Abstract
Wettability of an iron surface is crucial for the wide applications of iron in practice. In this work, a hierarchical structure highly similar to that of the underside of a bamboo leaf was constructed on an iron surface via the template method and controllable etching. After modification by stearic acid, the iron surface with hierarchical structure showed excellent water repellency, with an average contact angle of 156° and a sliding angle of 3°. X-ray diffraction (XRD) techniques and Fourier transform infrared spectroscopy (FTIR) are applied to examine the chemical components of an iron surface.
Export citation and abstract BibTeX RIS
Corrections were made to this article on 15 April 2014. The DOI has been corrected.
1. Introduction
In recent years, living organisms possessing extreme water-repellent attributes, such as the lotus leaf, the butterfly wing, the water striders' leg, and many others, have drawn considerable attention from scientists in diverse fields, due to their excellent properties of self-cleaning, anti-fouling, drag reduction, high loading capacity, directional movement of water, and so on [1–10].
Research on natural super-hydrophobic surfaces shows that hierarchical surface structure is the crucial factor in generating super-hydrophobicity [11–13]. Meanwhile, scientists have discovered that super-hydrophobic surfaces can greatly improve the anti-corrosion property of metals such as copper, iron, stainless steel, and so on [14–16]. Thus, preparations of super-hydrophobic metal surfaces with hierarchical structure have attracted much interest in recent years. For example, Yao [17] and coworkers demonstrated a hierarchical structure that was composed of a dendritic microporous surface with nanostructured porosity on copper foils. They fabricated this structure by dynamic gas-bubble template-assisted electrochemical deposition. After modification with alkyl thiols, the prepared surfaces showed super-hydrophobicity with low water adhesion. Song [15] and his group reported a simple and reliable two-step process to render magnesium alloy surfaces super-hydrophobic via primary cell corrosion, and subsequently cover them with a fluoroalkylsilane film. Luo et al [18] obtained a super-hydrophobic surface with a porous micrometer–nanometer-scale binary structure roughness on stainless steel and engineering materials.
Although most of the reported methods have successfully fabricated hierarchical structures on metal surfaces, few of them can obtain surface structures very similar to those of natural species. The special hierarchical structures of living organisms often possess multiple functions [19–21]. For example, the hierarchical micro- and nanostructures on the surface of the butterfly wing not only endow the wing surface with super-hydrophobicity but also contribute to the directional adhesion and the beautiful colors on the wing surface [22, 23]. Thus, it is both important and necessary to develop an easy method of fabricating surfaces with super-hydrophobic micro- and nanostructuring very similar to those of living species to improve the practical application of metal materials.
In this paper, an easy method is proposed to fabricate a complex surface microstructure very similar to the underside of a bamboo leaf on an iron surface by combining template and chemical etching. The iron surface obtained showed excellent water repellency after it was modified by stearic acid. To the best of our knowledge, this is the first report of the fabrication of a super-hydrophobic iron surface with a micro- and nanostructuring similar to the underside of a bamboo leaf.
2. Experimental method
2.1. Materials
A fresh bamboo leaf was washed by ultrasonic cleaning in water for 10 min. Ethanol (95%) was obtained from the Changsha Huihong Chemical Reagent Plant of China. Iron sheets (10 mm × 15 mm × 1 mm) were washed in a hydrochloric acid solution (0.29 mol L−1) and ethanol by ultrasonic cleaning. Liquid PDMS and its catalyzer were bought from Shanghai Jing Xi Chemical Technology Co.; Ltd. FeCl3 was obtained from a chemical reagent of Jinshan Chengdu Co., LTD; and stearic acid was obtained from a chemical reagent of Kemaou Tianjin Co., LTD.
2.2. Preparation of the iron surface with a hierarchical structure
Preparation procedures for the iron surface with a hierarchical structure are illustrated in figure 1. Part A of figure 1 shows that the liquid polydimethylsiloxane (PDMS) and its catalyzer were cast on the cleaned underside of a bamboo leaf to obtain the PDMS template. Part B of figure 1 indicates that the PDMS template was pressed on glass substrates wetted by a very thin layer of FeCl3 solution (0.125 g mL−1). After being peeled off the glass substrates, the PDMS template was stained with a thin layer of FeCl3 solution. Part C of figure 1 shows that the PDMS template with FeCl3 solution was put on the iron surface with a pressure of 24.5 ± 0.4 N for 24 h. After the stress was released, an iron surface with a hierarchical structure similar to that of a bamboo leaf was obtained.
Figure 1. Preparation of the iron surface with a hierarchical structure similar to that of the underside of bamboo leaf.
Download figure:
Standard image High-resolution image2.3. Modification of the iron surface
First, 1 g stearic acid was dissolved in 100 ml ethanol to form a uniform solution. Then, the iron obtained from the above procedure was immersed in the uniform stearic acid solution for 60 min. After it was drawn out of the stearic acid solution, a super-hydrophobic iron surface with a unique hierarchical surface structure similar to the underside of a bamboo leaf was obtained.
2.4. Characterization
2.4.1. Scanning electron microscopy (SEM)
Morphologies of the surface structures were observed by scanning electron microscopy (SEM) of HITACHI S-3000N and FEI Quanta 200.
2.4.2. Testing of chemical components
The chemical composition of the iron surface was tested by XRD techniques (D8 Advanced) and FTIR (Niclote 380).
2.4.3. Testing of contact angles
Contact angle measurements were performed using a sessile drop method on a Data Physics OCA 20 contact-angle system at ambient temperature using 5 μl droplets. Five different places were tested on each sample, and the average value was taken as the final result.
2.4.4. Testing of sliding angles
The sliding angle was measured by tilting the sample stage from 0° to higher angles and then placing a droplet on the sample, using a micro-gauge. When the droplet rolled off the surface, the angle of the sample stage was the sliding angle.
3. Results and discussion
The panoramic morphology is shown in figure 2(a). In figure 2(a), two types of papillae can be clearly seen. The larger are elliptic papillae with sharp ends, almost all of which are almost facing in one direction. The lengths along the long axis of the elliptic papillae are 35 to 40 μm, and the lengths along the minor axis of elliptic papillae are 20 to 30 μm. The smaller papillae are round in shape, with a diameter range of 5 to 10 μm. The magnified images (figure 2(b) and (c)) show that there are many nanostructures on the surfaces of the papillae (both the larger and smaller types), as well as on the bottom between the papillae. By casting liquid PDMS and its catalyzer on the underside of a bamboo leaf, the solidified PDMS template is obtained. SEM images of the PDMS template are exhibited in figure 3.
Figure 2. SEM image of the underside of a bamboo leaf. Figure 2(a) shows the SEM image of the overall structure of the underside of a bamboo leaf. Figure 2(b) and (c) are the SEM images of the larger papilla and smaller papilla, respectively, on the underside of a bamboo leaf.
Download figure:
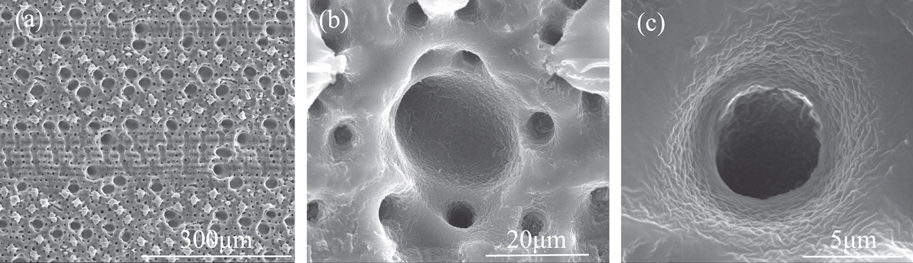
Standard image High-resolution imageFigure 3. SEM image of a solidified PDMS template. Figure 3(a) shows the SEM image of the overall structure of the solidified PDMS template. Figure 3(b) and (c) show a larger pore and a smaller pore, respectively, on a PDMS template at higher magnification.
Download figure:
Standard image High-resolution imageComparing figure 3(a)–(c) with figure 2(a)–(c), we see that the size and distribution of the holes on the PDMS template are consistent with the two main types of papillae on the underside of a bamboo leaf; and the protrusions on the PDMS template are consistent with the concave parts on the underside of a bamboo leaf. The pictures indicate that the surface structure of the PDMS template is complementary with the inherent characteristics of the underside of a bamboo leaf. Figure 4 shows the SEM images of the iron surface prepared as described in the experimental method section B. Figure 4(a) displays the overall structure of the iron surface. Figures 4(b) and (c) show the two main types of papillae, respectively, on the iron surface at higher magnification.
Figure 4. SEM image of the iron sheet with a hierarchical structure before modification by stearic acid. Figure 4(a) shows an SEM image of the overall structure on the prepared iron sheet with a hierarchical multiscale structure. Figures 4(b), (c) are the SEM images of the larger papilla and smaller papilla, respectively, on the prepared iron sheet surface.
Download figure:
Standard image High-resolution imageComparing figure 4 with figure 2, the structures on the prepared iron surface are very similar to those on the underside of a bamboo leaf, but there are some discrepancies that cannot be overlooked. In figure 2, the nanopieces are seen to be distributed everywhere on the underside of the bamboo leaf, whereas in figure 4 nanopieces are rarely found. The method of the hierarchical structure formation on the iron surface is explained as follows:
As shown in part B of figure 1, the FeCl3 solution mostly adheres to the top surface of pores and protrusions on the porous PDMS template. As soon as the FeCl3 solution touches the iron, a reaction occurs, as shown in equation (1):
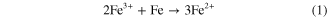
Therefore, the FeCl3 solution first corrodes the iron surface that directly contacts the top surface of the pores and protrusions on the porous PDMS template, and the corroded iron section will become thinner than the uncorroded section. Then the protruding parts of the PDMS template slowly penetrates into the corroded iron sheet section, and the uncorroded iron sheet section penetrates into the pores of the PDMS template. At the same time, some of the FeCl3 solution that adhered on the top surface of the pores is diffused into the pores under pressure and wets the rough walls and bottom of the pores on the PDMS template. At this point, the unreacted iron section that penetrated into the pores of the PDMS template is slowly configured along the walls and bottoms by the FeCl3 solution. Hence, nanostructures form on the iron sections that penetrated into the pores of the PDMS template. In the end, a hierarchical multiscale structure similar to the underside of a bamboo leaf is obtained on the iron surface.
We then tested the water contact angle and sliding angle of a cleaned iron surface and the iron surface obtained with a hierarchical structure. The clean, smooth iron surface is hydrophilic, with a water contact angle less than 10° (figure 5(a)), whereas the contact angle and sliding angle obtained on the iron surface are 146° (figure 5(b)) and 47.8°, respectively.
Figure 5. Contact angle on the iron surface. Figure 5(a)–(c) represents contact angles on a clean iron surface, an iron surface with a hierarchical structure, and a stearic acid–modified iron surface with a hierarchical structure, respectively.
Download figure:
Standard image High-resolution imageAccording to the Wenzel model, the apparent contact angle θw on a rough surface in the homogeneous regime can be expressed as equation (2):
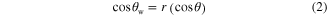
where θ is the contact angle on the flat surface and r is the roughness ratio, defined as the ratio of the true area of the solid surface to its projection area. Because roughness is always larger than 1, from equation (2) it is clear that the enhanced roughness will make an originally hydrophobic surface (θ > 90°) more hydrophobic. Otherwise, the surface will become more hydrophilic, which indicates that the iron surface with the hierarchical structure should be more hydrophilic; however, the contact angle of the iron surface the with hierarchical surface structure increased to 146° (figure 5(b)). To understand why the wettability on the iron surface changed unexpectedly, we analyzed the obtained iron surface by XRD and FTIR. XRD and FTIR results on the cleaned iron surface and the prepared iron surface with the hierarchical structure are shown in figures 6 and 7, respectively.
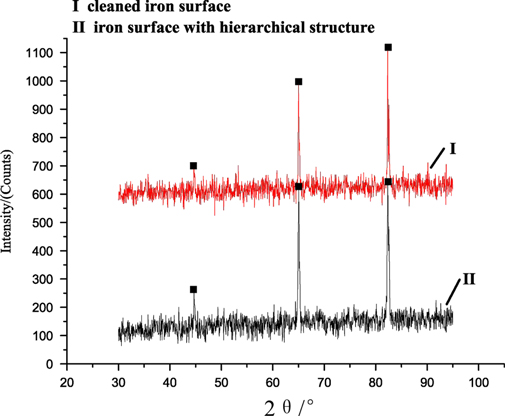
Figure 6. XRD results of the iron surface. Lines I and II represent cleaned iron surface and the iron surface with a hierarchical structure, respectively.
Download figure:
Standard image High-resolution imageFigure 7. FTIR results of the iron surface. Line I is assigned to the iron surface corroded by FeCl3 for 24 h without using a PDMS template; line II is assigned to the iron surface with a hierarchical structure.
Download figure:
Standard image High-resolution imageIn figure 6, lines I and II represent XRD results of the cleaned iron surface and the iron surface with the hierarchical structure, respectively. Comparing lines I and II, we know that three main peaks appear at the same diffraction angles, namely 44°, 65°, 82°, respectively. The three peaks are assigned to the three characteristic peaks of Fe. However, some impurity peaks also appear in lines I and II. By researching the PDF card, we find that the impurity peaks are ascribed to the diffraction angles of Fe2O3. The XRD results indicate that the main chemical composition of the hierarchical structure is Fe.
FTIR results of the iron surface are shown in figure 7. In figure 7, line I is assigned to the iron surface corroded by FeCl3 for 24 h without using a PDMS template; line II is assigned to the iron surface with the hierarchical structure. By comparing lines I and II, we see that the peaks of line II, appearing at 3308.28 cm−1, 1259.65 cm−1, and 805.47 cm−1, are assigned to the stretching vibration of C-H, O-Si-O, and C-Si-C groups, respectively, whereas line I doesn't show these peaks. Thus, we conclude that a few unsolidified PDMS monomers have transferred to the iron surface during preparation, and they decrease the free energy of the surface, which explains the increase in the contact angle (from 10° to 146°) of the roughened iron surface. Nevertheless, PDMS monomers do not completely cover the iron surface, and there are some minor discrepancies between the surface structure of the prepared iron sheet and the underside of a bamboo leaf (though their panoramic morphologies, shown in figure 2 and figure 4, are similar); therefore, the iron surface did not reach super-hydrophobicity.
According to the early work of Wang [24] and coworkers, when an iron surface with a hierarchical structure is immersed in a stearic acid solution, stearic acid develops, and nanosheets and small clusters will form in a short time on the iron surface. Therefore, we immersed the iron obtained with a hierarchical surface structure into stearic acid to modify it. Figure 8 shows the morphology of the iron surface with a hierarchical structure after modification.
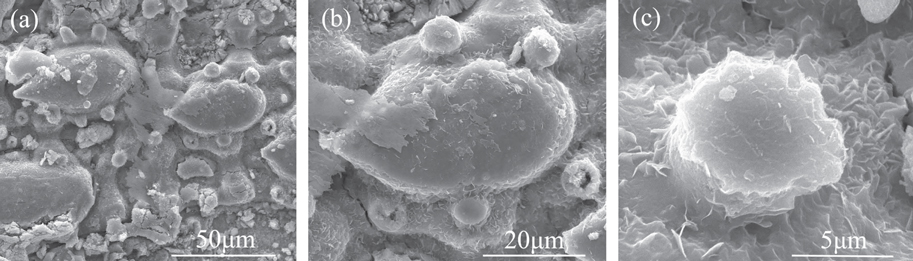
Figure 8. SEM images of an iron sheet with a hierarchical structure after modification by stearic acid. Figure 8(a) shows the SEM image of the overall structure on the modified iron sheet with a hierarchical multiscale structure. Figure 8(b) and (c) show the SEM images of the larger papilla and smaller papilla on the modified iron sheet surface, respectively.
Download figure:
Standard image High-resolution imageFigure 8(a)–(c) shows the panoramic morphology, the larger papilla, and the smaller papilla, respectively, on a modified iron surface. In figure 8, we see many nanosheets, which could not be seen before modification, form on the iron surface. They are distributed everywhere on the papillae and at the bottom between the papillae, in a manner very similar to the nanopieces on the underside of a bamboo leaf. This indicates that the surface structure on the iron surface becomes more similar to the underside of a bamboo leaf after modification with stearic acid. The contact angle and sliding angle of the modified iron surface were tested after the surface was dried, and the results were 156° (figure 5(c)) and 3°, respectively.
To further understand the reason why the iron surface with the hierarchical structure becomes super-hydrophobic after modification, chemical components on the modified iron surface were also examined by FTIR (figure 9). Figure 9(a) shows the results of an FTIR test of the modified iron surface.
Figure 9. The FTIR results of the modified iron sheets and stearic acid powder. The line in figure 9(a) and the line in figure 9(b) represent the modified iron surface and stearic powder, respectively.
Download figure:
Standard image High-resolution imageIn figure 9(a), we see that the peaks assigned to O-Si-O and C-Si-C are not obvious compared with those in line II of figure 7. This is because stearic acid reacts with the Fe2O3 on the iron surface to generate Fe(CH3(CH2)16COO)3, which has been proven by Wang [24] and coworkers, and most of the PDMS monomers are finally covered by the Fe(CH3(CH2)16COO)3. To further prove the existence of Fe(CH3(CH2)16COO)3 on the iron surface, we also performed an FTIR test of stearic acid powder (the result is shown in figure 9(b)). Comparing the lines in figure 9(a) and (b), the free COO band from stearic acid at 1702 cm−1, shown in figure 9(b), also appears at the same place in the line of figure 9(a), which indicates that Fe(CH3(CH2)16COO)3 does exist on the iron surface. Therefore, it is easy to understand the reason for the contact angle increase of the modified iron surface. First, the newly formed nanosheets increased surface roughness on the iron surface with a hierarchical structure. In addition, more stearic acid and Fe(CH3(CH2)16COO)3 equally distribute on the iron surface, which greatly lowers the surface energy. The two factors make the iron surface super-hydrophobic.
The change of the sliding angle can be explained according to the viewpoints of Miwa [25] and Öner et al [26]. Miwa et al [25] believed that the hierarchical structure, which can trap air, is the essential reason why a solid surface achieves a low sliding angle. Öner [26] holds that the discontinuous, unstable, and contorted three-phase (solid-liquid-air) contact line is necessary for a solid surface to realize a low sliding angle. In this case, the micro- and nanostructure of the iron surface with a hierarchical structure (shown in figure 4 and figure 8) satisfy the two conditions. According to Miwa and Öner, the iron surfaces with hierarchical structures should have a low sliding angle. From the values of the sliding angle test, we know that the modified iron surface has a low sliding angle of 3°. However, the unmodified iron surface with a hierarchical structure (figure 4) shows a high sliding angle of 47.8°. A possible reason may be that the unsolidified PDMS monomers transferred to the iron surface (figure 4) are very few, and the iron surface cannot be continuously and completely covered by the PDMS monomers. When the iron surface contacts with a water droplet, those parts without PDMS monomers on the iron surface are hydrophilic; the hierarchical structure of such parts is easily penetrated by water and changes to the Wenzel state. In the Wenzel state, the sliding angles of the super-hydrophobic surfaces are often high. After the iron surface with a hierarchical structure is modified by stearic acid, the surface is completely and equally covered by Fe(CH3(CH2)16COO)3, with no hydrophilic part exposed; thus water cannot penetrate into the hierarchical structure, and the interface of water, air, and iron surface tends to form a Cassie state [27]. In a Cassie state, the surface often shows a low sliding angle. Thus, the modified iron surface with the hierarchical structure (figure 8) showed a lower sliding angle.
4. Conclusions
In summary, an iron surface with micro- and nanostructures similar to that of a bamboo leaf was obtained through a method combining a template and etching. After modification by stearic acid, the obtained iron surface showed excellent water repellency, with a contact angle of 156° and sliding angle of 3°. The method is rather simple and novel, and also provides an easy way to construct various complex micro- or nanostructures on metal surfaces.
Acknowledgments
The authors would like to acknowledge the support of the Natural Science Foundation of China (No. 51103036, 51203183), the Youth Foundation of Hunan Educational Committee (No. 11B035), and the Science Foundation of Hunan University of Technology (No. 2011HZX06).