude compared to the matrix. Cr and Mo elements were aggregated by one order of magnitude. They would greatly consume the fluoride medium and affect the discharge condition of the laser. The Ni element with low solubility and high corrosion resistance owned relatively low aggregation. The stability to fluorine made it a good choice of anticorrosive layer.
Figure 3. Trace elements distribution of the corroded aluminum (a) surface content >600 ppm (b) surface content between 20–600 ppm (c) surface content between 1–20 ppm (d) surface content <1 ppm (e) surface content and aggregation degree (f) aggregation degree (the surface content/the matrix content) and matrix content of the trace elements.
Download figure:
Standard image High-resolution imageAll elements diffused within 5 μm, but some elements like Cu, Fe and Mg diffused tens of microns. Fe, Mg and Cu elements decreased and then rose to the matrix content within the range. The lowest content of them appeared at 3.6, 8.4 and 13.2 μm, respectively. An important factor influenced the aggregation was the solubility of elements in aluminum. The solubility of Cu, Fe, Mg, Zn element in aluminum material was 0.18%(227 °C), <0.01%(427 °C), 1.9%(27 °C) and 5.6%(125 °C). Fe element mainly exists in precipitation phase and is difficult to diffuse compared with the dissolved elements. Therefore, its content on the surface was 1.5 times that of the matrix, and the diffusion depth was relatively low, despite the high chemical activity and high content. Although the surface content was affected by the residual electrode particles, the reduced and then elevated trend still indicated the difficulty of diffusion. The Mg element demonstrated high solubility and activity, and it aggregated highly on the surface despite the low matrix content. Products of these metal elements were in solid state, so they might induce the isolation between the fluorine and impurity elements. They affected the structure and corrosion rate of the material but did not damage the output energy.
However, the fluorination products of certain elements are gases. The surface aggregation degree of S, P, C, B and Si elements was 124, 15, 9, 6 and 3.4 times, respectively. Fluoride of these elements would escape from the surface layer, so their surface content was relatively reduced. The solubility of Si element in aluminum material was 0.01%(227 °C), it mainly exists in the precipitation state. The 1050 ppm matrix content and 3570 ppm surface content showed serious aggregation. As 10 ppm SiF4 would reduce the output energy of ArF excimer laser by 10%, although the existing fluoride layer exerted a certain isolation effect, the Si content in the material should be strictly controlled.
The content of Al, C, O, N and other impurity elements at different depth ranges is presented in table 1. The depth of the element diffusion zone was below the detection interval of quantitative analysis (2.4 μm) as the deeper content was basically the same. The surface and the newly exposed layer in the process would adsorb and react with O and C elements, so the result reflected the carbon oxidation of the material with the air. The contaminated surface layer reacted with the fluorine medium to generate O2, CF4 and other impurity gases. According to the relevant research, the fluorine oxygen compounds is relatively stable. The F–O substitution reaction may not finish in a short time but may continue during laser operation process and become a harmful factor for the output energy and stability. This study analyzes the influence of element aggregation and solubility, but the elements are not independent, so further research on the interaction and other factors is needed. A quantitative study with finer resolution is important to show accurate element distribution.
Table 1. Contents of Al, C, O, N and other impurity elements at different depth ranges/ω, %.
| Depth/μm | Al | O | C | N | Others |
|---|---|---|---|---|---|
| 0.0–2.4 | 86.06 | 7.51 | 2.57 | 0.28 | 3.59 |
| 2.4–4.8 | 95.95 | 2.97 | 0.58 | 0.10 | 0.41 |
| 33.6–36 | 96.29 | 2.91 | 0.28 | 0.11 | 0.42 |
3.3. Analysis and discussion
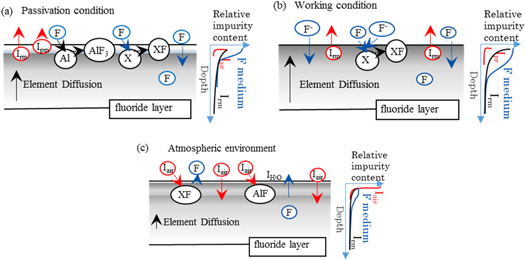
The corrosion behavior and main influence factors of the aluminum are clear. During passivation, the surface composition especially the hydrocarbon oxide contamination is corroded chemically with dry fluorine gas, as H2O is fluorinated into HF and O2. The main restricting factor is the fluorination rate. The contaminants and harmful elements on the surface fluorinate to vaporize off the surface and the surface aluminum fluorinate to form AlFx or AlOxFy species [19]. The uphill diffusion of F element and the downhill diffusion of internal impurity elements occur. Excessive aggregation of harmful elements affect the passivation effect and the subsequent life of the aluminum. As depicted in figure 4(a), only when the surface contamination is eliminated by fluorination and the diffusion rate of the harmful elements is low can a stable fluoride surface with low harmful impurity be obtained. Therefore, the passivation time should be selected while comprehensively considering the contamination thickness, fluorination rate and element diffusion rate.
Figure 4. Corrosion behavior and element distribution of aluminum in each process (a) passivation process (b) operation process (c) Air exposure process Irm–Impurity in the raw material produce gaseous fluoride, Ipr–Impurity introduced in the preparation process produce gaseous fluoride, Iair–Impurity introduced by air exposure produce gaseous fluoride, X–elements which produce solid fluoride.
Download figure:
Standard image High-resolution imageDuring operation, the aluminum is not consumed like the copper electrodes but rather pitted by the fluoride medium slowly, consistent with the normal corrosion mode of metals by halogens. The micron scale corrosion depth suggests the contamination formed in the atmosphere had been destroyed. The internal impurity elements continually diffuse to the surface and become the main reactant, as showed in figure 4(b). Most fluorides are solid, but the fluorides of elements like Si, O, C are harmful gases for the laser output. According to element distribution result, the diffusion rate of the harmful elements should be higher than their fluorination rate. Therefore, the reaction is still controlled by fluorination rate rather than the decreasing diffusion rate. This can be proved by the constant gas generation rate measured by Sumitani A, when the electrodes are not consumed [19]. The increased rate when the electrodes are consuming [19] further proves the content of harmful elements at the interface greatly affect the gas generation.
The internal of the discharge chamber will be exposed to air during component replacement. The stable surface is destroyed by carbon oxidation reaction [29], as showed in figure 4(c). The reaction of C and O with the sample has electrochemical corrosion characteristics due to humidity. According to the law of atmospheric corrosion, the corrosion depth should be proportional to Atn, while A and n are related to the material and environment, and t stands for the exposure time. Therefore, limited exposure time is important because long-time exposure results in deeper contamination.
Working gas must be replaced when the output energy of the laser is strongly reduced. The increased effective fluorine medium reacts with the aggregated harmful elements to generate gases at a faster rate. If the aggregation of harmful elements is great, the output energy will be reduced greatly again within a short time. Therefore, the stability of the aluminum during operation is mainly ensured by the low content of the internal harmful elements and their low diffusion degree. Three effective measures for reducing the content or diffusion of the harmful elements are recommended: (1) Reduce the impurity elements introduced in the raw material and preparation process; (2) Improve the surface quality of the material; (3) Select proper heat or surface treatment.
4. Conclusion
The corrosion behavior of aluminum in fluoride-containing discharge condition was studied. The aluminum was fluorinated mainly by pitting corrosion and did not generate a dense passivation layer. It had typical two zones, and the loose zone with many 2–4 μm corrosion pits owned higher fluorination and carbon oxidation degree than the dense zone. The internal elements diffused to the surface with different degrees. The elements of IA, IIA and VIB groups within 2.4 μm from the surface aggregated over 10–1000 times, but Fe, Ni and Si, C elements aggregated only a few times. The serious element diffusion zone was about 5 μm, and some elements diffused tens of microns. Considering the stability to fluorine medium and the ability to improve thermodynamic stability, the Ni element should be a good choice for anticorrosive layer. The aggregation of elements like Si greatly increases the possibility to form harmful gases, and their content should be strictly controlled. The violent fluorinations of the surface impurities and matrix dominate the passivation. Slowly diffused impurity elements become the main reactant during operation. The carbon oxidation which destroy the stable fluorinated surface is the main reaction during air exposure. Therefore, the passivation effect largely depends on the surface content and state of harmful elements, but the long-term chemical stability is related to the surface quality, internal content of harmful elements, and diffusion rate.
Acknowledgments
This research financially support by the National Natural Science Foundation of China, grant No. 61705235.