Abstract
Oral tablets with tunable release profiles have emerged to enhance the effectiveness of therapies in different clinical conditions. Although the concept of tablets with adjustable release profiles has been studied before, the lack of a fast and scalable production technique has limited their widespread application. In this study, a scalable fabrication method was developed to manufacture controlled-release polyanhydride tablets. A new polymeric core–shell tablet design is also proposed, that in conjunction with a micro-fabrication procedure, allows for achieving tunable release profiles required in personalized medicine in small-size tablets. Utilizing a surface-erodible polymeric carrier in the fabrication of the new tablet design resulted in achieving adjustable release profiles and improvements in the drug-loading capacity of the delivery system which allows for delivering a flexible amount of therapeutics with desirable patterns to patients. The proposed fabrication techniques allow for scalable production of personalized tablets with the high resolution required in precision medicine and hence have a high potential for clinical translation.
Export citation and abstract BibTeX RIS
1. Introduction
Oral drug delivery systems (DDSs) comprise more than 50% of the drug delivery market [1, 2]. Patients and clinicians alike prefer the oral route for drug administration because it is economical, noninvasive, and does not require expertise for the administration [3, 4]. Conventional means of oral DDSs release the entire therapeutic substance immediately after administration. This leads to an instant increase, followed by a rapid decrease (to a sub-therapeutic level) in the drug concentration in the bloodstream. Therefore to keep the drug concentration at therapeutic levels, multiple administrations at regular intervals are required.
Controlled-release DDSs have emerged to enhance the patients' compliance and convenience by improving drugs' efficacy, reducing the chances of drug toxicity, and eliminating the need for multiple administrations [5]. Biodegradable polymers have been widely used in controlled-release DDSs since they reduce the need for surgical removal of the delivery devices after releasing their therapeutics and also because of their tunable mechanical properties [5, 6]. Synthetic biodegradable polymers, in particular, are of interest due to their unlimited availability [7].
Erosion is the mechanism governing a broad range of the chemically mediated controlled-release systems (CRSs) and can occur in the form of bulk and/or surface erosion. Most of the biodegradable polymers used in CRSs, e.g. polyesters, undergo bulk erosion [8]. The bulk-erodible polymers erode from their interior and exterior simultaneously, resulting in low predictability of the drug release kinetics from these polymers. In contrast, surface-erodible polymers lose the polymer moieties from their surface, and therefore their initial geometry is preserved while the size of the polymer decreases. Besides, the mechanical properties of the surface-erodible polymers do not change during the erosion. The highly reproducible and predictable release kinetics of the surface eroding polymers make them desirable options in manufacturing controlled-release DDSs [5].
Polyanhydrides (PAs) predominantly undergo surface erosion because of their hydrophobic backbone and hydrolytically unstable anhydride bonds. PAs have been shown to maintain their surface eroding behavior at sizes as small as 100 μm, which is the smallest value reported for surface-eroding polymers [9]. Unlike other surface-erodible polymers such as polyorthoesters that undergo surface erosion only in acidic environments, PAs have shown surface erosion in acidic, alkaline, and neutral environments [10]. Despite their attractive properties for controlled-release DDSs, PAs have not been used as often compared to other biodegradable polymers such as polyesters [11]. This can be attributed to the difficulties involved in their synthesis. In 2009, a new photocrosslinkable polyanhydride (PA) was synthesized [12] under straightforward conditions using thiol-ene polymerization. The polymerization process was also fast compared to the synthesis processes of other PAs. Therefore, this type of PAs has shown a high potential to be used in fabrication of controlled-release DDSs [13].
Controlling the temporal profile of drug release is vital for achieving the optimal therapeutic effect. Despite the substantial progress in developing controlled-release tablets, most of the existing designs only offer monotonic or sustained release profiles [14]. However, different types of clinical circumstances necessitate different release profiles for their optimal treatment. The temporal patterns are mostly defined by the effect of circadian rhythm on different clinical circumstances, giving rise to specific rhythms of medical conditions during the 24 h of the day [15]. Besides, various factors such as the patient's condition, age, and gender call for patient-specific treatments via personalized medicine. The increased awareness of individualized therapy and introduction of various biodegradable polymers with adjustable physicochemical properties, encourage the development of fabrication methods for manufacturing advanced controlled-release DDSs that enable releasing of drugs at controllable rates.
The traditional compression tableting that is used by pharmaceutical industries for manufacturing tablets and capsules [14] does not provide the flexibility required for fabricating controlled-release DDSs. Micro-fabrication has been used in some studies owing to its ability to manufacture tiny features while providing control over the shape or geometry of the delivery devices [4]. The combination of micro-fabrication techniques such as standard photolithography, with repeated replica molding steps, has been reported which allows for easy, rapid, and precise manufacturing of drug delivery devices at relatively low cost [4, 16]. These methods have been used to fabricate microchips as CRSs to generate long-term pulsatile release [17]. Although the microfabrication methods provide a high-resolution release pattern and have the potential for scalable manufacturing, the limitations associated with using implantable microchips have left the oral controlled-release DDSs the preferred option [1, 18].
Three dimensional (3D) printing is another technology that has been studied extensively by researchers for fabricating polymeric oral tablets with adjustable release profiles. Most studies fabricated tablets with monotonic or constant release profiles of drugs, with some reporting more complicated (e.g. pulsatile) release profiles [19–22]. The complicated release patterns were achieved by elaborate tablet designs and specific fabrication techniques, demonstrating the feasibility of creating tablets with various release patterns of drugs.
Although the above-cited studies showed that the fabrication of personalized tablets is no longer a dream, there exist some limitations in the current fabrication methods used for the tablets that hindered their widespread implementation. Some of these limitations can be addressed by improving the tablet design and/or the appropriate choice of the fabrication method. As an example for the latter, the resolution of the fabrication method is an important parameter that can determine the precision of the release rate. Inexpensive and commercially available 3D printers have relatively low resolution, while higher resolution 3D printers are limited by their small range of resins (printable materials) [22–26]. On the other hand, microfabrication techniques such as photolithography are capable of creating miniaturized fine features with high resolution. Besides resolution, scalability and throughput manufacturing is a critical characteristic of any potentially successful fabrication method, which is currently a challenge for producing advanced controlled-release DDSs with adjustable release rates. Unlike high-volume production of drug tablets to treat a disease, the emergence of personalized medicine requires the consideration of individual variability to design both the dose and release profile of the chosen drug. In this case, having a cost-effective, throughput platform for manufacturing sufficient numbers of tablets for each patient is essential. Most studies reported the fabrication of a single tablet in each experiment, reducing the chances of their practical use in clinical settings. Manufacturing each tablet can take a considerable amount of time, and the reproducibility level is low [19–25, 27–29].
We aim at addressing the challenges in the fabrication of controlled-release tablets with adjustable release profiles of drugs by proposing a scalable fabrication method that manufactures tablets to deliver accurate amount of therapeutics to patients with specific patterns. In this study first we propose a tablet design through which we program a surface-erodible PA to release its cargo with specific patterns. Then we develop a throughput fabrication platform to manufacture the tablets with a fast and large-scale technique using the combination of the microfabrication and 3D printing methods. We also improve the drug loading capacity of the delivery system by modifying the tablet designs.
2. Materials and methods
4-Pentenoic anhydride (PNA), 2, 2-(Ethylenedioxy) diethanethiol (3,6-Dioxa-1,8-octane-dithiol, EGDT), Pentaerythritol tetrakis(3-mercaptopropionate) (PETMP), 1-Hydroxycyclohexyl phenyl ketone, Trichloro(1H, 1H, 2H, 2H-perfluorooctyl)silane and acid orange 10 (orange G) were purchased from Sigma Aldrich Chemical Co., Milwaukee, USA, and were used as received. SU-8 2050 photoresist was purchased from MicroChem Corporation, MA, USA. 3D printer acrylate-based resin (Clear 2005T) was purchased from MiiCraft, Germany. Both the Sylgard 184 Silicone Elastomer pre-polymer and the curing agent were purchased from Ellsworth Adhesive Chemical Co., ON, Canada, and were used for making Polydimethylsiloxane (PDMS). 3.3 mm poly (methylmethacrylate) (PMMA) sheets were purchased from McMaster-Carr, NJ, USA. Tyfon Microbore tubes (ID 0.079 in) were purchased from Cole-Parmer, Inc. Canada.
2.1. Polymer preparation procedure
1-Hydroxycyclohexyl phenyl ketone (photo-initiator, 0.1 wt%) was weighed and placed into a 15 ml Falcon tube. Then, PNA was transferred into the tube, followed by the mixture of EGDT and PETMP. The solution was mixed to obtain a homogenous mixture. The initial mole ratio of PNA to the total amount of monomers containing thiol groups (EGDT and PETMP) was 100:100. In this study, four different initial mole ratios of PNA to PETMP and EGDT were prepared and used: PNA: PETMP: EGDT = 100:100:0, 100:75:25, 100:50:50, and 100:25:75. The homogenous pre-polymer solution was then purged under the inert gas (nitrogen or argon) for 3 min and was transferred to PDMS molds. PDMS molds with different designs and geometries were fabricated and used according to the desired geometry of each polymer. The pre-polymer solution in the molds was exposed to ultraviolet (UV) light (CL-1000 UVP Cross-linker) equipped with 365 nm UV lamps (intensity =  5 mW cm−2) for 5 min. To study the release profiles of dye-loaded tablets, a model compound (orange G) was added to the pre-polymer solution of some of the tablets. Orange G (1–3 wt%) was weighed and added to the purged solution and mixed using sonication for 25 min. Similar to the pre-polymer without the model compound, the orange G dye-loaded solution was transferred to PDMS molds and synthesized under the same UV light for 5–15 min. The reaction scheme is presented in the Supplementary file (supporting figure S1 (available online at stacks.iop.org/BF/12/045007/mmedia)).
5 mW cm−2) for 5 min. To study the release profiles of dye-loaded tablets, a model compound (orange G) was added to the pre-polymer solution of some of the tablets. Orange G (1–3 wt%) was weighed and added to the purged solution and mixed using sonication for 25 min. Similar to the pre-polymer without the model compound, the orange G dye-loaded solution was transferred to PDMS molds and synthesized under the same UV light for 5–15 min. The reaction scheme is presented in the Supplementary file (supporting figure S1 (available online at stacks.iop.org/BF/12/045007/mmedia)).
2.2. Fabrication of 3D printed master molds
3D printed master molds were used to fabricate the PDMS molds on which the pre-polymer solutions were polymerized. The master molds were printed by a commercial digital light processing (DLP) 3D printer (PICO2, ASIGA). Desired 3D models were designed by AutoCAD software and were printed layer by layer from a UV-curable resin (Clear 2500T). The layer thickness was set to 250 µm, and the UV-curing time for each layer was 5 s. The 3D printed objects were treated using sonication in ethanol for 10 min and washed by deionized (DI) water to remove any uncured polymer.
2.3. Tablet design
The tablet has two main parts: the core and the shell (figure 1(A)). The core is made of a surface eroding polymer loaded with a drug (shown in orange in figures 1(A) and (B) while the same polymer without any drug is used for the shell (the grey color in figure 1). The core is enclosed by the shell and is placed closer to the top surface compared to the other faces. When the thickness of the shell is 'large enough' for all sides except the top, the release of the drug is ensured only from the top surface of the tablet (figure 1(B)). This design relies on the surface eroding behavior of the polymer which preserves the tablet structure and integrity during erosion and ensures the erosion of the polymer at a similar rate from all sides. An experiment was conducted that verified the latter (see section 2.4). Therefore, in this design, the water first reaches the drug at the top. Because the thickness of the shell is large enough, the drug is released completely from the top before the water reaches it from the other sides. Accordingly, the release profile of the drug is only governed by the variations in the surface area of the drug-loaded core polymer along with its height (figure 1(C)). As an example, in figure 1, the core polymer's surface area from the top is increasing along its height, showing that this tablet is designed for obtaining an increasing release profile (figure 1(D)). A similar approach was used to design the constant (figure 7(A)) and decreasing-increasing (figure 8(A)) release profile tablets.
Figure 1. Schematic illustrations of core–shell tablet design for an 'increasing' release profile. The core is shown in orange, and the shell is the grey part. (A) Three dimensional (3D) view and (B) front view showing that the core is closest to the top surface. The shell is made of the same polymer, with larger thickness than the core's height on the other sides. (C) Release of the drug-containing part when the tablet is immersed in phosphate-buffered saline (PBS). (D) The expected release profile of a drug for a sample tablet design with linear increasing erodible surface area along the height of the tablet.
Download figure:
Standard image High-resolution imageThe 600 µm distance from the upper edge of the tablet core to the top surface is considered to prevent the initial burst release of the drug upon contact with biological fluids. The surface eroding PAs used in this work showed an induction period in which they absorb water by diffusion before surface erosion starts. The erosion front (the thickness that water diffuses to the thiol-ene PA prior to the start of the surface erosion process) is around ∼400 µm. The 600 µm distance also ensures that both the drug release and the surface erosion occur concurrently.
2.4. Tablet characterization: height and diameter reduction rates
To ensure that the polymer erodes at similar rates from all sides, the erosion of cylindrical thiol-ene PAs was studied. Four thiol-ene PA cylinders with initial mole ratios of 100:100:0, 100:75:25, 100:50:50, and 100:25:75 were synthesized in cylindrical PDMS molds. The PDMS molds were fabricated using 3D printed master molds with the diameter and height both equal to 8.7 mm. The synthesized polymers (cylindrical tablets) were immersed in 10 ml PBS (pH = 7.4) in glass vials, before being placed on a shaker (VWR micro-plate shaker). The shaker was set up at room temperature and the shaking rate of 120 rpm. The diameter and height of the tablets were measured frequently (approximately one every hour) during the erosion. For each measurement, tablets were removed from the solution and their surfaces were slowly wiped using disposable paper wipers. The dried tablets were then put on a printed coloured graph paper, and photos were taken using a Canon SX201 IS digital camera from four different sides of each tablet, before putting the tablet back in PBS. Images from the top and bottom of the tablet were used to monitor the diameter reduction, while the two images from the sides provided information on variations in the tablet's height. The NIH ImageJ software (version 1.8.0) was used to measure tablet dimensions from the photos taken. Experiments were repeated three times for each polymer tablet.
2.5. Micro-fabrication of the tablet core
A microfluidic network that was fabricated using the conventional standard photo- and soft-lithography techniques [30, 31] was utilized to create the dye-loaded polymers used as the core of the tablets. The SU-8 photoresist was poured on a four-inch silicon wafer, and a 300 μm-thick layer of photoresist was spin-coated (Laurell Tech Corp.) on the top surface of the silicon substrate. Considering the photo- and soft-lithography procedure for microfabrication, a photomask that replicated the final desired shape is used. In the design for the core of the tablet, instead of using the shape of a single tablet core for the photomask, a throughput pattern of tablet cores was designed using AutoCAD software which was then printed as the photomask on a high-transparent sheet (supporting figure S2(A)). The SU-8 photoresist (with the desired thickness spin-coated on the substrate) was covered by the photomask and exposed to high-intensity UV-light (AB-M Inc.) for 80 s. When using a photo-mask, the UV-light only passes through the UV-transparent parts, crosslinking only the desired areas on SU-8. Therefore, the SU-8 photoresist polymer hardened only at the designed pattern and the other parts remained uncross-linked. Lastly, SU-8 developer was used to remove the uncross-linked photoresist from the wafer, yielding the final SU-8 mold (supporting figure S2(B)).
Figure 2(A) shows an example of the design for scalable manufacturing of the tablet core for an increasing release profile. First, the SU-8 master molds were fabricated using the above-mentioned steps (figure 2((A)i–ii)). To fabricate the microfluidic device, the PDMS polymer mixture containing the 1:10 ratio of base to curing agent was thoroughly mixed. To remove the trapped air bubbles created in the PDMS sample, the mixture was degassed in a vacuum chamber for 15 min. As shown in figure 2((A)iii), the negative PDMS mold is developed and peeled off from the SU-8 positive mold. To facilitate the peel-off process, the SU-8 mold was silanized to make the mold's micrometer-sized channels more hydrophobic. The positive SU-8 mold was silanized using (tridecafluoro-1,1,2,2- tetrahydrooctyl) trichlorosilane by putting the mold in a sealed petri-dish on a hot plate for 2 hrs at 65 °C. To make the PDMS replica mold, the mixture was poured on top of the SU-8 mold and were cured in an oven for 2 hrs at 80 °C. Finally, the cured PDMS was easily peeled off from the SU-8 master mold, yielding the throughput patterns connected through the microfluidic network (figure 2((A)iii)).
Figure 2. Scalable fabrication platform for manufacturing tablets with adjustable release profiles. (A) Microfabrication of a throughput design to create the tablet core. (i) Ultravilet (UV) light exposure to the SU-8 layer covered with the printed photomask containing the throughput patterns of the tablet core (in this case, the increasing release profile design). (ii) The positive SU-8 mold containing the embossed features. (iii) Pouring the PDMS on top of the SU-8 mold and peeling off the final negative PDMS mold once cured in the oven. (iv) Two polydimethylsiloxane (PDMS) layers sandwiched between two rigid poly (methylmethacrylate) (PMMA) sheets equipped with holes designed to embed screw-nuts for applying a uniform force on PDMS layers. Squeezing the two PDMS layers using the uniform force eliminates the potential leakage of the solutions introduced into the microfluidic network. (B) Schematic representations for the throughput fabrication of the core–shell tablet using (i) A throughput set up to manufacture the tablet core. Microfluidic channels were filled by orange G dye-containing polymer solution which was injected from a reservoir. The vacuum pump helped the solution flow through the network. The first round of the UV-light exposure was used to cure the connected features. (ii) Micro-fabricated features were placed and aligned in PDMS wells. Empty spaces were filled with the same polymer without any orange G dye. The final tablets were fabricated using the second round of UV-light exposure. (C) Fabricated PDMS microfluidic chip for creating the tablet core. (i) SU-8 master mold fabricated to make PDMS replica molds. (ii) Putting two PDMS layers with the mirror patterns on top of each other to create microfluidic cavities with 600 µm depth in between. (iii) Using clamps for squeezing two PDMS layers for the elimination of potential leakage. (iv) Using two PMMA sheets to apply uniform forces on PDMS layers under uniformly distributed screws.
Download figure:
Standard image High-resolution imageA platform for using the fabricated PDMS negative molds to make the polymeric tablet cores was designed (figure 2((A)iv)). Another PDMS negative mold with a pattern mirrored to that of the original was fabricated and placed on top of the original PDMS negative mold, making a microfluidic network with 600 µm in thickness. The top PDMS negative mold was punched on two sides with a 2 mm biopsy punch (EMS-Core Sampling Tools) to create inlet/outlet ports through which solutions flow into the channels created by the two PDMS negative molds (figure 2((A)iv)). The two PDMS layers were sandwiched between the two rigid PMMA sheets (3.3 mm thickness). Uniformly distributed holes were designed using AutoCAD software and were cut on PMMA sheets using the CO2 laser (SUNCOO K40 laser cutter). These holes were designed for using screw-nuts through which a uniform force was applied to squeeze the two PDMS layers together to prevent any leakage. At the center of the top PMMA sheet, a pattern was cut using the laser cutter to allow exposure of the solution in the PDMS layers to UV-light (figure 2((A)iv)) for curing the tablet cores.
2.6. Throughput setup for the fabrication of core–shell tablets
Pre-polymer solutions were prepared as described in the polymer preparation procedure section. In this study, two pre-polymer solutions with initial mole ratios of PNA: PETMP: EGDT = 100:100:0, and 100:75:25 were prepared. Approximately 5 ml of solutions containing 1 wt% of orange G model compound were transferred into a vial. The solution was continuously injected into the microfluidic network from the inlet port using the Tygon Microbore tubing (0.079''ID) (figure 2((B)i)). A vacuum pump was connected to the outlet, helping draw the solution to fill the chambers. After the solution filled the chambers completely, the features were exposed to UV-light (UVP Crosslinker, intensity =  5 mW cm−2) for 5 min. The screws were then opened and the three connected dye-loaded tablet cores were easily peeled off by separating the two PDMS layers.
5 mW cm−2) for 5 min. The screws were then opened and the three connected dye-loaded tablet cores were easily peeled off by separating the two PDMS layers.
The cured micro-fabricated features (the three connected tablet cores) were then placed in PDMS wells (figure 2((B)ii)) fabricated using master molds printed by commercial DLP 3D printer. The procedures for the fabrication of the 3D printed master molds were the same as the steps described in section 2.2. The 3D model of the wells was designed so that when the throughput micro-fabricated cores are placed in the wells, each of the cores stays at the center (from the sides, horizontally) and at a pre-determined distance from the bottom and the top of the well (vertical alignment). Particularly, as explained in the tablet design section, the cores were closest to the top and far enough from all other sides of the wells. The dimensions of wells (cylindrical molds with diameter × height equal to 8.7 × 6.6 mm, 8.7 × 8.7 mm, and 14.1 × 12 mm) were determined based on dimensions of the tablet cores to provide desired thickness as tablet shells. After placing the orange G dye-loaded cores in the wells, the empty spaces around the polymers inside the wells were filled using the same pre-polymer solution as the core but without the orange G dye. Another 5 min UV-exposure was conducted to cure the newly added polymer solution. Finally, three tablets were peeled off from the PDMS wells (figure 2((B)ii)) and simply were separated using a blade by cutting the connecting part. To continue reusing the PDMS molds, the channels were washed after peeling off the dye-loaded polymer using the mixture of water and ethanol. After washing the channels carefully, they were dried using high-pressure air for 2 min which ensures cleaning the molds from dirt and possible residues.
The designs of tablet cores, shown in supporting figure S3, were printed as photomasks and the SU-8 master molds were fabricated through photo-lithography steps (figure 2((C)i)). The PDMS molds were successfully fabricated via a standard soft-lithography process using the SU-8 master molds (figure 2((C)i)). The depth of the chambers on PDMS negative molds was measured using a microscope (Nikon 334 Eclipse Ti-E). The measured depths ranged from 298 µm to 301 µm for different molds that are very close to the designed value (300 µm), showing successful fabrication of PDMS negative molds.
The original PDMS layer and the one with the mirrored pattern were put on top of each other to create 600 µm cavities between them (figure 2((C)ii)). Typically, two PDMS layers irreversibly bound to each other or a PDMS layer bonds to a glass slide using standard oxygen plasma activation process to make a microfluidic network [32–34]. The pattern can be destroyed by the formation of an irreversible seal between the PDMS mold and a glass slide [35]. However, in this study, there was a desire to access the features and remove the orange G dye-loaded PA solution once it is cured in the network. Therefore, to seal the channels and at the same time prevent the leakage of the fluid, at first, small clamps were used to squeeze the two layers together as shown in figure 2((C)iii). However, leakage of the polymer solution from the microfluidic channels was observed using the clamps. Alternatively, two rigid PMMA sheets were utilized to sandwich the PDMS layers. Screws and nuts were used to apply uniform force and to secure the alignment. This platform was designed and fabricated for creating the tablet cores. The successful injection of the pre-polymer solution containing the orange G model compound via inlet/outlet ports is shown in figure 2((C)iv).
2.7. Tablets for achieving adjustable release profiles
The ultimate goal of this study is to achieve different release profiles through a new tablet design fabricated by a throughput fabrication platform. To meet this goal, different tablet geometries were designed using AutoCAD software. Different designs of the throughput tablet cores which are connected to each other are shown in supporting figure S3. These tablet cores were designed for increasing (supporting figure S3(A)), and constant release profiles (supporting figure S3(B)). The width of the tablet cores is increasing and uniform from the top along their heights in the increasing and constant tablets, respectively. Supporting figure S3(C) shows different designs for the tablet core with constant release profiles that have two, three, or four constant release cores in each connected feature (this design is described in details in the next section). These designs were printed as photomasks and went through soft-lithography steps for making the PDMS molds containing connected features as a microfluidic network.
The micro-fabricated cores for the increasing and constant release profiles were placed into PDMS wells where the pre-polymer solution without drug was poured and polymerized under the UV-light. The three tablets for each geometry were placed in 5 ml PBS at pH = 7.4 separately before being placed on a shaker with a shaking rate of 120 rpm and at 37 °C. Almost every hour, 750 µl of each PBS solution was withdrawn, transferred into three wells of a 96-well plate (each well contained 250 µl), and analyzed using a microplate reader (Asys UVM 340). The light intensity of the orange G model compound in a 250 µl sample was measured using the absorbance measured by a the microplate reader. The concentrations were then calculated using the concentration-absorption calibration curve of orange G in the wavelength with the maximum absorbance of UV light (475 nm) (Supporting figure S4). For the UV microplate reader device, the absorbance-concentration relation was calculated using  where A is the absorbance of the UV light by the model compound and C is the concentration of the model compound in the sample. The absorbance is linearly related to the concentration. The slope (α in
where A is the absorbance of the UV light by the model compound and C is the concentration of the model compound in the sample. The absorbance is linearly related to the concentration. The slope (α in  ) was calculated for the micro-plate reader to be 27.13 by fitting a linear line to absorbance-concentration data.
) was calculated for the micro-plate reader to be 27.13 by fitting a linear line to absorbance-concentration data.
The absorbance of each sample was measured using the UV plate reader, and the concentration of the model compound in the sample was calculated using  . An equivalent amount of the fresh PBS (750 µl) was added after withdrawing the same amount of solution. After collecting the concentration data of the model compound at certain time intervals, the fractional release (equation (1)) and the release rates (equation (2)) of the model compound from each tablet were calculated. In equation (1), M0 is initial amount of the orange G dye dispersed in the tablet (which is known), Ci is concentration of the model compound calculated for the ith sample at time t, Vt is total volume of the sample (e.g. 5 ml in this study), and Vi is withdrawn sample volume (250 µl per well or 750 µl per sample), and Fi% is fractional released percentage of the dye.
. An equivalent amount of the fresh PBS (750 µl) was added after withdrawing the same amount of solution. After collecting the concentration data of the model compound at certain time intervals, the fractional release (equation (1)) and the release rates (equation (2)) of the model compound from each tablet were calculated. In equation (1), M0 is initial amount of the orange G dye dispersed in the tablet (which is known), Ci is concentration of the model compound calculated for the ith sample at time t, Vt is total volume of the sample (e.g. 5 ml in this study), and Vi is withdrawn sample volume (250 µl per well or 750 µl per sample), and Fi% is fractional released percentage of the dye.
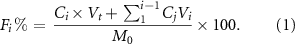
In equation (2), Ri is release rate of the model compound at time t for the ith sample, and Ti is time at which the ith sample was withdrawn.
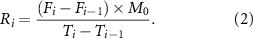
2.8. Modified tablet designs to increase the loading capacity of the delivery system
The tablet designs were modified to improve the capacity of the tablet in containing higher quantities of therapeutics. One-arm, two-arm, and four-arm designs for the core of the tablets were devised to make use of extra spaces in the shell of cylindrical tablets. In the modified designs, instead of using only the axial direction for releasing the dye, the radial directions were used as well by loading the dye in two-arm and four-arm tablet designs. Figures 3(A)–(C) shows the schematic front view illustrations of the one-, two-, and four-arm tablet designs, respectively. Although in the one-arm design, the core is closest to the top of the tablet (same as the tablet designs described so far), in the two and four-arm designs, the core is as close to the right, left, and bottom of the tablet as to the top side. It means the water could access the orange G dye from one, two, and four sides for the tablets shown in figures 3(A), (B) and (C), respectively.
Figure 3. Schematic representations of modified tablet design to improve the loading capacity. Front view of (A) one-arm (B) two-arm (C) and four-arm tablet designs.
Download figure:
Standard image High-resolution imageAs was shown in supporting figure S3(C), similar to the increasing and constant designs, the modified tablets are designed in a throughput connected pattern. The tablet cores were printed as photomasks and fabricated using the throughput microfabrication method previously discussed. Similar to what was described above, the tablets' shells were fabricated with the help of PDMS cylindrical wells. Three separated tablets for each design were immersed in PBS solution for the release test using the same procedure mentioned above. The concentration of the model compound was calculated using the absorbance values measured by the microplate reader. The fractional percentage model compound released and the release rates for these three modified designs were calculated using equations (1) and (2) and then compared to each other.
3. Results and discussion
3.1. Tablet characterization: height and diameter reduction rates
Several studies have shown that PAs predominantly undergo surface erosion [36, 37]. It has been shown that the linear mass loss profile of PAs results in a near zero-order drug release from these polymers [9, 38]. Most of these studies used PAs in the form of a slab (a thin layer of polymer). Using the new type of PAs synthesized by thiol-ene photopolymerization, a rectangular slab (2 × 10 × 10 mm) made of PNA and PETMP showed a linear mass loss profile. More complicated mass loss profiles were observed for a cube made of the same polymer [39]. The non-linearity observed in the mass loss profile of the more complex thiol-ene PA geometries (such as cubes and cylinder) does not contradict the surface-eroding behavior of the polymer. Surface erosion behavior is defined as the quality to preserve the tablet geometry during the erosion time. This unchanging geometry of tablets is attributed to a similar dimension reduction rates in all directions. In our study, the diameter and height variations for each tablet were monitored and calculated from the photos that were taken at specific time intervals (approximately once every hour).
The average of the measured diameters and heights from triplicate experiments for each tablet were plotted over time (figure 4) Where the linear behavior of the dimension reduction for cylindrical tablets is shown. An increased initial mole ratio of EGDT to PETMP in the thiol-ene PAs network results in higher erosion rates. The rates of the reduction for both diameter (figure 4(C)) and height (figure 4(D)) of all the tablets were calculated by finding the slope of the linear curves fitted to the measured data. Table 1 summarizes the results of the reduction rates for diameters and heights of these four tablets. The dimension reduction profiles in figure 4 showed that the reduction of diameters and heights for each tablet is linear. In addition, similar diameter and height reduction rates are observed for tablets made of the same PA. Having the same reduction rate in different dimensions is a very crucial characteristic for a polymer because it increases its applications in controlled-release DDSs. The adjustable release rates from the tablet designs rely on similar reduction rates in different dimensions.
Figure 4. Linear reduction profiles of (A) diameters of four cylindrical tablets. (B) Heights of four cylindrical tablets. Schematic illustrations of (C) horizontal direction of a cylindrical tablet monitored to measure the diameter reduction rates and (D) vertical direction of a cylindrical tablet monitored to measure the height reduction rates.
Download figure:
Standard image High-resolution imageTable 1. Diameter and height reduction rates measured for cylindrical tablets.
| PETMP: EGDT | Diameter Reduction Rate (mm h−1) | Height Reduction Rate (mm h−1) |
|---|---|---|
| 100: 0 | 0.16 | 0.15 |
| 75:25 | 0.25 | 0.25 |
| 50:50 | 0.37 | 0.37 |
| 25:75 | 0.65 | 0.54 |
The linear behavior of the reduction rates (the calculated correlation coefficients R2 ≈ 1) observed in both dimensions (shown in figures 4(A) and (B)), allowed calculating the expected dimension reduction rate of the same polymer for a new cylindrical design. Ideally, this expected reduction rate can be calculated by measuring the dimensions of the tablet at least at two arbitrary time points during erosion and fitting a linear function to dimension versus time data. The slope of the dimension versus time represents the reduction rate. This linear behavior of the reduction rate in this study eliminates the need for monitoring tablet dimensions during the erosion time. For example, by having the initial and final dimensions and the erosion time, the reduction rates can be calculated and used in the tablet design for releasing pre-determined patterns of drugs. The diameter and height reduction rates for the PAs used to fabricate tablets in this study at physiological temperature (37 °C) were monitored and calculated using the same approach. The reduction rates for the heights and diameters of the thiol-ene PA cylindrical tablet with initial mole ratio of PNA:PETMP:EGDT = 100:100:0 were calculated to be similar and equal to 0.34 mm hr−1 and 0.66 mm hr−1 for the PA cylindrical tablet with the initial mole ratio of PNA:PETMP:EGDT = 100:75:25.
3.2. PDMS wells for fabrication of tablet shells
To create the tablet shell, PDMS wells were successfully fabricated using 3D printed master molds. Firstly, cubic wells were designed in a way that the distance between every two adjacent cubes was the same as the distance between the two adjacent micro-fabricated tablet cores. The orange G dye-loaded tablet cores were placed at the center of the wells at a certain height using external aligners. Two aligners were used to hold the fabricated dye-containing part at the specific x,y,z position. Figure 5(A) shows the printed cubic wells, the PDMS negative molds, and the three cubic surface eroding tablets created in the cubic PDMS wells (from left to right). Figure 5(B) shows an alternative design for the cubic wells in AutoCAD in which two aligners were added at both ends of the 3D printed object. Two aligners were designed with certain heights and a notch on them where the micro-fabricated tablet cores hang to ensure the right x,y,z position. These two aligners facilitate the correct placement of the fabricated dye-containing part in the right location inside the well, without the need for external aligners. All the tablet shell designs were changed to cylindrical objects (figure 5(C)) since tablets with cubic shapes have sharp corners that are inconvenient to swallow when used as oral tablets. The optimized design was the cylindrical wells with two aligners in both sides and two more aligners between every two adjacent wells (figure 5(D)). Aligners between wells were added to prevent the micro-fabricated part from bending or collapsing due to gravity.
Figure 5. Development of PDMS wells fabricated using the 3D printed master molds. (A) Cubic 3D printed master mold, PDMS cubic wells created using the master mold, and three surface-eroding polymers in a cubic shape fabricated in the cubic PDMS mold. (B) The revised version of the cubic well design with two aligners in both ends of the mold and the printed master mold. (C) Modified cylindrical wells design and the printed object. (D) Final optimized cylindrical wells design with four aligners to hold the tablet core.
Download figure:
Standard image High-resolution imageThe resolution of the method used for fabrication of the tablet core is of higher importance than that of the tablet shell. The geometries of the micron-sized tablet cores determine the release profile of the orange G model compound and are also more complex than the geometries of the milimeter-sized PDMS wells that form the tablet shell. However, our tablet design benefits from a high-resolution technique for producing PDMS wells (DLP 3D printing). This 3D printing method is also capable of printing various geometries with sharp corners or curvatures, including cubes, cylinders, and spheres as the common form of tablets in the pharmaceutical industry. The higher surface quality of the tablet shells also ensures more precise control over the erosion rate of the tablet in the novel design proposed in this study. Lithography-based 3D printers, including stereolithography (SLA) and DLP 3D printers can create objects with more complex designs at a higher speed and higher resolutions compared to the other commercially available 3D printers such as fused deposition modeling (FDM) machines [40]. In this study, a DLP 3D printer (PICO2, ASIGA) was used to fabricate the master molds because of its fast and high-resolution manufacturing process compared to the SLA counterparts. The limitation of the lithography-based 3D printers is the limited number of the available UV-curable resins. Clear 2500T resin was used after evaluating its thermal stability in an oven for 2 hrs at 80 °C, which is the same thermal condition for curing the PDMS on top of the printed master molds.
3.3. Throughput fabrication of core–shell tablets
The cores and shells of the tablets were fabricated using the developed platform described above for the throughput manufacturing of the tablets. As an example, the results of the throughput fabrication of the increasing release profile tablets are shown in figure 6(A), where it shows the final device developed for fabricating the orange G dye-loaded cores of the tablets. Three tablet cores for the increasing release profile that were fabricated and peeled off from the μm-sized PDMS mold are also shown in figure 6. The cross-linked dye-loaded features were set in place using the notch aligners embedded in the PDMS wells. These features were created using the 3D printed master molds (figure 6(B)). Empty spaces in the wells were filled by the same surface eroding polymer without the orange G model compound. All three wells were then exposed to the UV-light, and the final connected tablets were peeled off from the PDMS wells (figure 6(C)). These connected tablets were simply separated to obtain three individual tablets for the increasing release profile (figure 6(D)).
Figure 6. Throughput fabrication of the increasing release profile core–shell tablets. (A) The throughput platform to create the micrometer-sized tablet cores. (B) Submerging the cured micro-fabricated tablet cores on PDMS wells to create the tablets' shells. (C) Three connected core–shell tablets peeled off from the PDMS wells. (D) Three separated increasing release profile tablets.
Download figure:
Standard image High-resolution image3.4. Tablets with adjustable release profiles
Two of the PA compositions (PETMP:EGDT = 100:0, and 75:25) were used in fabrication of the oral tablets with adjustable release profiles. The other two polymers (PETMP:EGDT = 50:50, and 25:75) indicated fast erosion rates in comparision to the polymers were used (∼10 h vs 24 h). The 24 h window allows to release therapeutics in about one day in a controllable manner. The proposed tablet design in this study was tested at first for the constant release profile. The constant release profile tablets were designed such that the surface area of the tablet core from the top along its height was constant (figure 7(A)). Figure 7(B) shows the constant release profile core–shell tablet made of PA (initial mole ratio PNA:PETMP:EGDT = 100:100:0) in PBS solution after the first half of the core is eroded. The concentration of the orange G model compound in the sample was measured approximately once every hour and the fractional cumulative release of the model compound was calculated and plotted over time (figure 7(C)). The results show the linear fractional release of the model compound. On the top left on the graph in figure 7(C), the release rate of the orange G model compound is plotted. The release rate remain unchanged over time which agrees with the pre-determined constant release profile intended by the tablet design.
Figure 7. Constant release profile of a core–shell tablet. (A) A schematic representative of the tablet core with the constant surface area from the top along its height and the position of the tablet core are shown in tablet front view. (B) The fabricated constant release profile core–shell tablet in PBS while it is eroding. (C) The linear fractional drug release after the ∼5 hrs induction period and the constant drug release rate on the top left side.
Download figure:
Standard image High-resolution imageTo demonstrate the versatility of the tablet design, a different core–shell tablet was designed and fabricated (figures 8(A) and (B)). The core was fabricated for a decreasing and then increasing release profile of the orange G model compound. The surface area of the tablet core from the top along its height is decreasing and then increasing. Figure 8(C) shows the fabricated core–shell tablet for decreasing-increasing release profile (figure 8(B)) made of PA (initial mole ratio of PNA:PETMP:EGDT = 100:75:25) in PBS solution after the first half of the erosion. The fractional cumulative release of the model compound was calculated and plotted (figure 8(D)) over time. The fractional release of the model compound shows two distinct sections, one during the erosion of the decreasing part and the other happens during the erosion of the increasing part. The data for these two parts were fitted to two quadratic models using MATLAB MathWorks software. On the top left on the graph in figure 8(D), the release rates of the orange G model compound were plotted which shows a decreasing and then an increasing release rate over time.
Figure 8. The decreasing-increasing release profile core–shell tablet. (A) Schematic representative of the tablet core with decreasing and then increasing surface area from the top along its height and the location of the tablet core shown in front view. (B) The fabricated decreasing-increasing tablet core. (C) The fabricated decreasing-increasing release profile core–shell tablet in PBS while it is eroding. (D) The fractional orange G model compound release after the ∼4.5 hrs induction period and the decreasing-increasing release rates on the top left side.
Download figure:
Standard image High-resolution imageRelease rates calculated for the constant and the decreasing-increasing release profile core–shell tablets showed the feasibility of tuning the pattern of release of the orange G model compound from the polymer using the proposed core–shell tablet design. The release profile patterns were only dependent on the tablet's core geometry, particularly the variations in its surface area from the top along its height as well as the location of the tablet core inside the shell. The linear reduction rates of the erodible tablets' dimensions make it possible to estimate the release profiles of the drugs from the tablets. Compared to other studies conducted on obtaining desired release profiles from polymeric tablets [41–43], the presented method is more straightforward and does not require complex modeling and/or solving of complicated equations for designing tablets with adjustable release profiles.
3.5. Modified tablet designs towards increasing the loading capacity of the delivery system
The drug-loading capacity of a polymeric delivery system is the total amount of drug that can be loaded in the system and depends on the polymer, drug, fabrication method, and the design of the DDS. In the tablet design, a higher intensity UV-light source was required to cross-link the polymers containing higher amounts of the orange G model compound (or any other therapeutics). The high-intensity UV-light transfers very high energy which may damage the loaded therapeutics. By modifying the tablet design and utilizing the extra space in the shell polymer, higher amounts of the model compound can be loaded into the tablets without increasing the total tablet volumes.
The height and diameter reduction rates for polymers used in this study were measured before and results showed the similarity of the reduction rates. One-arm, two-arm, and four-arm core–shell tablets were fabricated (figure 9(A)) using the throughput platform. The same weight percentage of the orange G was added to these three tablet designs so that the total initial amount of the orange G dye loaded in the two-arm and four-arm tablets were two and four times higher than the one-arm loaded dye, respectively. The cumulative release of the orange G from all three tablets was calculated and the mean values from triplicate experiments were plotted as shown in figure 9(B). Linear curves were fitted to data and slopes were calculated, showing the constant release rates as expected (considering the tablet core designs). Figures 9(C)–(E) showed the tablets fabricated for constant release profiles while they are eroding in PBS.
Figure 9. Modified core–shell tablet designs to increase the loading capacity of the delivery system. (A) Three core–shell tablets, fabricated for constant release profile with one-arm, two-arm, and four-arm cores from left to right, respectively. (B) The cumulative amount of orange G released after the induction period and linear fitted curve to the one-arm (the blue line), two-arm (the green line), and four-arm (the red line) release data. The slopes show the constant release rates in mg/hr. The core–shell tablets fabricated for constant release profiles while eroding in PBS for (C) one-arm, (D) two-arm, (E) and four-arm tablets.
Download figure:
Standard image High-resolution imageCumulative release of the orange G over time was linear for all three fabricated tablets which provided evidence for a constant release profile. The slopes of the linear lines were calculated to show the constant release rates (figure 9(B)). The release rate of the four-arm tablet was almost four times higher than the release rate of the one-arm tablet, and the slope of the linear cumulative release of the two-arm tablet showed an almost two fold increase compared to the one-arm tablet. These findings also imply that using the four-arm design, it was possible to release the same amount of the drug by loading almost four-times lower weight percentage of the drug compared to the one-arm tablet. Consequently, the intensity of the UV light source required for curing the photocrosslinkable polymeric tablet cores is reduced which lowers the risk of damages to loaded drugs. Moreover, the results of this experiment showed that higher amounts of drug could be released using the other sides of the tablet as well as the top side, while preserving the desired release profile.
Previous studies have shown an increasing loading capacity of controlled release DDSs by changing the polymer compositions [44]. Various fabrication techniques such as hot-melt extrusion and injection molding were also used to increase the loading capacity of the polymeric tablets [45–47]. In this study, using the modified tablet designs which utilize more dimensions of the tablet for releasing the therapeutics, the loading capacity of the designed tablets was increased by approximately four times, in a straightforward fashion.
4. General discussion
This study presented a new core–shell tablet design in which the core is made of a surface eroding polymer loaded with a model compound, while the same polymer without any model compund was used for the shell. The position of the tablet core inside the shell is such that the core is closest to the top, and far enough from the other sides, ensuring that the release of the drug happens only from the top surface of the tablet. Accordingly, the release profile of the drug is only governed by the variations in the surface area of the drug-loaded core polymer along its height. This design relies on the surface eroding behavior of the polymer to preserve the tablet structure and integrity during erosion, as well as the similarity of the erosion rate at all sides of the tablet.
The tablet design requires only one type of surface eroding polymer in contrast to other designs that utilized multiple polymers in tablet fabrication [19, 20, 23, 25, 48, 49]. For example, PLA has been used in form of layers or containers for achieving delayed, sequential, and pulsatile release profiles as well as designs with adjustable release profiles [25, 50–52]. Using multiple polymers for manufacturing the tablets increases the time and complexity of the fabrication process. Besides, the interaction between different polymers used in a DDS may change their properties, and hence their erosion behavior. If not accounted for, this may result in erroneous estimations of the release profile of drug from the DDS. Therefore, in addition to characterizing the erosion behavior of each of the polymers separately before manufacturing the tablet, studies on characterizing the erosion behavior of each of the polymers from the multi-polymer DDS after it is manufactured is also needed. In addition, some polymers used in the multi-polymer DDSs have slow degradation rates in the human body environment, e.g. PLA that is usually utilized for fabricating outer layers or containers can take several months to degrade [53, 54]. The tablet design developed in this study overcomes the aforementioned issues by utilizing only one surface eroding polymer.
The design for achieving adjustable release rates relies on the similarity of the dimension reduction rate of different sides of the tablet, i.e. the independence of the dimension reduction rate from the orientation of the tablet. In this study, the diameter and height reduction rates of cylindrical tablets made of thiol-ene PAs were obtained from mass loss experiments and found to be linear and very close to each other. The linearity of the dimension reduction rates allows for estimating the dimension reduction rate of a new cylindrical design made from the same polymer, without the need for monitoring the tablet dimensions during the entire erosion time.
Scalable manufacturing is currently a challenge for producing advanced controlled-release DDSs with adjustable release rates. Most of the studies reported the fabrication of a single tablet in each experiment [23–25, 47, 55–57]. In this study, a platform for throughput manufacturing of core–shell tablets was developed to provide adjustable release profiles. Although we fabricated three tablets in one round of the fabrication process to demonstrate the feasibility of our platform, many tablets can be produced at once. These tablets are envisioned to be designed and manufactured for specific patients with individual variability factors. Therefore, the number of patient-specific tablets needed for each individual is considerably lower than the number of tablets that are mass-produced to treat an affected population. Currently, our design can fabricate 50–60 tablets using a 4-inch radius silicon wafer. As each of our tablets releases its content over a long time, this platform can provide sufficient numbers to treat patients in a personalized medicine context.
Tablet cores were fabricated using a microfluidic network consisting of two PDMS layers manufactured using conventional soft-lithography techniques. To prevent the leakage of the pre-polymer solution from the microfluidic network, two rigid PMMA sheets were used to sandwich the PDMS layers and to apply a uniform force through a screw/nut configuration. The successful injection of the pre-polymer solution via the inlet/outlet ports was achieved. To create the tablet shell, PDMS wells were successfully fabricated using 3D printed master molds. Aligners were designed to ensure the right positioning of the micro-fabricated tablet cores in the shell. High-resolution tablet shells were fabricated using DLP 3D printing for producing PDMS wells. DLP was chosen because it can create objects with more complex designs at a higher speed and higher resolutions compared to the other commercially available 3D printers such as FDM machines which are previously used for manufacturing tablets with adjustable release profiles [47, 55, 56]. The DLP printer has an accuracy of 96%–98% within the CAD design.
Results of the release rates measurements of the tablets designed for constant and the decreasing-increasing release profiles showed the feasibility of achieving tunable patterns of release using the proposed core–shell tablet design. Compared to previous studies, our method provides a more straightforward translation of the desired release profiles to the tablet design, owing to the linearity of the surface erosion rate observed in these experiments, along with the core–shell tablet design. Unlike other studies [41–43], complex modeling and/or solving of the complicated equations for designing tablets with adjustable release profiles was not required.
By modifying the tablet design and utilizing the extra space in the shell polymer, higher amounts of the orange G was loaded into the tablets without increasing the total tablet volumes. The modified design reduces the chances of UV-induced damage to therapeutics loaded in the tablet. One arm, two-arm, and four-arm designs for the core of the tablets were devised by using the radial directions for erosion. The two- and four-arm tablets preserved the estimated release pattern. The release rate of the four-arm tablet was almost four times higher than the release rate of the one-arm tablet and the slope of the linear cumulative release of the two-arm tablet showed an almost two-fold increase compared to the one-arm tablet. These findings demonstrated that using the four-arm design, it is possible to load (and release) the same amount of therapeutics at an approximately four-times lower weight percentage of drug loading compared to the one-arm tablet. This, in turn, decreases the required UV light energy for cross-linking the tablet cores and hence reducing the chances of damage to the loaded drugs. In this study, using the modified tablet designs, the loading capacity of the tablets was increased by approximately four times using a straightforward method.
5. Conclusion
In this work, we developed a new tablet design for achieving tunable release profiles of drugs using only one erodible polymer. We also developed a scalable fabrication platform for manufacturing personalized tablets. Utilizing microfabrication techniques allows for manufacturing tablets with complicated geometries and small sizes that could be explored for personalized pediatric patients. By modifying the tablet design, the drug loading capacity of our delivery system was improved by a factor of four. The proposed fabrication method which is a combination of the microfabrication and 3D printing techniques in conjugation with the new tablet design allows for the production of personalized tablets with tunable release profiles and hence is of high potential to be used in clinical settings.
It is noteworthy that the simultaneous fabrication of three tablets (with either constant or decreasing-increasing release profiles) was presented to demonstrate the potential of the proposed method for mass production of the personalized tablets. Testing a design that includes a higher number of simultaneously fabricated tablets is important to further validate the feasibility of the proposed method for mass production. In addition, the design for achieving adjustable release profiles should be used and tested for achieving other clinically relevant and in-demand release profiles with actual drugs. Finally, to improve the efficiency of the delivery system and to further increase the drug loading capacity, other sides of the tablet shell can also be utilized for drug release. Optimization of the design and the fabrication technique is required to realize this goal.
Acknowledgments
This work was supported by the Natural Science and Engineering Research Council (NSERC) of Canada and Canada Research Chairs Program.











