Abstract
The aim was to evaluate the effects of adding different functional monomers to experimental self-adhesive composites (SACs) on polymerization kinetics, cell metabolic activity, and sealing ability. SACs were formulated using urethane dimethacrylate as the base monomer and triethylene glycol dimethacrylate. Additionally, 10 wt.% of distinct functional monomers were added - 10-methacryloyloxydecyl dihydrogen phosphate, glycerol phosphate dimethacrylate (GPDM), 2-hydroxyethyl methacrylate (HEMA) or hydroxyethyl acrylamide (HEAA). ATR-FTIR was used to determine real-time polymerization kinetics (20 min, n = 3). The final extrapolated conversion and polymerization rates were determined (DC,max;Rp,max). The DC,max values were employed to calculate volumetric shrinkage. The MTT assay was performed on MDPC-23 cells using disc extracts at different concentrations (n = 8). Class V cavities were prepared in 60 sound human molars, assigned to six groups (n = 10), depending on the composite used and aging type (T0 or TC, if thermocycled for 10 000 cycles). One-way ANOVA, two-way, and Kruskal–Wallis tests were employed to treat the data (ɑ = 0.05). Varying the functional monomers had a large impact on DC,max, as confirmed by one-way ANOVA (p<0.001). The highest was obtained for HEMA (64 ± 3%). The HEMA and HEAA formulations were found to be significantly more toxic at concentrations below 100%. For microleakage, having a functional monomer or not did not show any improvement, irrespective of margin or aging period (Mann–Whitney U, p > 0.05). Larger functional monomers MDP and GPDM affected polymerization properties. Conversely, their acidity did not seem to be detrimental to cell metabolic activity. Regarding sealing ability, it seems that the functional monomers did not bring an advantage to the composites. Varying the functional monomer in SACs had a clear impact on the polymerization kinetics as well as on their cytotoxic potential. However, it did not confer better microleakage and sealing. Claiming self-adhesiveness based only on functional monomers seems dubious.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Conventional restorations that use resin-based materials require pretreatment of the enamel or dentin surface to increase their bonding ability. This encompasses the use of multiple steps or simplified adhesive systems. However, these systems, featuring several or just one application step, are still technique-sensitive and prone to degradation owing to their hydrophilic nature [1]. For this reason, following the trend of simplifying procedures, there is a need to find new, efficient, and long-lasting alternatives in the field of restorative dentistry [2, 3]. The Minamata Convention in 2013, which phased out amalgam use, sparked the drive to research new general-purpose dental restorative materials with competitive properties [4]. The demand for this type of material is increasing [5].
Self-adhesive composites (SACs) were introduced to simplify the restorative procedure, providing reduced application time, facilitated handling and technique sensitivity, and no need for a separate adhesive or pretreatment to bond to the substrate [1, 6]. The composition of such self-adhesive materials is similar to that of typical composite methacrylate systems, but is enriched with acidic functional monomers. These can be glycerophosphate dimethacrylate (GPDM) or 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP), which promote self-etching features and chemical bonding potential [7]. Enriching SACs with functional monomers is intended to establish chemical adhesion following superficial etching without requiring an adhesive system [3, 8–10]. This simplification aims to reduce the variability in the bonding procedure, the risk of clinical errors (because fewer steps are needed), the need for moisture control, and eventually also reduce postoperative sensitivity [11, 12]. This procedure also saves chair time and improves the cost-effectiveness of restorative treatment [1, 13]. However, despite numerous investigations and advances made to increase the longevity of dental polymers, SACs still fall short of their clinical objective [14, 15].
Ideally, the adhesive effectiveness of SAC resins should be similar to that of conventional restorative systems [16, 17]. However, they have shown significantly lower bond strengths compared to conventional restorative materials, applied using etch-and-rinse (E&R) or self-etch (SE) techniques, particularly when there is aprismatic enamel or dentin covered by a smear layer [13, 18–20]. The interaction between these self-adhesive resins and the substrate (specifically dentin) is very limited, as they demonstrate very little retention and poor sealing, resulting in significant clinical failure rates [3, 14, 21]. Delgado et al [22] also showed that these materials cannot form a proper hybrid layer as conventional adhesives. In contrast to conventional composites, SACs have a lower amount of filler particles, thus having lower elastic modulus and, consequently, lower mechanical strength and greater polymerization shrinkage [20, 21]. Additionally, hydrophilic monomers increase the rate of water absorption, leading to matrix expansion and polymeric disintegration [13]. Clinical studies have reported low success rates. Therefore, it is necessary to improve existing formulations on the market [13, 23, 24].
Few studies have compared the variation in functional monomers and their impact on viscous materials, that is, SACs. Varying the type of functional monomers will affect numerous crucial properties, such as polymerization, strength, bonding capacity, and water uptake potential, all of which are relevant to the longevity of self-adhesiveness. In addition, the cytotoxicity of leached products is a concern. Monomers such as 10-MDP have been suggested to have a high potential for causing cell membrane damage due to their strong interaction with phospholipid layers and other resin monomers, such as triethylene glycol dimethacrylate (TEGDMA) [18]. Other acidic monomers have also been shown to induce cytotoxic effects; however, research on this is scarce. Thus, assessment of the polymerization profile and toxicity of acidic material formulations is fundamental.
Although adhesive functional monomers are acidic, their acidity may not be sufficient to promote a relevant pattern of demineralization in dental substrates, which guarantees bonding and sealing of the cavity [3]. Previous studies have concluded that SACs present higher microleakage in enamel and dentin margins compared to when they are restored with an SE adhesive [25]. The same study showed that microleakage in dentin margins when using SACs was also higher than that of a conventional three-step E&R adhesive. This may be attributed to the hydrophilic/hydrophobic incompatibility of these materials, poor wettability, and consequently, very low bond strengths. Other studies also reported that using an adhesive with these composites improved dentin margin sealing [11, 23, 26, 27]. Varying the functional monomer in the formulation of these composites may improve their bonding outcomes, but this requires further evaluation.
Therefore, it is important to study the effect of varying the functional monomers on the properties mentioned so far. The polymerization profile, toxicity, and sealing appear to be monomer-dependent, and an optimal formulation and ratio should be investigated to improve the self-adhesiveness and bulk properties of upcoming materials. The aim of this study was to formulate different experimental SACs by varying the functional monomers included, and to test whether this would affect their (1) polymerization kinetics, (2) cytotoxicity, and (3) microleakage in enamel and dentin surfaces.
2. Materials and methods
2.1. Materials
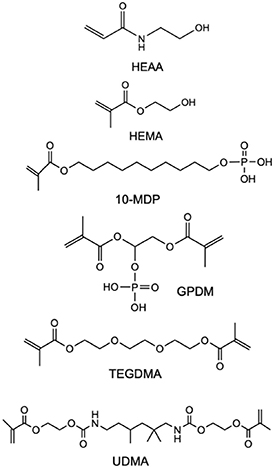
The monomers used in this study were 10-MDP from DM Healthcare, San Diego, CA, USA; 2-hydroxyethylmethacrylate (HEMA), obtained from Tokyo Chemical Industry, Tokyo, Japan; hydroxyethyl acrylamide (HEAA) (Tokyo Chemical Industry, Tokyo, Japan), glycerol phosphate dimethacrylate (GPDM; DM Healthcare, San Diego, CA, USA), triethylene glycol dimethacrylate (TEGDMA) from Polysciences (Warrington, PA, USA), and urethane dimethacrylate (UDMA) (Sigma-Aldrich, Schnelldorf, Germany). Camphorquinone was obtained from PCM Products GmbH (Krefeld, Germany), silica nanoparticles were obtained from Evonik Operations GmbH (Essen, Germany), and barium glass filler particles from SDI (Victoria, Australia). The latter items were donated by Dentsply Sirona. The chemical structures of the monomers are shown in figure 1.
Figure 1. Chemical structure of the functional monomers and conventional methacrylates used throughout the study.
Download figure:
Standard image High-resolution image2.2. Experimental composite preparation
In this study, five distinct experimental self-adhesive flowable composites were formulated by varying the functional monomer used in each formulation. The composites were prepared by combining a powder mixture containing a hybrid filler phase of silica nanoparticles and barium aluminosilicate glass and a liquid mixture containing different dimethacrylate and functional monomers. Monomer mixtures were prepared by combining the base monomer UDMA (60 wt. %) with 24 wt. % TEGDMA as a diluent. Different functional monomers were then added to four of the five experimental groups: 10-MDP, GPDM, HEAA or HEMA at 10 wt%. An additional control group was prepared, without functional monomers, but with an extra 10 wt% of UDMA, making up to 70%. Camphorquinone was added at a concentration of 1 wt. %. Magnetic stirring was then performed at 150 rpm for 24 h. The hybrid powder mixture was prepared in parallel. It included glass filler particles of different sizes: barium glasses at 1.5 μm and 0.4 μm and silica nanoparticles (>100 nm), added at a 60 wt%, 30 wt% and 10 wt% ratio, respectively. The liquid was then poured onto the powder at a powder-to-liquid ratio of 1.2/1 ratio (powder-to-liquid), in opaque pots and mixed at 1500 rpm for 45 s in a centrifugal speed mixer (Flacktek Speed Mixer, Landrum, South Carolina). The filler particles and powder-liquid ratio remained the same in all formulations, with the only variable being the functional monomer. The composites were stored in opaque pots at 4 °C until use. This originated five different formulations, as shown below.
- 1)UT_CTRL = 79 wt% UDMA + 20 wt% TEGDMA (control group)
- 2)UT_MDP = 69 wt% UDMA + 20 wt% TEGDMA + 10 wt% 10-MDP
- 3)UT_GPDM = 69 wt% UDMA + 20 wt% TEGDMA + 10 wt% GPDM
- 4)UT_HEAA = 69 wt% UDMA + 20 wt% TEGDMA + 10 wt% HEAA
- 5)UT_HEMA = 69 wt% UDMA + 20 wt% TEGDMA + 10 wt% HEMA
The choice of using weight percentages for formulating the composites is commonly employed in dental materials research and industry due to its simplicity and ease of measurement, and any potential bias resulting from the differences in molar mass of the functional monomers is likely to be minimal compared to the total molar mass of the composite.
2.3. Viscosity measurements
The viscosities of the five non-polymerized formulations were studied using a rheometer (MCR 92, RhemCompassTM software (Anton Paar, Virginia, USA)) with a cone-plate geometry (CP50) at 25 °C. Viscosity curves were obtained in the shear rate range of 0–100 s−1. The measurements were performed in triplicate for each formulation.
2.4. Polymerization kinetics
ATR-FTIR (Spectrum 65, Perkin-Elmer, MA, USA) was used to determine real-time polymerization kinetics for 20 min of 2 mm-thick composite discs (10 mm internal diameter) made by dispensing the composite into carbon-steel clips, at a resolution of 8 cm−1 (∼2 s temporal resolution). The discs were covered with an acetate sheet, and their top surfaces were individually irradiated for 20 s using an LED blue light curing unit (LCU DB686; COXO Medical Instrument Co., Guangzhou, China), with a measured peak irradiance of 950 mW cm−2, contacting the acetate (zero distance). Irradiance was monitored every three exposures using an analog radiometer. Final extrapolated conversions (DC ,max) were determined, and rates of polymerization (Rp ,max) were derived following the method described in [28]. The DC ,max values were calculated using equation (1):
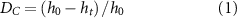
h0
and ht
were taken as the peak absorbance at 1319 cm−1 wavenumber, above background at 1352 cm−1 initially, and at time t after the start of the mixing. The final degree of conversion ( ) was obtained by linear extrapolation of late-time DC
values versus inverse time to zero (as the inverse of zero is infinity). These final DC
values were further employed to calculate the theoretical polymerization shrinkage, which are linearly related values [29]. The same method already employed in previous publications was used [28, 30]. It was assumed that one mole of polymerizing carbon-carbon double bonds in methacrylates resulted in a volumetric shrinkage of 23 cm3. The number of moles of reacted double bonds per unit volume can then be calculated using equation (2):
) was obtained by linear extrapolation of late-time DC
values versus inverse time to zero (as the inverse of zero is infinity). These final DC
values were further employed to calculate the theoretical polymerization shrinkage, which are linearly related values [29]. The same method already employed in previous publications was used [28, 30]. It was assumed that one mole of polymerizing carbon-carbon double bonds in methacrylates resulted in a volumetric shrinkage of 23 cm3. The number of moles of reacted double bonds per unit volume can then be calculated using equation (2):
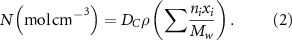
Σ indicates the sum of all monomers present in the liquid phase. For each monomer, Mw is molecular weight (g mol−1), ni number of double bonds per molecule, xi their mass fraction of the composite, ρ (g cm−3) is the composite density. Polymerization shrinkage as a percentage was then estimated using equation (3):
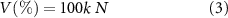
where k is 23 cm3.
2.5. Metabolic activity—MTT assay and pH measurement
Cytotoxicity tests were performed according to the ISO 10993-5 [31]. The mouse odontoblast MDPC-23 cell line, kindly provided by Professor Jacques Nör (University of Michigan, Ann Arbor, MI, USA), was used as an experimental model. The cell line was cultured in adherent conditions using Dulbecco's Modified Eagle's medium (DMEM) (Sigma-Aldrich D8900), supplemented with 10% heat-inactivated fetal bovine serum (Gibco 2010–09) and maintained at 37 °C in a humidified atmosphere with 95% air and 5% CO2 in a cell incubator (Heraeus HeraCell 150 CO2–BridgePath Scientific, MD, USA). Polymerized discs were made using O-Ring-type silicone rings (10 mm diameter, 1 mm thickness; n= 8), with the aid of a spatula. Each disc was covered with an acetate sheet and polymerized in a standardized approach following an overlapping pattern on four corners, as described in ISO 10993-5 [31], for 20 s at each corner, on both sides. The discs were covered with an acetate sheet and polymerized as described in the previous section. The discs were then subjected to UV sterilization using a UV sterilization device (Ultragen, Viseu, Portugal). Conditioned cell culture media were obtained for each group following a previously described protocol [30]. The sterilized discs were placed in Falcon® tubes, and an adequate amount of DMEM was added to obtain a 250 mm2 ml−1 contact surface. The tubes were kept on rotation for 24 h (Sarstedt 62.554.502, Nümbrecht, Germany) in a HeraCell 150 incubator (Thermo Electron Corporation, Palm Beach, FL, USA) with 95% relative humidity, 5% of CO2, and temperature of 37 °C.
After incubation, the obtained medium was considered 100%, and serial dilutions were performed with fresh DMEM to obtain the 50%, 25%, 12,5% and 6.25% concentrations.
Metabolic activity was determined using the colorimetric 3-(4,5-dimethyl thiazolyl-2)2,5- diphenyltetrazolium bromide (MTT) assay [32]. A 250.000 cells ml−1 cell suspension was plated and left overnight to allow cell adherence. The next day, conditioned media at different concentrations were added. Cells treated with DMEM alone were used as controls. Metabolic activity was evaluated 24 h after exposure by adding MTT (0.5 mg ml−1, Sigma, St. Louis, MO, USA) in phosphate buffer solution (PBS), pH 7.4, in the dark at 37 °C for 4 h. The formazan crystals were solubilized with a 0.04 M solution of hydrochloric acid in isopropanol, and the absorbance was measured using a Perkin-Elmer Enspire plate reader. Cytotoxicity was calculated as a percentage of the metabolic activity inhibition in treated cultures, correlated with the control group, which was considered as 100%. A minimum of three independent experiments were performed. pH measurements were performed in triplicate for each composite and the respective concentrations of the conditioned media using a pH meter that was previously calibrated with pH solutions (ISE 710 A, Orion Research Inc., Boston, MA, USA).
2.6. Microleakage
Sixty extracted sound human third molars were obtained with approved consent and were approved by the Ethics Committee of Instituto Universitário Egas Moniz (Proc. No. 1048) were randomly assigned to six experimental groups (n= 10) according to the composite used in the restorative procedure. The five groups were the composites described in 2.2, while the sixth group was a positive commercial control group, in which the restorations were performed with a gold standard etch-and-rinse adhesive, Optibond FL® (Kerr, Orange, CA, USA), followed by 3 M™ Filtek™ Z250 (3 M ESPE, St Paul, MN, USA), A2 shade, to simulate a conventional restorative procedure (OBFL_Filtek). Class V standardized cavities with 6 mm width, 2 mm height and 1.5 mm depth were prepared in each sample using a rotatory instrument calibrating platform and a tapered cylindrical diamond bur (#842-018), mounted in a dental piece. Restoration was performed immediately after cavity preparation.
The restorative procedure for the experimental SACs was performed without acid etching or any adhesive system by directly applying a thin layer of the composite (∼1 mm) with the aid of a spatula. The composites were packed and compacted within preformed cavities. Each composite increment was cured for 20 s at a distance of ∼0 mm, with the LED blue light curing unit operating at a peak irradiance of 950 mW cm−2.
In the OBFL_FILTEK group, each tooth cavity surface was pre-etched with 37.5% orthophosphoric acid (Gel Etchant, Kerr, Orange, CA, USA) for 15 s, and then washed for 15 s, and dried carefully to maintain dentin hydration. The primer was applied with a microbrush, rubbed on the cavity surface for 15 s, and dried using an air stream for 10 s. Bonds were applied and light-cured for 10 s. Subsequently, 2 mm increments of Filtek™ Z250 were applied to the cavity and light-cured until the cavity was filled.
All samples were stored in distilled water in an incubator at 37 °C for 24 h. Samples were further subdivided into two additional groups: artificial aging simulation by thermocycling (TC) and no aging (24 h). Aging was performed using a thermocycler (SD Mechatronik GmbH, Feldkirchen-Westerham, Germany) for 10 000 cycles, oscillating between 5 °C and 55 °C temperature baths. All samples were sealed and immersed in 0.5% basic fuchsine for 24 h [33, 34]. Each sample was sectioned in the occlusal–cervical direction under running water using a cut-off machine (Accutom-50, Struers, Ballerup, Denmark), and observed in each margin, occlusal and cervical, with a stereoscopic magnifier allowing score classification through a semi-quantitative scale: score 0—no dye penetration; score 1—dye penetration to half the depth of the cavity wall; score 2—dye penetration exceeding half the depth of the cavity wall without reaching the axial wall: score 3—dye penetration reaching the axial wall, as described in [35].
2.7. Statistical analysis
Statistical hypothesis testing was performed using OriginPro 2021 for Windows (OriginLab, Northampton, MA, USA). Tests included one-way ANOVA for polymerization properties, followed by Tukey's HSD and two-way ANOVA for inferential analyses of the cell metabolic influence, with formulation and concentration as factors (and Bonferroni as the chosen post-hoc test). These were employed following the normality and heteroscedasticity tests. Since microleakage data were heteroscedastic, the Kruskal-Wallis test (α = 0.05), followed by the Mann-Whitney U test, was used.
3. Results
3.1. Viscosity analysis
All formulations demonstrated shear-thickening flow behavior as their viscosity increased with increasing shear rates. The viscosity values at a shear rate of 1 s−1 are depicted in table 1.
Table 1. Viscosity values for the non-polymerized formulations at a shear rate of 1 s−1. The error bars are the ± standard deviation (n = 3).
| Non-polymerized formulation | Viscosity (mPa s, at 1 s−1) |
|---|---|
| UT_CTRL | 1.99 × 104 ± 7.01 × 103 |
| UT_MDP | 2.00 × 106 ± 3.01 × 105 |
| UT_GPDM | 3.51 × 104 ± 1.17 × 104 |
| UT_HEAA | 1.44 × 105 ± 2.27 × 104 |
| UT_HEMA | 5.69 × 103 ± 3.28 × 102 |
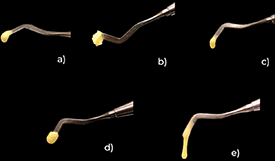
The UT_MDP formulation showed the highest viscosity values, whereas UT_HEMA was less viscous. UT_GPDM and UT_CTRL had values within the same order of magnitude.
The inclusion of different functional monomers resulted in different viscosities, which are portrayed in figure 2.
Figure 2. Different handling viscosities of the five experimental self-adhesive resins. (a) UT_CTRL, (b) UT_10MDP, (c) UT_GPDM, (d) UT_HEAA and (e) UT_HEMA.
Download figure:
Standard image High-resolution image3.2. Polymerization kinetics
The polymerization kinetic results are presented in table 2. The different monomer compositions had an impact on the overall DC ,max, mean (shown in figure 3), as confirmed by one-way ANOVA (p < 0.001).
Figure 3. DC ,max means for the five different experimental formulations.
Download figure:
Standard image High-resolution imageTable 2. Polymerization properties of the five distinct experimental composites, shown as means and standard errors (n = 3). Different letters within the same column indicate statistically significant differences (Tukey's HSD, p < 0.05).
| Polymerization properties | |||
|---|---|---|---|
| Material | DC ,max (%) | Rp ,max (%s−1) | Shrinkage (vol%) |
| UT_CTRL | 63 ± 2A | 2.2 ± 0.1A | 5.8 ± 0.1A |
| UT_HEMA | 64 ± 2A | 2.4 ± 0.1A | 6.1 ± 0.3A |
| UT_HEAA | 49 ± 12B | 3.3 ± 0.1B | 5.1 ± 0.3B |
| UT_GPDM | 45 ± 2B | 0.9 ± 0.1C | 4.4 ± 0.1C |
| UT_MDP | 45 ± 2B | 0.6 ± 0.1C | 4.2 ± 0.2C |
The highest value for DC ,max was obtained in the UT_HEMA formulation (64 ± 3%), which was also comparable to the control UT_CTRL (63 ± 2%), without any functional monomer. These groups differed significantly different to UT_HEAA (49 ± 12%, p< 0.05), UT_MPD (45 ± 2%, p< 0.05), and UT_GPDM (45 ± 2%, p < 0.05). The polymerization rates, results were largely material-dependent and all differed from each other (p < 0.01), except for UT_MDP and UT_GPDM, which were comparable (0.6 vs. 0.8%/s, respectively). Again, UT_CTRL and UT_HEMA outperformed the others, with a trend similar to that of DC ,max.
The volumetric shrinkage ranged from 4.2 to 6 vol%, in the following order: HEMA > CTRL > HEAA > GPDM > MDP. The experimental composite UT_HEMA reported the highest shrinkage, significantly different from UT_MDP (4.2 ± 0.2 vol%, p = 0.047) and UT_GPDM (4.4 ± 0.2 vol%, p = 0.031).
3.3. Metabolic activity—MTT and pH measurement
Paired comparison plots of cell metabolic activity for different extract concentrations are shown in figure 3. Differences were only registered for concentrations below 100% (50, 25, 12.5 and 6.25%). These were between the experimental groups containing HEMA and HEAA as functional monomers and the other groups. UT_CTRL, UT_MDP, and UT_GPDM were not significantly different (Bonferroni, p> 0.05).
Metabolic activity significantly decreased upon exposure to higher concentrations (two-way ANOVA model, factor: concentration; F = 240.4, p < 0.001). At a concentrations of 12.5% and below, almost all materials registered metabolic activity values higher than 50%. This trend is illustrated in figure 4.
Figure 4. (A) Metabolic activity in percentage, resulting from the MTT assay results, divided by extract concentration to enable rapid group comparison. Differences were observed at concentration ⩽50% (Bonferroni post-hoc). Error bars represent standard error of the mean. (B) shows a trend line for each concentration, which shows higher metabolic activities at more diluted concentrations of disc extract.
Download figure:
Standard image High-resolution imageThe pH of the retrieved extracts was above the neutral range spectrum. The maximum mean was 8.4 for the extract containing HEAA at 100% HEAA. No significant differences were observed between the measurements.
3.4. Microleakage
The distribution of microleakage scores, ranked from no microleakage (0) to involvement of the pulpal wall (3), in both tested margins (occlusal and cervical), is presented in table 3(A)/(B) after 24 h and after 10 000 TC cycles.
Table 3. (A)/(B). Frequency of each classification obtained, in percentages, for all experimental groups at 24 h of test and after 10.000 cycles of thermocycling. In table (A) the occlusal margin and in table (B) the cervical margin.
| A | OCCLUSAL MARGIN | |||||||
| 24 h | TC | 24 h | TC | 24 h | TC | 24 h | TC | |
| CLASSIFICATION | [0] | [0] | [1] | [1] | [2] | [2] | [3] | [3] |
| UT_CTRL | 80% | 20% | 0% | 0% | 0% | 0% | 20% | 80% |
| UT_MDP | 20% | 0% | 0% | 0% | 0% | 20% | 80% | 80% |
| UT_GPDM | 20% | 40% | 0% | 0% | 0% | 0% | 80% | 60% |
| UT_HEAA | 60% | 0% | 0% | 0% | 0% | 20% | 40% | 80% |
| UT_HEMA | 0% | 0% | 0% | 0% | 20% | 20% | 80% | 80% |
| OBFL_FILTEK | 100% | 100% | 0% | 0% | 0% | 0% | 0% | 0% |
| B | CERVICAL MARGIN | |||||||
| 24 h | TC | 24 h | TC | 24 h | TC | |||
| CLASSIFICATION | [0] | [0] | [1] | [1] | [2] | [2] | ||
| UT_CTRL | 60% | 20% | 0% | 0% | 40% | 80% | ||
| UT_MDP | 20% | 0% | 0% | 20% | 80% | 80% | ||
| UT_GPDM | 20% | 40% | 0% | 0% | 80% | 60% | ||
| UT_HEAA | 40% | 0% | 20% | 0% | 40% | 100% | ||
| UT_HEMA | 0% | 0% | 20% | 20% | 80% | 80% | ||
| OBFL_FILTEK | 100% | 100% | 0% | 0% | 0% | 0% | ||
All experimental SAC groups had similar microleakage scores. Differences were only registered when comparing the positive control OBFL_FILTEK to the experimental composites, as this group did not present microleakage in any margin or aging method studied. Thus, OBFL_FILTEK was significantly different from the UT_HEMA, UT_10-MDP, and UT_GPDM groups (p < 0.05) for any margin and time, except for the cervical margin at 24 h in the UT_CTRL (Mann-Whitney U, p = 0.19) and UT_HEAA (Mann-Whitney U, p = 0.11) groups. In addition, in the occlusal margin at 24 h, there were no differences between OBFL and UT_CTRL (Mann-Whitney U, p = 0.153) and UT_HEAA (Mann-Whitney U, p = 0.21). Although not statistically significant, UT_CTRL had a higher percentage of scores without microleakage compared to the other experimental groups at 24 h, which was also seen for UT_GPDM after aging.
4. Discussion
Studies on experimental SACs are scarce. Existing studies have focused on investigating the effects of varying the photoinitiator system [36], bond strength [16, 37], or the addition of functional particles to a new formulation. Recently, Delgado et al studied the addition of varying concentrations of 10-MDP to SACs and its effects on polymerization and mechanical properties [28]. To the best of our knowledge, the effects of adding different monomers to SACs have not yet been investigated.
The development of the polymerization reaction is determined by the chemical structures of the monomers. The presence of certain functional groups, molecular weight, and flexibility influences the polymerization rates and extent [38]. For this reason, flexible monomers with less bulky structures are added as reactive diluents to the mixture to improve viscosity and reactivity [39]. In this study, a UDMA/TEGDMA mixture was modified by the addition of different monomers. It is well documented that this matrix combination achieves reasonable conversion rates, as seen in the control group (UT_CTRL) in the present study.
The addition of monomethacrylates such as HEMA or 10-MDP, or an acrylamide such as HEAA, contributes to the formation of linear chains, which precedes the crosslinking stage in the earliest stages of the polymerization reaction [28]. However, as seen in earlier reports, MDP are also known to contribute to crosslinking [28]. Nonetheless, in this study, the MDP formulation in this study did not reach the same conversions as the ones seen in UDMA/PPGDMA systems, which can reach DC ,max values above 70%. These systems are more reactive due to increased flexibility and lower molecular weight of PPGDMA [28, 40]. UT_CTRL and UT_HEMA achieved the highest conversions, most likely because of the overall system viscosity. With lower initial viscosities, the reaction media mobility is improved, favoring polymerization rates and conversion levels [38]. This was further corroborated by the maximum rates of polymerization observed in the present study, which were lower for the UT_MDP and UT_GPDM formulations. This may be due to the increased viscosity of the system promoted by the structure of these monomers, which are large and bulky compared to that of HEMA, and/or steric hindrance effects [28]. GPDM is a dimethacrylate with methacrylate groups on both ends and a central phosphate group similar to 10-MDP [41]. As a dimethacrylate, it can contribute to crosslinking at a later stage of the reaction, but owing to its size, molecular mobility is also reduced, as with MDP, ultimately decreasing the chances of active site collisions during polymerization [28, 41]. This and the overall system viscosity could explain the polymerization rates and conversion levels observed for these mixtures. Additionally, 20 s of light-curing seems to be insufficient to guarantee decent polymerization rates. While fast near infra-red spectroscopy techniques could have been used to improve the temporal acquisition of data, as shown by previous authors [42], the ATR-FTIR technique was preferred due to the simplicity of the method, convenience of sample preparation and reliability of data adequate for an initial material property screening study.
The higher volumetric shrinkage observed in the UT_HEMA and UT_CTRL formulations is tightly linked to the increased conversion witnessed in these formulations, compared to the remaining formulations, while the lower shrinkage in formulations such as UT_MDP and UT_GPDM is due to the molecular weight compensation, which is higher for these monomers (322 and 356 g mol−1, respectively), and less methacrylate reactive groups per unit volume (cc), compared to UDMA/TEGDMA and HEMA [38].
The cytotoxicity study was developed following ISO 10993-5 [31] to simulate the appropriate conditions. Indirect cytotoxicity through material extracts was chosen as a model to simulate a restorative procedure placed in dentin, which is not in direct contact with the pulp complex [43]. However, in deep dentin cavities, diffusion of monomers present in these materials may occur through the dentinal tubules, as reported, eventually reaching the pulp tissues [44]. If this occurs, the odontoblastic layer will be the first to be affected. This justifies the use of MDPC-23 cells, which are an excellent model for cytotoxicity studies of restorative materials [38]. A 24-hour time point was chosen because the release of residual monomers, during the dark cure phase of polymerization, to oral fluids occurs gradually and mainly during the first 24 h [45, 46].
The harmful effect of resin composites on cells has been attributed to the release of residual monomers as a result of an incomplete polymerization reaction that is almost always inevitable, or to the by-products of resin matrix degradation processes [47]. It is well known that resin composites that reach a greater polymerization extent, with higher conversion levels, are less cytotoxic than those with impaired polymerization [48]. Although acidic functional monomers are frequently used in adhesive formulations, the effects of different monomer concentrations and chemistries on cytotoxicity remain largely unknown [18], and the acidity of the resulting materials can induce cytotoxic responses, such as in the case of luting materials, as verified in vitro [49].
At the highest tested concentration, all monomers showed an accentuated cytotoxic response. However, at lower concentrations, it was possible to discern differences in the cytotoxic behavior when the monomers were varied. These differences were observed between the experimental groups containing HEMA and HEAA as functional monomers and the other groups. A possible explanation for this is the relationship between the monomer structure and the degree of cytotoxicity [50]. The diffusion rate of a molecule is proportional to the square root of its molecular weight. Low molecular weight monomers such as HEMA and HEAA are more diffusible to cells and tissues [51]. HEMA and HEAA had lower molecular weights than the other monomers studied. Therefore, it is possible that the diffusion of these monomers occurred in greater amounts, leading to a greater reduction in metabolic activity. Another possible explanation is that different monomers cause cell death through different mechanisms, showing cytotoxicity at different concentrations [7]. The extracts from SACs containing GPDM and 10-MDP resulted in similar metabolic activity values for every studied concentration, and at lower concentrations, the metabolic activity values were higher than those of the other groups, suggesting that the decrease in metabolic activity occurred through similar mechanisms, not resulting in large variations between them.
Monomers such as HEMA and HEAA have a hydroxyl group (OH) at the end, conferring them with hydrophilic behavior and rapid ionization when in contact with water. This chemical behavior can easily generate hydrolysis phenomena, causing monomer leaching [52], which may lead to the lower metabolic activity values in this study. On the other hand, 10-MPD has a larger chemical structure, with a phosphate group and a co-polymerizable methacrylate group separated by a large spacer carbon chain. This structure confers hydrophobicity to this monomer, promoting more efficient interactions with molecules, such as collagen [49]. Compared to HEMA and HEAA, GPDM is also a larger molecule, with two co-polymerization methacrylate groups, which are able to participate in the crosslinking of the polymer network [11], which justifies its lesser diffusion compared to more hydrophilic, small monomers.
At all extract concentrations, UT_HEMA and UT_HEAA were the most cytotoxic monomers. The low levels of metabolic activity observed in the extract containing HEMA are consistent with those reported by Ferracane [5], that is, a high amount of unreacted free HEMA leached from the adhesive in deep demineralized dentin during resin infiltration, leading to cytotoxic effects.
According to the available literature [53], lower DC values may result in higher eluate cytotoxicity owing to the release of unbound free monomers. These results also reflect those of [54] and other authors, who tested the toxicity of the resin composite toxicity band and concluded that composites with lower conversions presented a higher degree of cytotoxicity. Therefore, the results observed in the extract containing HEAA discs can also be explained the low DC ,max this composite reached, of 49%.
It was hypothesized that the acidity of monomers might play a role in their toxic behavior. However, in this study, the pH did not seem to be important, since the results did not show substantial variations when comparing the pH before and after preparation of the extracts.
In contrast with this study's observations [55], other reports of self-adhesive resin cements, through an MTT assay in 3T3 fibroblast cells, concluded that the acidity caused by HEMA's ionization, present in their formulation, might have been responsible for the diffusion of toxic components. Accordingly, Şişmanoğlu et al [56] also studied the influence of pH variation in HL-60 cells and concluded that pH reduction is responsible for the increase in caspase activity and consequent cellular apoptosis. However, the results obtained in this study were different. The buffering capacity of the cell medium did not allow large pH variations during the first 24 h. As a future perspective and to enrich this screening test, it would be interesting to investigate cell viability, cellular death pathways via cytometry, protein content, or ROS production.
Considering that functional monomers are commonly used in dental resin formulations and taking into account the need for comparative studies between functional monomers, these results shed light on self-adhesive dental materials and their potential role in cytotoxicity.
Concerning the microleakage test, the difficulties of securing durable adhesion to enamel, mainly dentin, as dental substrates are currently the main sources of concern in dental adhesion research [57]. The correct infiltration of resin monomers and subsequent polymerization in the interfibrillar gaps of the collagen network must be ensured to achieve stability and longevity of adhesive treatments in dentin by creating a highly crosslinked and cohesive layer [58–60]. This is difficult to achieve in self-adhesive materials, especially in SACs, which are viscous pastes. While it is not a direct bond test, microleakage testing is widely accepted as a reliable and validated method for evaluating the quality of the adhesive interface in dental composites, presently used as a rapid screening test to assess these experimental formulations in regard to sealing [61].
Indeed, no differences were found between the experimental SACs formulated in terms of marginal microleakage scores at both margins and within each time point. This suggests that varying the functional monomer did not improve the sealing of the SACs, and the scores were poor for all SACs. The reason for the differences between SACs and E&R may be their poor wettability, influence of viscosity, poor hydrophilicity [62], and insufficient removal of the smear layer. It can also be derived from inadequate micromechanical retention between the restoration and tooth structures caused by the lower etching capacity of the SACs and possible lower flowability of SACs compared to dentin bonding agents [27, 63–66]. Therefore, E&R outperformed the remaining composites in terms of microleakage for both aging periods.
Several functional monomers were investigated in this study. Monomers such as 10-MDP, GPDM, HEMA, and HEAA have relatively hydrophilic amide, hydroxyl, phosphate, and ester groups [67], which make them more susceptible to hydrolysis in the oral environment. The water sorption phenomena at the adhesive interface, which may act as a permeable membrane, can result in the formation of water channels in the adhesive layer, where water can flow more freely, accelerating the degradation of the hydrophilic domains at the adhesive interface and in the oral cavity. The other primary process involved in adhesive failure, which compromises the long-term integrity of the bonded interface, is collagen degradation. Resin hydrolysis is also responsible for exposing collagen, which is then degraded by host-derived enzymes, such as matrix metalloproteinases, present in the oral environment and activated by acidity, which makes this cycle difficult to break [64]. Self-adhesive materials have been described to have an even greater propensity to absorb water than conventional composites, which causes the matrix to inflate and may further increase the penetration of salivary esterases that can accelerate the hydrolytic process and polymer chains to break [64]. SACs are characterized by a high hydrophilic monomer content, contributing to the increased permeability of the hybrid layer to water movement [65, 68], leading to hydrolytic degradation phenomena [65, 69, 70]. This may have contributed to the poor sealing seen in these materials, justified by water ingress and hydrolytic degradation, especially at the composite-dentin interface.
The experimental SACs used in this study also displayed different levels of viscosity (see table 1) owing to differences in the functional monomers included. Varying levels of viscosity may hamper the sealing ability of the composite, and the shear thickening behavior shown by the formulations can further complicate technical processes, namely, filling and molding processes. Additionally, flowable materials often have a higher water sorption rate than those with higher filler content [63]. Clinical failure of resin composite restorations due to disruption of the bonded dentin-composite interface continues to be a common occurrence. Such interfacial flaws may appear as a result of persistent thermal and mechanical strains or even during a deficient restorative process. This may be due to the stress caused by shrinkage during polymerization [64, 71]. Flowable composites, such as those evaluated in the present study, exhibit higher volumetric shrinkage than hybrid composites because of their reduced filler content and increased resin matrix [58, 72, 73]. The cavity configuration factor (C-factor) is related to polymerization shrinkage and is particularly relevant when considering class V cavities [63]. The high C-factor of class V cavities (C-factor = 5) may make them more susceptible to microleakage. Volumetric shrinkage that takes place in constraints due to surrounding walls/substrates will induce considerable shrinkage stress, putting the material/substrate interfaces at high risk. This is especially true when the C-factor increases and has been verified theoretically and experimentally [70], justifying microleakage gaps. Thus, microleakage studies in class V lesions have high clinical relevance, as reported in past studies [73, 74].
The microleakage results at immediate observation (24 h) were poor, showing that all experimental SACs portrayed microleakage in both margins, which may translate into unsuccessful restorations when they are used without any surface pre-treatment or adhesive. As expected, the results obtained after TC followed the same trend. Indeed, owing to thermal stresses and water sorption, the TC process might intensify debonding at the resin-enamel contact. The functional acidic monomers used in SAC's significantly enhance the hydrophilicity of the resin. This causes water sorption by the hydrophilic resin, which over time causes plasticity and raises the likelihood of filler debonding and matrix–filler bond breakdown [74]. The microleakage results of all the experimental SACs tested in this study indicate a weak adhesive interface, which suggests that their composition is somewhat unstable and susceptible to degradation. This, in turn, may be responsible for partial failure of the bond to the tooth, leading to marginal leakage and subsequent loss of retention of the restoration [27, 74].
These results highlight the fact that these functional monomers in SACs do not seem to be sufficient to achieve marginal sealing in enamel and dentin, making self-adhesiveness illusory and below expectations.
5. Conclusion
From the present study, it can be concluded that the functional monomers present in self-adhesive resin composite formulations influence polymerization reaction kinetics and cellular metabolic activity. This is factor-dependent on their chemical structure, size, molecular weight, and chain flexibility, which influence their molecular reactivity. The DC ,max and shrinkage (vol%) in these formulations ranged from 62%–45% and 4.5%–6.1%, respectively. In extracts <50%, formulations containing HEMA and HEAA were found to be the most detrimental to metabolic activity, while the acidic functional monomers were comparable to the control. Finally, adding a functional monomer, such as MDP, GPDM, HEMA, or HEAA, to the chemical composition of a flowable composite did not seem to be sufficient to improve their sealing ability and adhesiveness, since microleakage was present in 40%–60% of all surfaces, occlusal and cervical. The observed monomer-dependent effects on viscosity, polymerization, cellular response, and sealing highlight the need for a tailored approach in material design to optimize clinical outcomes. While SACs have made place in the market, this study highlights that further advancements are needed to match the properties and bonding capabilities of established gold standard adhesives.
Acknowledgments
The authors acknowledge Professor Jacques Nör (University of Michigan, Ann Arbor, MI, USA) for providing the mouse odontoblast MDPC-23 cell line, the company Dentsply Sirona Restorative and Dr Oliver Elsner for kindly supplying the filler particles and camphorquinone used for the formulation of the experimental self-adhesive composites. This work was funded by Fundação para a Ciência e a Tecnologia (FCT) through the unit Projects from CQE—UIDB/00100/2020, UIDP/00100/2020, IMS—LA/P/0056/2020, and CiiEM—UIDB/04585/2020.
Data availability statement
The data cannot be made publicly available upon publication because they are not available in a format that is sufficiently accessible or reusable by other researchers. The data that support the findings of this study are available upon reasonable request from the authors.
Data access statement
Research data supporting this publication are available from the authors upon reasonable request.
Conflict of interest
The authors declare that they have NO affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.