Abstract
Infectious keratitis is still one of the major causes of visual impairment and blindness, often affecting developing countries. Eye-drop therapy to reduce disease progression is the first line of treatment for infectious keratitis. The current limitations in controlling ophthalmic infections include rapid precorneal drug loss and the inability to provide long-term extraocular drug delivery. The aim of the present study was to develop a novel ophthalmic formulation to treat corneal infection. The formulation was prepared by constructing moxifloxacin (MFX) and dexamethasone (DEX)-loaded nanostructured lipid carriers (Lipo-MFX/DEX) mixed with a collagen/gelatin/alginate (CGA) biodegradable material (CGA-Lipo-MFX/DEX) for prolonged ocular application. The characteristics of the prepared Lipo-MFX/DEX nanoparticles were as follows: average size, 132.1 ± 73.58 nm; zeta potential, −6.27 ± 4.95 mV; entrapment efficiency, 91.5 ± 3.5%; drug content, 18.1 ± 1.7%. Our results indicated that CGA-Lipo-MFX/DEX could release an effective working concentration in 60 min and sustain the drug release for at least 12 h. CGA-Lipo-MFX/DEX did not produce significant toxicities, but it increased cell numbers when co-cultured with ocular epithelial cells. An animal study also confirmed that CGA-Lipo-MFX/DEX could inhibit pathogen microorganism growth and improve corneal wound healing. Our results suggest that CGA-Lipo-MFX/DEX could be a useful anti-inflammatory formulation for ophthalmological disease treatment.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Corneal infections usually occur because of exposure to bacterial, fungal, or other microbiological agents. Contact with contaminated hands, handkerchiefs, water, toys, and various utensils has been found to be the major cause that induces ocular inflammation. Conjunctivitis is the most common ophthalmic inflammatory illness [1] caused by pathogenic microorganisms such as viruses, bacteria, or fungi [2, 3]. Further, cataract surgery and corneal transplantation were also found to potentially result in postoperative inflammation or other complications. Severe corneal inflammation leads to ulceration and can even lead to blindness [4]. Completely eliminating the pathogens and controlling the immune response are the major steps involved in treating such ophthalmic inflammatory diseases.
The currently available delivery systems for ophthalmic drugs used to treat postoperative infection include ophthalmic formulations, such as eye drops, emulsions, hydrogels, or ointments [5]. The topical application of eye drops is the most conventional type of treatment used to reduce disease progression [6]. Based on the different characteristics of pathogenic microorganisms, there are various antibiotics available for treating ophthalmic inflammatory diseases. Among these, neomycin [7], erythromycin [8], and chloramphenicol [9] are generally used depending on the nature of the pathogen. The major concern associated with the current delivery methods of ophthalmic medications is that an effective drug concentration can only be sustained in the eyes for a short period of time [10]. To overcome this disadvantage of eye drops, therapeutic strategies, such as increasing the frequency of topical administration or altering the formulation of the medication, including changing it to an ointment form, have been considered [11]. However, frequent application and high drug concentrations may decrease patient compliance owing to increased levels of irritation and may increase the occurrence of severe local or systemic side effects. On the other hand, using ophthalmic ointments may increase the ocular bioavailability of the medication compared with using conventional eye drops; however, the high viscosity of ointments results in a sticky sensation on the eyelids, increased blinking reflex, and blurred vision, which also reduce the level of patient compliance [11]. Therefore, developing novel application techniques that could prolong the drug retention time in the local area would yield very promising results in terms of improving the clinical application of treatments for infective ophthalmological illnesses.
The application of liposomal nanoparticles in drug delivery has been significantly investigated over the last decade, and they have been found to result in various advantages, such as increasing the cellular uptake of drugs and stabilizing the physicochemical properties and bioavailability of both water-soluble drugs and drugs with poor water solubility [12]. Sustained-release drug delivery systems have been used in the treatment of several diseases, including in cancer and arthritis therapy [13–16]. Regarding ophthalmological illnesses, drug-loaded liposomal nanoparticles have been used in glaucoma therapy [17, 18]. However, the currently used liposomal nanoparticle formulation cannot stay on the precorneal surface for a long time for sustained drug release. Therefore, there is a need for the development of an improved slow-releasing formulation for treating ophthalmological infective illnesses.
Moxifloxacin (MFX) is a fluoroquinolone analogue that has been approved for the treatment of bacterial conjunctivitis, keratitis, and keratoconjunctivitis and has been in use for decades [19]. MFX can be used to treat ocular infections owing to its favorable tolerability and safety profile and broad spectrum and excellent antibacterial efficacy against microorganisms [19]. Because of the rapid loss and lower ocular bioavailability of topically applied drugs, frequent application or higher drug concentrations have been proposed. MFX-loaded liposomal nanoparticles have also been developed as a drug delivery system [20, 21]. However, there is a need for another carrier or biomaterial polymer that can be added to liposomal eye drops or to a constitutive-release formulation in order to prolong the attachment of the nanoparticles onto the surface of the cornea. Developing a new slow antibiotic-releasing formulation for treating ophthalmic infective illnesses would improve the therapeutic responses and quality of life (QOL) of patients.
The use of the conventional liposomal formulation or hydrogel that is applied in the case of ophthalmological illnesses and especially in that of infective diseases has been investigated for decades [10, 22, 23]. In our previous study, a novel ophthalmic formulation was developed by combining a collagen/gelatin/alginate (CGA) biomaterial with liposomal chloramphenicol in order to control infection [24]. In the current study, we aimed to develop a delivery system consisting of a dexamethasone (DEX) and MFX encapsulated liposomal nanoparticle-containing biodegradable film (CGA-Lipo-MFX/DEX) for application in ophthalmological disease treatment. This new ophthalmological formulation, CGA-Lipo-MFX/DEX, developed by combining liposomal nanoparticles with a hydrogel was not only able to release an effective dose/concentration of MFX and DEX in a short time but was also able to sustain the release of MFX and DEX for a long period. This novel formulation did not induce significant toxicities in the ocular epithelial cells. The results of an in vivo study also indicated that CGA-Lipo-MFX/DEX dramatically reduced microorganism growth and improved corneal wound healing. The aforementioned evidence indicates the safety and efficiency of CGA-Lipo-MFX/DEX in treating and promoting healing in corneal infection. This novel formulation could potentially be applied in ophthalmological disease treatment in the future.
2. Materials and methods
2.1. Cell culture
Human corneal epithelial cells (HCECs) and all the culture reagents were purchased from the American Type Culture Collection (ATCC) (Manassas, VA). The cells were maintained in fresh corneal epithelial cell basal medium (ATCC) supplemented with apo-transferrin (5 mg ml−1), epinephrine (1 mM), extract P (0.4%), hydrocortisone hemisuccinate (100 ng ml−1), L-glutamine (6 mM), recombinant human insulin (5 mg ml−1) corneal epithelial growth factor, and 10 U ml−1 penicillin/10 µg ml−1 streptomycin in a CO2 incubator at 37 °C and with 5% CO2. The cells were subcultured by trypsinization (0.25%; Invitrogen, Carlsbad, CA) and were dissociated at approximately 80% confluence. The culture media were changed every 2–3 d.
2.2. Material preparation
Collagen (type I), gelatin, sodium alginate, calcium chloride, sodium citrate, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and chloramphenicol powder were all purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO).
2.3. Preparation of MFX and DEX containing liposomes (Lipo-MFX/DEX)
The protocol involved in preparing liposomal antibiotics has previously been described [25]. Briefly, 50 mg of MFX combined with 7.7 mg of cholesterol and 110.6 mg of DSPC (molar ratio = 14:15:2) were dissolved in 5 ml of methylene chloride. The solvent was evaporated in a rotary evaporator at 50 °C, and the solvent traces were removed by placing the mixture in a 60 °C oven for 30 min. The phospholipid film was hydrated with either 5 or 10 mg of DEX-containing double-distilled water using a probe-type sonicator at 35 W for 30 min, which facilitated the self-assembling of Lipo-MFX/DEX. The liposomal system was then filtered using a 0.2 μM filter and was purified using a PD-10 column to deplete the non-encapsulated MFX in the supernatant.
2.4. Preparation of the biodegradable film (CGA)
The currently available liposomal nanoparticle-based eye drops or suspensions are not able to stay on the corneal surface for sustained drug release. To overcome this limitation, we investigated the use of a biodegradable film as a carrier to load Lipo-MFX/DEX. The biodegradable film that we used was the material that facilitated the attachment of Lipo-MFX/DEX for sustained drug release, as described previously [26]. To prepare the biodegradable film, collagen, gelatin, sodium alginate calcium chloride, and sodium citrate powder were dissolved in phosphate-buffered saline (PBS) for further experimentation, as described previously [26]. Briefly, a stock solution of gelatin and alginate was added to a 1 mg ml−1 collagen solution in order to adjust the final concentration of the hydrogel to 1% alginate and 3% gelatin. Subsequently, 400 μl of the hydrogel was applied onto a 6-well cell culture plate (BD Falcon, Bedford, MA) to form a film with a thickness of ∼42 μm. The film was then chemically cross-linked by treating it with a 3% calcium chloride solution for 30 min, following which it was washed in PBS three times at room temperature.
2.5. Preparation of CGA-Lipo-MFX/DEX
Our first step at this stage was to establish the appropriate formulation of CGA-Lipo-MFX/DEX for ophthalmic use. Based on our degradation curve measurement, we developed a formula to determine the relationship between the molar ratio of sodium citrate to sodium alginate with the CGA decomposition time: Y = 12.3314X2 − 38.6691X + 29.2180 (where Y is the CGA decomposition time, and X is the molar ratio of sodium citrate to sodium alginate) [24]. Based on this degradation formula, we designed an 8 h degradable formulation by adjusting the sodium citrate/sodium alginate ratio in our investigation.
To generate CGA-Lipo-MFX/DEX, Lipo-MFX/DEX was mixed with a liquefied CGA solution at 50 °C and was allowed to stand at room temperature for 2 h in order for the CGA complex to solidify. The solidified CGA complex was rinsed with 3% calcium chloride and was stabilized to form CGA-Lipo-MFX/DEX. The final sodium citrate concentration was calibrated and determined to be 0.01%. Owing to the properties of the hydrogel, CGA-Lipo-MFX/DEX could attach onto the corneal surface for a long time. The released MFX or DEX from the liposomes was trapped in the CGA until the matrix degraded.
2.6. Microscopic analysis and transmission electron microscopy (TEM)
TEM examinations were performed for the direct visual monitoring of the individual particle sizes of the prepared liposomes. Briefly, a drop of a sample was placed onto a carbon-coated copper grid (Polysciences Inc. Warrington PA) along with a drop of 2% phosphotungstic acid to create a thin film, and the excess solution was drained off using filter paper. The samples were also subjected to negative staining with a 2% w v–1 aqueous uranyl acetate solution. The grid was allowed to air dry thoroughly, and the samples were then viewed using a Hitachi transmission electron microscope (Hitachi H-7650, Tokyo, Japan). The TEM images and electron diffraction patterns were visualized and collected by a soft imaging software program.
2.7. Zeta potential and particles size analysis of liposomes
To obtain the mean size and polydispersity index (PDI), the zeta potential values were calculated using the Malvern Zetasizer Nano ZS90 system (Malvern Instruments Ltd., Malvern, UK) based on the electrophoretic mobility by means of the Helmholtz-Smoluchowski relationship [27]. The size and surface charge of the MFX and DEX loaded liposomes were measured by dynamic light scattering (DLS; Delsa™ Nano Particle Analyzer; Beckman Coulter, Fullerton, CA, USA) and zeta potential (Beckman Coulter, Brea, CA) calculations, respectively. The autocorrelation function of the scattered light was analyzed via the cumulate method, as described previously [28].
2.8. The measurement of loading capacity (LC) and encapsulation efficiency (EE)
Both LC and EE are indications of the quantity of drugs entrapped within liposomal formulations. The amount of entrapped MFX was measured by spectrophotometry at a detection wavelength of 295 nm that is the maximum absorbance wavelength of MFX and was analyzed quantitatively by comparing the result with the standard curve.
To detect the quantity of DEX, samples from each group were collected and tested using a DEX enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource, San Diego, CA) according to the manufacturer instructions. The plates were read at 450/630 nm dual wavelengths. The samples were tested in triplicates, and the mean values were calculated. The LC % and EE % were calculated using the following equations [29, 30]:
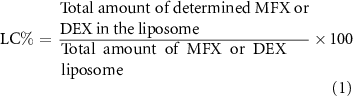
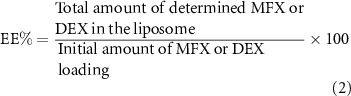
2.9. Measurement of in vitro release
Based on our previous investigation, we established the formula to determine the relationship between the molar ratio of sodium citrate to sodium alginate with CGA decomposition time [24]. The CGA complex completely degraded in 0.01% sodium citrate. The in vitro release profiles of MFX or DEX from CGA-Lipo-MFX/DEX were studied, as described previously [31]. Briefly, a 2 ml volume of CGA-Lipo-MFX/DEX was put in a dialysis bag (molecular weight cutoff 3.5 kDa) with 0.01% sodium citrate, and the dialysis bag was incubated in 50 ml of PBS (pH ∼ 7.4) and gently stirred at 37 °C, and the release medium was collected at predetermined time intervals. To detect the amount of released MFX, the samples were quantified via spectrophotometry at a detection wavelength of 295 nm and were analyzed quantitatively by comparisons with the standard curve, as described above.
To detect the amount of released DEX, the samples were quantified using a DEX ELISA kit (MyBioSource, San Diego, CA) according to the manufacturer's instructions, as described above. The samples were tested in triplicates, and the mean values were calculated. All the results were expressed as mean ± standard deviation.
2.10. Culture of Bacillus strains and growth inhibition assays
Bacillus strains (Escherichia coli (E. coli)) were cultured in sterilized glass flasks containing 250 ml of BG-11 medium, as described previously [32]. The temperature was maintained at 37 °C in a standard incubator. No bacterial contamination was detected during a microscopic examination of the culture. The growth curve of E. coli was determined by spectrophotometry at a wavelength of 600 nm.
To determine the growth inhibition of E. coli induced by CGA- Lipo-MFX/DEX, 50 ml E. coli suspensions were treated with the control, CGA only, or the following formulations: CGA-Lipo-MFX, CGA-Lipo-DEX, or CGA-Lipo-MFX/DEX. E. coli suspensions were collected at predetermined time intervals, and the concentration was measured by spectrophotometry, as described above. The inhibition efficiency was calculated using the following standard formula:

To further investigate the growth inhibition effects induced by CGA- Lipo-MFX/DEX on E. coli, the direct colony counting method was also performed, as described previously [33]. Briefly, 10 μl E. coli suspensions collected from the samples with the various slow antibiotic-releasing formulations at different time intervals were added to 500 μl of BG-11 medium and then cultured on a BG-11 agar plate in an incubator at 37 °C for 12 h. The colony-counting numbers were calibrated using the BioCapt v.11.02 software program (Vilber Lourmat, Cedex, France).
2.11. Detection and quantification of corneal epithelial cell cytotoxicity by direct counting
To evaluate and quantify corneal epithelial cell cytotoxicity resulting from CGA-Lipo-MFX/DEX, a traditional direct cell counting method was performed, as previously described, with some modifications [34, 35]. Briefly, CGA, CGA-Lipo-MFX, CGA-Lipo-DEX, and CGA-Lipo-MFX/DEX were first placed in 6-well plates, onto which 1 × 105 corneal epithelial cells were seeded. The cells were then incubated in complete corneal epithelial cell growth medium for 12 h. After incubation, the cells were trypsinized, and cell suspensions in 1 × PBS were incubated with trypan blue (0.4%) (Sigma-Aldrich, St. Louis, MO) (1:1 volume) for 5 min and were assessed using a hemocytometer. The cell numbers in each sample were counted in five random experiments, and the percentage of cytotoxic cells was calculated as the number of CGA-Lipo-MFX, CGA-Lipo-DEX, or CGA-Lipo-MFX/DEX cells/the number of CGA-treated cells that were counted.
2.12. Mouse model of corneal wound healing
To evaluate and quantify the wound healing effect of CGA-Lipo-MFX/DEX in vivo, the CGA-Lipo-MFX/DEX treatment was administered to a central corneal epithelial debridement mouse model, as described previously [36, 37]. Briefly, C57BL/6 mice were anesthetized via intraperitoneal injections of ketamine hydrochloride (2 mg g−1 body weight) and xylazine (0.4 mg g−1 body weight). After the topical application of one drop of proparacaine (Alcaine; Alcon Laboratories, Inc. Fort Worth, TX) in each eye, the central cornea was marked using a trephine with a diameter of 2 mm, and the epithelium was debrided using a corneal rust ring remover with a 0.5 mm burr (Algerbrush IITM; Alger Equipment Co., Inc. Lago Vista, TX) under a stereomicroscope (SV11; Carl Zeiss Meditec, Dublin, CA). The animals were sacrificed, and the eyeballs were isolated and then cultured in a 96-well plate. CGA, CGA-Lipo-MFX, and CGA-Lipo-MFX/DEX were seeded onto the top of the eyeballs. Subsequently, the specimens were cultured in sodium citrate-containing Dulbecco's modified eagle medium (DMEM) (Invitrogen-Gibco, Grand Island, NY) containing 1% fetal bovine serum (FBS) without antibiotics in a humidified atmosphere of 5% CO2 at 37 °C. After incubation, the extent of corneal wound closure was examined by fluorescein staining and was photographed with a digital camera (Axiovision; Carl Zeiss Meditec GmbH, Oberkochen, Germany). The eyeballs were then fixed in 4% paraformaldehyde in PBS and were embedded in paraffin for further experimentation.
2.13. Histopathological examination
The epithelium-debrided eyeballs from the mice were isolated and treated with CGA, CGA-Lipo-MFX, or CGA-Lipo-MFX/DEX and were cultured as described above. The specimens were then fixed in 10% buffered neutral formalin and were embedded in paraffin. Sections were cut at a thickness of 3–5 μm and were stained with hematoxylin and eosin. The histopathological changes, including cell morphology and the presence of metastatic tumor cells, were examined by light microscopy (40X and 200X).
2.14. Statistical analysis
Statistical analyses were carried out using SPSS version 6.0 for Windows and involved a one-way analysis of variance (ANOVA) and the Mann–Whitney U test. A P value of <0.05 was considered to be statistically significant.
3. Results
3.1. Characteristics of the Lipo-MFX/DEX complex
We synthesized Lipo-MFX/DEX at various doses based on different ratios of MFX/DEX, including 50:5 and 50:10, as described previously. The Lipo-MFX/DEX nanoparticle was observed by TEM (figure 1(A)). The TEM images of the Lipo-MFX/DEX morphology indicated that the sizes and spherical shapes of these nanoparticles were consistent with those revealed in the DLS analysis (figure 1(B) and table 1). The sizes of the Lipo-MFX/DEX particles with the ratios of 50:5 and 50:10 were 213.4 ± 171.8 nm and 435.3 ± 316.6 nm, respectively (figure 1(C) and table 1). The majority of the particles were approximately 0.2 nm in size, demonstrating that the solutions were homogeneous with a narrow size distribution. In further experiments, the EE of the designed MFX/DEX-loaded nanoparticles was examined. As shown in table 1, the EE of Lipo-MFX/DEX was 94.5 ± 3.8% (50:5) and 84.6 ± 5.2% (50:10), the drug content was 18.1 ± 1.4% (50:5) and 12.4 ± 2.2% (50:10), and the zeta potential was −4.8 ± 3.3 mV (50:5) and −3.7 ± 3.1 mV (50:10) (table 1).
Figure 1. Characteristics of various dosages of MFX/DEX-loaded liposomes. (A) Representative figures of MFX/DEX-loaded liposomal nanoparticles captured by transmittance electron microscopy. The left panel depicts an empty liposomal nanoparticle. The right panel depicts a liposomal nanoparticle that was loaded with MFX/DEX. (B) The representative figures of the size distribution of various dosages of MFX/DEX-loaded liposomal nanoparticles acquired by DLS analysis. (B1: 50 mg MFX/5 mg DEX-loaded liposomal nanoparticle, B2: 50 mg MFX/10 mg DEX-loaded liposomal nanoparticle). (C) Bar figures of size distribution of various dosages of MFX/DEX-loaded liposomal nanoparticles. Note: The average sizes of the MFX/DEX-loaded liposomal nanoparticles were approximately 200–400 nm.
Download figure:
Standard image High-resolution imageTable 1. Characteristics of various dosages of MFX/DEX-loaded liposomes.
| MFX/DEX (mg) | Encapsulation efficiency (%) |
Drug content (%) |
Mean size nm (PDI) |
Zeta potential (mV) |
|---|---|---|---|---|
| 50/5 | 94.5 ± 3.8% | 18.1 ± 1.4% | 213.4 ± 171.8 (0.266) | −4.8 ± 3.3 |
| 50/10 | 84.6 ± 5.2% | 12.4 ± 2.2% | 435.3 ± 316.6 (0.278) | −3.7 ± 3.1 |
aMFX/DEX encapsulation efficiency (%) = (weight of MFX/DEX in the liposomal nanoparticle/weight of the feeding MFX/DEX) × 100%. bDrug content (%) = (weight of MFX/DEX)/(weight of MFX/DEX + weight of polymer) × 100%. cAs determined by DLS after filtration through a 0.22 μm filter.
These results indicate that all the designed nanoparticles were successfully prepared at the nanoscale level; however, in comparison with the 50 mg MFX combined with 10 mg DEX-loaded liposomal nanoparticles, the 50 mg MFX combined with 5 mg DEX-loaded liposomal nanoparticles were superior in terms of EE and drug content. Consequently, we used the 50 mg MFX combined with 5 mg DEX-loaded liposomal nanoparticles as the standard preparation for further studies.
3.2. Lipo-MFX/DEX release profiles
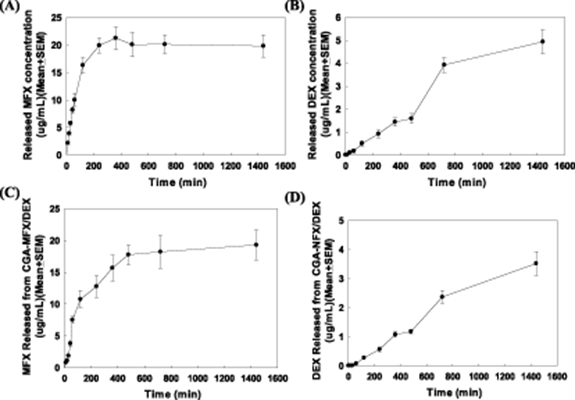
Next, we investigated the release profiles of MFX and DEX from Lipo-MFX/DEX in vitro. The release profile of MFX from the Lipo-MFX/DEX nanoparticles was evaluated by the dialysis bag diffusion method in PBS at 37 °C. It took 10 min for the Lipo-MFX/DEX formulation to release an effective dose of MFX (MIC90 = 1.5 μg ml−1). The following are the correlations between the timepoints and doses of MFX released: control, −0.01 ± 0.0 μg ml−1; 10 min, 2.2 ± 0.3 μg ml−1; 20 min, 3.9 ± 0.3 μg ml−1; 30 min, 5.8 ± 0.4 μg ml−1; 45 min, 8.2 ± 0.5 μg ml−1; 60 min, 10.0 ± 1.2 μg ml−1; 120 min, 16.3 ± 1.4 μg ml−1; 240 min, 19.9 ± 1.4 μg ml−1; 360 min, 21.3 ± 2.0 μg ml−1; 480 min, 20.1 ± 2.3 μg ml−1; 720 min, 20.1 ± 1.7 μg ml−1; and 1440 min, 19.8 ± 2.0 μg ml−1 (figure 2(A)).
Figure 2. The release profiles of various Lipo-MFX/DEX formulations in vitro. (A) MFX release concentrations from Lipo-MFX/DEX at different timepoints. Data were expressed as mean ± standard error of the mean (SEM). (B) DEX release concentrations from Lipo-MFX/DEX at different timepoints. (C) and (D) MFX/DEX release concentrations from CGA-Lipo MFX/DEX at different timepoints were also measured. Data were expressed as mean ± SEM.
Download figure:
Standard image High-resolution imageThe time-based DEX release profile of Lipo-MFX/DEX was also measured, as described. It took 30 min for Lipo-MFX/DEX to release an effective dose of DEX (40 ng ml−1). The correlations between the timepoints and doses of DEX released were similar to those of MFX (from −0.00 ± 0.00 to 4.9 ± 0.5 μg ml−1 at each timepoint, respectively, figure 2(B)).
These results reveal that the MFX and DEX encapsulated liposomal nanoparticles could release increasing concentrations of both the drugs. Not only could the Lipo-MFX/DEX complex release the effective dose/concentration of MFX and DEX in a short time, but it could also sustain the MFX and DEX release for a long period.
3.3. CGA-Lipo-MFX/DEX release profiles
We established the validity of the CGA complex degradation formula in our previous investigation. We also confirmed the EE and drug release profiles of Lipo-MFX/DEX. To develop a new effective MFX/DEX formulation for ophthalmic application, we combined the CGA complex with Lipo-MFX/DEX to form CGA-Lipo-MFX/DEX, a biomatrix loaded with liposomal MFX and DEX, which was formulated to degrade in 8 h. We then investigated the release of both MFX and DEX from CGA-Lipo-MFX/DEX. Figure 2 illustrates the results of the investigation of MFX and DEX-release from CGA-Lipo-MFX/DEX. As shown in figure 2, the release of MFX (figure 2(C)) and DEX (figure 2(D)) from CGA-Lipo-MFX/DEX resulted in an effective dose in 30 min (The timepoint-drug release correlation ranged from −0.0 ± 0.0 to 19.3 ± 2.4 μg ml−1 for MFX, figure 2(C)) and 60 min (The timepoint-drug release correlation ranged from −0.0 ± 0.0 to 3.5 ± 0.4 μg ml−1 at each timepoint for DEX, figure 2(D)), respectively.
Based on the results presented in figures 2(C) and (D), we confirmed that the release of both MFX and DEX from CGA-Lipo-MFX/DEX could be sustained stably. This formulation could not only generate an effective dose/concentration of MFX and DEX in a short time but could also sustain the release of MFX and DEX for a long period.
3.4. Bacterial growth inhibition ability of CGA-Lipo-MFX/DEX
We previously evaluated the release profiles of both MFX and DEX from CGA-Lipo-MFX/DEX in vitro. CGA-Lipo-MFX/DEX could generate an effective dose/concentration in 30 min and sustain the release of MFX and DEX for more than 12 h. We then investigated the efficacy of various CGA loaded MFX/DEX liposomal nanoparticles in inhibiting bacterial growth activity. An E. coli growth model was used to investigate MFX-inhibited pathogen proliferation in the current study. In the experiment, E. coli with the addition of normal saline at specific timepoints was used as the control group (100%). As shown in figure 4, CGA-Lipo-MFX/DEX could stably and constitutively suppress E. coli proliferation, which started in 2 h and was sustained for more than 24 h. The viability rate of E. coli at each timepoint was as follows: 2 h, 67.4 ± 15.7%; 4 h, 48.5 ± 10.2%; 6 h, 39.7 ± 5.9%; 8 h, 28.6 ± 4.2%; 12 h, 17.1 ± 3.3%; and 24 h, 17.4 ± 5.1% (figure 3(A)).
Figure 3. The proliferation inhibition ability of CGA-Lipo-MFX/DEX on E. coli treated in vitro. (A) Bar figure of the viability of E. coli treated with CGA-Lipo-MFX/DEX. CGA-Lipo-MFX/DEX could inhibit E. coli proliferation significantly. (B) Representative figures of colonies from E. coli treated with CGA-Lipo-MFX/DEX at different timepoints. (C) Bar figure of colony numbers of E. coli treated with CGA-Lipo-MFX/DEX at different timepoints. (★ indicates p< 0.05, ★★ indicates p < 0.01, one-way ANOVA).
Download figure:
Standard image High-resolution imageTo quantitate the inhibitory effect induced by CGA-Lipo-MFX/DEX on E. coli growth, direct colony counting experiments were conducted. The representative bacterial growth status at various timepoints is presented in figure 3(B). As shown in figure 3(C), CGA-Lipo-MFX/DEX significantly inhibited the number of E. coli colonies, which also started at the 2 h timepoint and was sustained for 24 h. The maximum inhibition effect was approximately 55.1% on allowing CGA-Lipo-MFX/DEX to act on the E. coli suspensions for 6 h.
The aforementioned evidence indicates that CGA-Lipo-MFX/DEX could not only generate an effective dose/concentration of both MFX and DEX in a short time and sustain MFX or DEX release for a long period, but it could also inhibit E. coli proliferation.
3.5. Biocompatibility analysis of CGA-Lipo-MFX/DEX
Our previous experiments have revealed the properties of CGA-Lipo-MFX/DEX in terms of rapidly generating an effective dose/concentration, sustaining the stable release of MFX/DEX, and inhibiting E. coli growth for a long period. We also investigated the biocompatibility of CGA-Lipo-MFX/DEX and whether this novel ophthalmic formulation produced corneal epithelial toxicity. Consequently, in the current study, we co-cultured corneal epithelial cells with CGA, CGA-Lipo-MFX, CGA-Lipo-DEX, or CGA-Lipo-MFX/DEX and then calibrated the viable cells in order to analyze the potential toxicity. Cells counts from the co-culture with CGA for 24 h were defined as the control group results (100%, figure 5). As shown in figure 4, the cell survival rate in the CGA-Lipo-DEX- and CGA-Lipo-MFX/DEX-treated groups were significantly higher compared to in the other groups (CGA-Lipo-MFX: 91.5 ± 3.5%, CGA-Lipo-DEX: 186.7 ± 18.1%, and CGA-Lipo-MFX/DEX: 216.2 ± 20.7, figure 4). No significant cell toxicity was noted in any of the groups. This evidence suggests that all the sustained MFX/DEX-releasing formulations including CGA-Lipo-MFX, CGA-Lipo-DEX, and CGA-Lipo-MFX/DEX did not induce significant toxicity in corneal epithelial cells. Moreover, with the addition of DEX, the epithelial cell proliferation increased. The proliferated cells might help in promoting corneal epithelial wound healing.
Figure 4. Biocompatibility analysis of CGA-Lipo-MFX/DEX. Bar figure of the viability of corneal epithelial cells co-cultured with CGA-Lipo-MFX/DEX. Corneal epithelial cell numbers were significantly increased on co-culturing with CGA-Lipo-DEX and CGA-Lipo-MFX/DEX. (★ indicates p< 0.05, ★★ indicates p < 0.01, one-way ANOVA).
Download figure:
Standard image High-resolution image3.6. Effects of CGA-Lipo-MFX/DEX on corneal epithelial wound healing
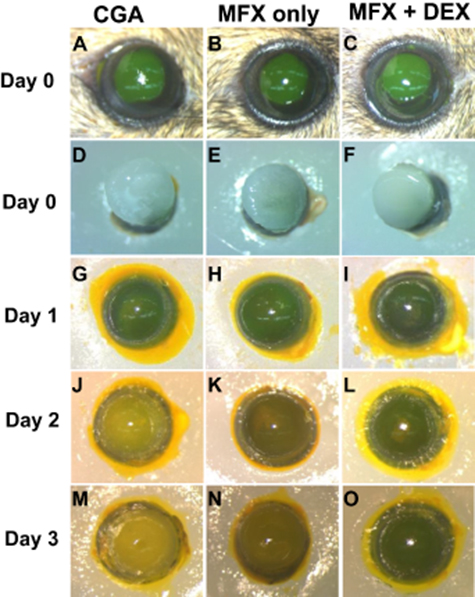
The previous evidence that we have presented has demonstrated that CGA-Lipo-MFX/DEX could not only suppress pathogenic microorganism growth but could also increase corneal epithelial cell proliferation. To validate these findings in vivo, we topically administered exogenous CGA-Lipo-MFX/DEX onto cultured corneal epithelial debridement mouse eyes in order to observe the anti-infection and corneal re-epithelialization effects. As shown in figure 5, the corneal epithelial cells were covered with CGA, CGA-Lipo-MFX, or CGA-Lipo-MFX/DEX on day 0. The eyeball became turbid in the CGA-treated group on day 1. The eyeballs in the CGA-Lipo-MFX or CGA-Lipo-MFX/DEX-treated groups remained clear until day 3. These results indicate that CGA-Lipo-MFX and CGA-Lipo-MFX/DEX could significantly inhibit microorganism growth in vivo.
Figure 5. CGA-Lipo-MFX/DEX inhibited bacterial growth and promoted corneal epithelial wound healing in wild-type mouse eyes. (A)–(C) Photographs depicting the representative results of fluorescein-stained eyes (green). An epithelial defect (2 mm in diameter) was created at the center of the corneas at D0. (D)–(F) The mouse eyes were enucleated and covered with CGA, CGA-Lipo-MFX, or CGA-Lipo-MFX/DEX and then cultured in DMEM with 1% FBS without any antibiotics for different time intervals. Re-epithelialization was observed at different timepoints as indicated (day 1 (G, H, I); day 2 (J, K, L); day 3 (M, N, O), respectively). (MFX only: CGA-Lipo-MFX; MFX + DEX: CGA-Lipo-MFX/DEX).
Download figure:
Standard image High-resolution image3.7. CGA-Lipo-MFX/DEX treatment reduced eyeball inflammation and edema
We subsequently determined if there were any differences in the levels of infiltration of leukocytes or lymphocytes within in the different eyeball samples treated with the various CGA loaded MFX/DEX liposomal nanoparticles. The corneal epithelial debridement mice were sacrificed, the eyeballs were excised, and tissue blocks were prepared using the CGA-, CGA-Lipo-MFX-, or CGA-Lipo-MFX/DEX-treated eyeballs, as described earlier. Representative figures of the infiltrating leukocytes within the eyeballs of the different treatment groups evaluated by hematoxylin and eosin staining are presented in figure 6. The corneal epithelial debridement eyeballs that were treated with CGA only exhibited increased inflammation and also edema progression without epithelial regeneration. As shown in figure 6, the corneal epithelial cells were scraped on day 1 in all groups. Epithelial cell growth was not enhanced in the mice that received only CGA as observed on day 3. In comparison with the CGA-treated group, the CGA-Lipo-MFX-treated group and especially the CGA-Lipo-MFX/DEX-treated group exhibited not only significantly decreased edema and inflammation but also enhanced epithelial cell regeneration. The number of infiltrating leukocytes was also dramatically reduced in the eyeballs treated with CGA-Lipo-MFX and especially in those treated with CGA-Lipo-MFX/DEX. This evidence indicates that the CGA-Lipo-MFX/DEX formulation produces dual effects including anti-bacterial growth and anti-inflammatory effects, which enhance corneal epithelial cell proliferation, thereby accelerating corneal wound healing.
Figure 6. CGA-Lipo-MFX/DEX inhibited bacteria growth and promoted corneal epithelial wound healing in wild-type mouse eyes. Left column ((A), (D), (G), (J), (M), (P)) hematoxylin and eosin staining revealed the histological changes in the CGA treated eyes (control group) on day 1 (A), (D), day 2 (G), (J), and day 3 (M), (P), respectively. Middle column ((B), (E), (H), (K), (N), (Q)); right column ((C), (F), (I), (L), (O), (R)) (scale bar (A)–(C); (G)–(I); (M)–(O) 40x: 200 μm; (D)–(F); (J)–(L); (P)–(R) 200x: 50 μm) (MFX only: CGA-Lipo-MFX; MFX + DEX: CGA-Lipo-MFX/DEX).
Download figure:
Standard image High-resolution image4. Discussion
MFX is a synthetic fluoroquinolone antibiotic agent that can be used to treat a broad spectrum of infections resulting from bacterial species [19]. Infections caused by both gram-positive and negative bacteria, including S. aureus, coagulase-negative staphylococci, S. pneumoniae, Enterobacteriaceae, and H. influenzae, can be treated using MFX [19]. Following treatment, MFX get widely distributed throughout various tissues and fluids, including saliva, mucosa of the sinuses, skin blister fluid, nasal and bronchial secretions, subcutaneous tissue, and skeletal muscle tissue, and the tissue concentrations often exceed the plasma concentrations [19]. The absorption and biodistribution of MFX are highly related to its formation concentration and particle size. The metabolism of MFX mainly depends on combining glucuronide and sulfate conjugation [38]. Approximately 14% of an oral dose of MFX is filtered through the glomerulus and discharged into the urine, thereby resulting in an effective anti-bacterial concentration [39].
MFX is generally well tolerated. Its most common side effects include nausea, vomiting, stomach pain, diarrhea, dizziness, and headache; however, these symptoms are usually mild [40]. Thus, MFX is an important treatment option for bacterial infections. With the advancement of medical research, it was made possible to use MFX eye drops as a topical medication. Owing to the topical application, the side effects of MFX were minimal. MFX hydrochloride ophthalmic solution (0.5%) is the most common ocular formulation/adaptation used in clinical practice. However, similar to the limitations of conventional eye drops, the need for frequent application may decrease the level of patient compliance. Moreover, high drug concentrations could also cause severe systemic and local side effects. Several approaches have been investigated to address the inherent drawbacks of the conventional types of delivery routes of ophthalmic MFX formulations [41, 42]. Our study was also focused on the development of new ophthalmic formulations to improve patient QOL. Based on the results of the current study, we developed a novel MFX/DEX-containing ophthalmic formulation, CGA-Lipo-MFX/DEX, which exhibited that it could release an effective concentration of the drugs in a short time and facilitate the sustained release for a long period. A biocompatibility analysis revealed that this new drug formulation did not result in significant cytotoxicity, but it increased the number of corneal epithelial cells. Thus, the subsequent development of CGA-Lipo-MFX/DEX has the potential to become the new therapeutic strategy for corneal infectious disorders including for corneal ulcers.
Liposomal nanoparticles have improved the therapeutic and delivery efficiency of various formulations and have been used in clinical applications for decades [43, 44]. In this study, a formulation consisting of CGA combined with liposomal MFX/DEX nanoparticles was developed and tested as a potential corneal infection treatment. In this study, we prepared DSPC for use as an emulsifying agent in order to improve the encapsulation and emulsification efficiency, as described previously. Tsotas and his colleagues have also demonstrated that the encapsulation of DEX was more effective in DSPC liposomes compared to in other phosphatidylcholine liposomes and that DEX could be displaced from the liposomes as the cholesterol content of the liposome membranes was increased [45, 46]. In addition, owing to the different drug delivery route, the new drug formulation developed in the current study will not directly enter the blood vessels and cause vascular occlusion.
The results of the current study were validated in both in vitro and in vivo experiments. E. coli was the only microorganism that was chosen as the infected model in order to demonstrate and test the anti-bacterial effects of the new ophthalmic formulations (figure 5). Apart from conjunctivitis caused by bacteria, E. coli is one of the most common pathogenic microorganisms responsible for eye infections [47, 48]. Additionally, S. aureus (25.88%) and coagulase-negative staphylococci (CoNS, 19.35%) may also result in ophthalmic infectious illnesses. These pathogenic microorganisms have been identified to have some antibiotic-resistant properties according to previous investigations [49, 50]. Due to these antibiotic-resistant properties, our new ophthalmic formulation, CGA-Lipo-MFX/DEX, could produce false negative results when used to treat bacterial infection-related diseases. To overcome this potential challenge, we used competent cells to confirm the anti-bacterial effects of our new formulation. Further, we used non-antibiotic media to culture the eyeball isolates from the central corneal epithelial debridement mice. Significant bacterial inhibition was demonstrated in this in vivo experiment. Further investigations will be conducted that are focused on the streptococcus and staphylococcus species in sequential studies in order to expand the applications of this novel slow-releasing pharmaceutical formulation.
The corneal stroma is composed of collagen fibrils with corneal keratocytes comprising approximately 90% of the entire structure. Foreign microorganisms that enter the eyes could digest collagen, resulting in corneal melting. Our previous investigations were focused on developing a biodegradable material containing collagen/gelatin/sodium alginate to be used as a scaffold for the liposomal nanoparticles to adhere onto the surface of the cornea. Previous studies have also demonstrated that materials with high biocompatibility may be a promising solution for use in tissue engineering applications [51, 52]. The results of both in vitro and in vivo experiments have confirmed that CGA-Lipo-MFX/DEX could not only generate an effective dose/concentration of MFX/DEX in a short time but could also sustain the MFX/DEX release for a long period (figures 5 and 6). Besides, owing to its high biocompatibility, the liposomal nanoparticle formulation could accelerate corneal wound healing (figure 6). Our formulation has the potential not only to enhance the medication adherence and QOL of patients but also to increase therapeutic efficiency.
Overall, the results of our current investigation on the efficacy of the new ophthalmic formulation that was developed to induce anti-bacterial effects demonstrate its potential as a treatment strategy for corneal infections. The beneficial effects of strategies based on similar mechanisms have been predominantly reported in the case of fungal keratitis-targeted therapy [53]. A biodegradable matrix involving liposomal antibiotic administration to the corneal surface has been found to be an appropriate option for treating cases involving keratitis, scleritis, iritis, and corneal ulcers and especially for preventing postoperative infection in patients. Our novel ophthalmic formulation could minimize the adverse responses that lead to acute antibiotic cytotoxicity in tissue that is not infected. However, there still are several challenges associated with the translation of this treatment modality to human clinical applications. A key issue is that the biomatrix should degrade at its setting time to reduce discomfort in order to enhance patient compliance. Moreover, the optimal dose, fraction, frequency, and timing of treatment must be optimized based on disease severity and location. Further research is warranted to facilitate the future application of this type of treatment.
5. Conclusion
In the development of ophthalmic formulations, the use of liposomal nanoparticles containing antibiotics that control pathogen growth by the sustained release of an effective antibiotic concentration has attracted considerable attention. Via the preparation of a thin collagen/collagen/gelatin film, a decomposition formulation was developed comprising a novel hydrogel with liposomal nanoparticles containing antibiotics that released an effective working drug concentration in a short time, and the drug release could be sustained for 12 h. The newly developed ophthalmic formulation did not exhibit any cytotoxic properties and effectively inhibited pathogenic bacterial proliferation. An animal study also confirmed that this novel ophthalmic formulation could inhibit pathogen microorganism growth and improve corneal wound healing. Thus, the novel drug releasing formulation has great potential as the future treatment strategy of ophthalmological diseases.
Acknowledgments
This study was funded by the Atomic Energy Council of Taiwan (Grant No. A-IE-01-03-02-02), Ministry of Science and Technology (Grant No. NMRPG3E6202-3), and Chang Gung Medical Research Project (Grant No. CMRPG3H1281). The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that no competing interests exist.