Abstract
The field of neural tissue engineering has undergone a revolution due to advancements in three-dimensional (3D) printing technology. This technology now enables the creation of intricate neural tissue constructs with precise geometries, topologies, and mechanical properties. Currently, there are various 3D printing techniques available, such as stereolithography and digital light processing, and a wide range of materials can be utilized, including hydrogels, biopolymers, and synthetic materials. Furthermore, the development of four-dimensional (4D) printing has gained traction, allowing for the fabrication of structures that can change shape over time using techniques such as shape-memory polymers. These innovations have the potential to facilitate neural regeneration, drug screening, disease modeling, and hold tremendous promise for personalized diagnostics, precise therapeutic strategies against brain cancers. This review paper provides a comprehensive overview of the current state-of-the-art techniques and materials for 3D printing in neural tissue engineering and brain cancer. It focuses on the exciting possibilities that lie ahead, including the emerging field of 4D printing. Additionally, the paper discusses the potential applications of five-dimensional and six-dimensional printing, which integrate time and biological functions into the printing process, in the fields of neuroscience.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The human body's sensory and motor functions are controlled by the nervous system, which is divided into two main categories: the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS comprises the brain and spinal cord, while the PNS consists of nerves throughout the body. Every year, millions of Americans suffer from central nervous injuries, such as traumatic brain injuries (TBIs) and spinal cord injuries (SCIs), which can lead to severe complications, including brain damage and neurodegenerative diseases [1, 2]. Meanwhile, peripheral nervous injuries are primarily caused by surgery and trauma and are common in clinical practice, occurring in 13–23 people out of every 1000 [3].
Damage or illnesses in the nervous system can result in the death of neural cells, disruption of neural networks, and interruption of information flow. These conditions can contribute to the development of various neurological disorders including neurodegenerative diseases such as Alzheimer's and Parkinson's disease, as well as strokes [4].
Despite extensive research efforts, currently, there is no treatment available that can fully restore neural function in the complex CNS due to the unfavorable environment surrounding the site of injury. While PNS regeneration is relatively more effective, it often does not result in complete restoration, leading to unsatisfactory functional outcomes [5, 6].
The objective of tissue engineering is to create functional replacements for damaged tissues and organs [7, 8]. Neural tissue engineering focuses on repairing, maintaining, and enhancing the function of neural tissue using biomimetic scaffolds and cells. To ensure the success of tissue-engineered neural scaffolds, which provide essential physical support for the engraftment of host tissue and the subsequent growth of new tissues, they should meet the following requirements: (1) support excellent cell adhesion and proliferation; (2) exhibit biocompatibility with low cytotoxicity; (3) degrade by producing cytocompatible metabolites; (4) possess high porosity to facilitate nutrient exchange; and (5) have three-dimensional (3D) mechanical stability [9].
In recent decades, various techniques have been studied to develop functional neural tissue scaffolds. Among these methods, 3D printing has gained considerable interest due to its ability to create scaffolds with precise and highly controlled spatial architectures that can be tailored to the specific requirements of patients. Conventional approaches for fabricating 3D scaffolds have demonstrated favorable outcomes in nerve regeneration. However, 3D bioprinting holds a distinct advantage over other conventional 3D tissue manufacturing techniques due to its unique capability of producing patient-specific scaffolds [10]. The emergence of smart materials science has led to advancements in 3D printing technology, specifically towards four-dimensional (4D) printing (figure 1(A)). This technology involves incorporating a time dimension, which enables 3D-printed products to change shape after printing [11]. In order to create a 4D print, several components are required including a 3D printing setup, a stimulus-responsive material, a stimulus source, an interaction mechanism, and mathematical modeling. The combination of these components enables the development of 4D-printed structures that can undergo controlled and predictable transformations [12]. The presence of functional blood vessels is crucial for effective neural tissue engineering as they facilitate the transport of nutrients, oxygen, and waste products. Four-dimensional bioprinting offers a promising approach to creating biomimetic blood vessels in a laboratory setting. Arteries can be generated using encapsulated cells or by utilizing the natural self-organizing properties of the cells [13]. The layer-by-layer technique in 4D bioprinting can be employed to print cell-scaffold mixtures into tube-shaped structures that resemble blood vessels. These structures can be stimulated with maturation factors to promote vascularization and facilitate rapid cell proliferation [14]. The emerging technology of five-dimensional (5D) bioprinting combines the benefits of 3D printing and bioprinting (figure 1(A)). In additive manufacturing, the print head and the printable object have five degrees of freedom, allowing for the production of curved layers instead of flat ones. Unlike 3D printers that move along a straight path, 5D printing occurs along the curved path of the printed part. This technology offers the advantage of creating parts with curved layers that possess improved strength while using fewer materials [15]. The curved surfaces of the human neural system pose a challenge in achieving the necessary strength using traditional flat printing techniques. Therefore, the emerging technology of 5D printing, which allows for the production of curved layers with improved strength and utilizes fewer materials, holds great potential in meeting the primary requirements of neural tissue engineering [16]. The aim of this review is to discuss advancements in the field of neural tissue regeneration, specifically focusing on the latest developments in advanced biomaterials and bioprinting.
Figure 1. The evolution of printing technologies from 2D to 6D. Reproduced from [17]. CC BY 4.0. Reproduced from [18]. CC BY 4.0.
Download figure:
Standard image High-resolution image2. Techniques for 3D printing
The field of neural tissue engineering is making rapid strides in its goal of repairing or replacing damaged tissues within the nervous system [19]. The potential of 3D printing technology lies in its remarkable capacity to produce intricate 3D structures with a high degree of precision and control. This technology can be applied to develop neural tissue scaffolds with particular characteristics to facilitate tissue regeneration and growth [20]. The following sections are some 3D printing techniques commonly utilized in neural tissue engineering:
2.1. Fused deposition modeling (FDM)
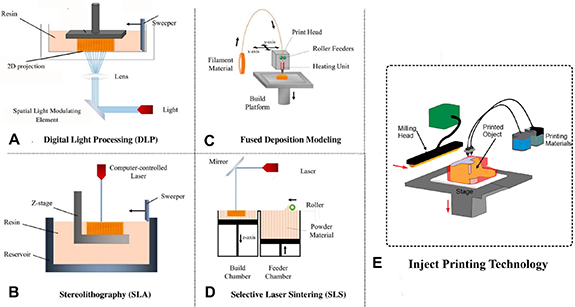
FDM is a widely used 3D printing method that involves the layer-by-layer extrusion of a heated thermoplastic filament to fabricate intricate 3D structures. This technique is widely applied in diverse areas like engineering, architecture, and medical applications, including tissue engineering. In FDM, the thermoplastic filament is melted using a heated nozzle and deposited in a series of continuous lines onto a build platform (figure 2(C)), following a pre-programmed design. As the nozzle moves along the X, Y, and Z axes, the molten plastic is deposited, forming the desired shape [21]. FDM is a technique that can produce precise and complex 3D structures, and it has become a useful tool in tissue engineering. One of the main applications of FDM is the creation of scaffolds with porous structures and adjustable pore sizes, which can be seeded with cells to promote tissue regeneration. The porosity of the scaffold can be controlled by adjusting the size and spacing of the extruded lines during printing. Additionally, FDM can be utilized to incorporate channels and cavities within the scaffold to facilitate the exchange of nutrients and oxygen for the growing cells [22]. As an illustration, a research paper by Mendoza-Buenrostro et al [23] introduced the idea of a machine tool that integrates FDM, electrospinning, and micromilling capabilities to create hybrid scaffold structures. The hybrid scaffolds, which were both biocompatible and biodegradable, could serve as a 3D supportive structure for cell cultures. With the use of this machine tool, various geometries for the FDM printed structure can be explored, and it enables multi-material, multi-scale manufacturing [23]. Although FDM offers several benefits, it has some drawbacks. One such drawback is the limited variety of materials that can be used, which may compromise the mechanical and biological properties of the scaffold produced. Moreover, FDM-generated scaffolds may show surface irregularities and structural imperfections that could adversely affect the adherence and proliferation of cells [24]. These factors must be taken into account when using FDM in tissue engineering applications.
Figure 2. Schematic comparison of popular 3D printing techniques including: (A) DLP. Reproduced from [44]. CC BY 4.0. (B) SLA. Reproduced from [45]. CC BY 4.0. (C) FDM, (D) SLS, and (E) inject 3D printing. Each method offers unique advantages with different precision, and complexity.
Download figure:
Standard image High-resolution image2.2. Stereolithography (SLA)
SLA is a 3D printing technique that employs a laser to cure a liquid resin to create a 3D structure. This method is known for its high level of accuracy and resolution, which allows it to produce intricate shapes and delicate details. It is used extensively in various sectors, including aerospace, automotive, and medical fields such as tissue engineering [25]. SLA is a 3D printing technique that uses a laser to selectively cure a layer of liquid resin based on a pre-programmed design (figure 2(B)). The build platform is lowered after each layer is cured to add the next layer, and the completed structure is then removed from the resin tank and cleaned with a solvent to remove any uncured resin. SLA is capable of producing complex geometries with high precision and resolution, resulting in a smooth surface finish. By adjusting the composition of the resin, SLA can create structures with different material properties. In tissue engineering, SLA can be used to create 3D scaffolds with controlled porosity and high precision, which can be seeded with cells to promote tissue regeneration [26]. The precise and accurate nature of SLA can be applied to fabricate complex details such as cavities, pores, and channels within the scaffold. These details can improve the exchange of nutrients and oxygen between the scaffold and the developing cells [27]. Arcaute et al [28] used SLA to create intricate 3D hydrogels made of poly(ethylene glycol) (PEG). They optimized the PEG solutions for use in SLA through photopolymerization experiments, which led to successful fabrication of hydrogel structures with varying internal channel orientations and multi-material structures. They also encapsulated human dermal fibroblasts in bioactive PEG, photocrosslinked using SLA, and assessed cell viability. The results of their study demonstrated the potential of SLA and photocrosslinkable biomaterials in creating complex bioactive scaffolds with living cells for tissue engineering purposes [28]. SLA has a major benefit of being capable of producing highly accurate structures with intricate details. However, compared to other 3D printing methods, SLA process may necessitate additional time and financial investment. Furthermore, the mechanical properties of scaffolds produced by SLA may be restricted due to the fragile nature of the cured resin [29].
2.3. Inkjet 3D printing
Inkjet 3D printing is a method that employs an inkjet printhead to accurately place droplets of bioink onto a surface layer-by-layer, resulting in the formation of a 3D structure. This technique has a high level of versatility, allowing for the creation of complex 3D structures with precision and control. Inkjet 3D printing has shown great potential in tissue engineering, particularly for the creation of functional tissues and organs [30]. Inkjet 3D printing involves the deposition of bioink layer-by-layer onto a substrate using an inkjet printhead (figure 2(E)). The bioink, which contains living cells, is loaded into the printhead and dispensed onto the substrate based on a pre-programmed design. After dispensing, the bioink is cured or cross-linked to create a stable 3D structure. Inkjet 3D printing is a versatile technique that allows for the creation of complex tissue structures with high resolution and control. It can be used to create patterns of different cell types, including neurons and glial cells, as well as multiple layers of cells with varying properties and functions [31]. Inkjet 3D printing offers a significant benefit in that it can accurately deposit several types of cells in specific patterns, which makes it possible to replicate the intricate structure of natural tissues and organs. Moreover, by changing the bioink's composition and printing settings, inkjet 3D printing can produce tissues with tailored functions and properties [32]. Xu et al [33] conducted a study on the fabrication of intricate cell patterns and structures using automated inkjet printing of primary embryonic hippocampal and cortical neurons. The study found that the printed neurons retained their fundamental cellular features and functions, including typical and healthy neuronal properties and electrophysiological traits [33].
Although inkjet 3D printing holds great promise for tissue engineering, it does have certain limitations. These include the requirement for specialized printers and restricted selection of compatible materials. The printing process can also be time-consuming, and air bubbles may form during droplet deposition, which can affect cell viability. Despite these restrictions, inkjet 3D printing is a valuable technique for tissue engineering and has the potential to transform regenerative medicine by facilitating the development of functional tissues and organs for transplantation and disease modeling [34].
2.4. Selective laser sintering (SLS)
SLS is a method for 3D printing that employs a high-power laser to selectively fuse powdered materials, such as plastics or metals, into a 3D structure. SLS is an extremely flexible approach that allows for the creation of intricate shapes and structures with excellent precision and resolution. To use SLS, a laser is directed at a particular layer of powdered material to selectively heat and fuse it, based on a pre-determined design (figure 2(D)) [35]. SLS is a 3D printing technique where powdered materials such as metals or plastics are selectively fused into a 3D structure using a high-powered laser. The process involves fusing each layer of the powdered material according to a predetermined design, while the build platform moves downwards to add the subsequent layer. After the printing process is completed, the structure is removed from the powder bed and cleaned to remove any excess powder. SLS is capable of producing complex geometries with high accuracy and resolution, and it can create structures with different material properties by adjusting the composition of the powdered material. In the field of tissue engineering, SLS can be used to fabricate precise 3D scaffolds with controlled porosity, which can be seeded with cells to promote tissue regeneration and growth [36]. The spacing and size of the powder particles can be altered to modify the scaffold's porosity, while the advanced precision of SLS can produce intricate features like pores, channels, and cavities inside the scaffold. These details can aid in the exchange of nutrients and oxygen for the developing cells. SLS has a significant advantage in producing highly detailed and accurate geometries. Moreover, it is a versatile technique that can manufacture structures with different material properties [37]. Wu et al [38] conducted a study on the fabrication of porous Polycaprolactone (PCL) scaffolds using SLS. The researchers aimed to optimize the critical processing parameters of SLS for PCL and apply post-processing techniques to enhance the performance of the PCL scaffolds. The PCL scaffolds were treated to produce PCL/alginate/polyacrylamide (PAAM) scaffolds. The mechanical properties of the scaffolds were evaluated, and the results showed that the PCL/alginate/PAAm scaffolds had better elastic modulus and elongation at break compared to PCL scaffolds. Cell culture experiments were also conducted in vitro, which demonstrated a high level of cell viability on the PCL/alginate/PAAm scaffolds [38]. Despite its advantages, SLS may be a more costly method of 3D printing compared to other techniques. Moreover, it demands specific equipment and safety measures because the process generates high temperatures and fumes. Furthermore, the mechanical properties of the scaffolds produced by SLS may be limited due to the properties of the powdered materials [39].
2.5. Digital light processing (DLP)
To create a 3D object using DLP, a liquid resin vat is used in combination with a digital projector. The projector displays each layer of the object onto the resin, which is then cured through photopolymerization. The UV light from the projector solidifies the resin in the shape of the projected layer (figure 2(A)). This process is repeated layer by layer until the object is complete. Afterward, the object is rinsed in a solvent to remove any remaining resin and then fully cured through UV light exposure to ensure complete hardening of the material [40].
DLP offers the benefit of producing objects with exceptionally high resolution and accuracy, which makes it suitable for applications like jewelry, dental molds, and microfluidic devices. Compared to the other techniques, DLP printers can also operate at relatively high speeds since the entire layer is cured at once rather instead of tracing it with a laser. Moreover, DLP is capable of using a diverse range of materials, including biocompatible, dental-grade, rigid and flexible resins [41]. However, a potential limitation of DLP is its suitability for larger objects, as the build volume is typically smaller compared to other 3D printing techniques. This means that printing larger-sized objects may pose some challenges when using DLP technology. Additionally, the cured resin may be brittle, which can limit the durability of parts. Finally, the cost of the resin can be relatively high compared to other materials used in 3D printing [42]. DLP 3D printing has been implemented in tissue engineering to produce scaffolds for growing new tissue. These scaffolds can be tailored to match the specific requirements of the tissue being grown, such as the size, shape, and mechanical properties. This technology has the potential to revolutionize the field of regenerative medicine, allowing for the creation of complex tissue structures that can be used to repair or replace the damaged tissues [43].
3. Materials for 3D printing in neural tissue engineering
The selection of the suitable materials for 3D printing in neural tissue engineering is crucial since it directly affects the mechanical, chemical, and biological properties of the resulting scaffold. It is crucial to choose materials that can closely resemble the native tissue, which should have features such as biocompatibility, biodegradability, electroconductivity, and mechanical strength, while also being compatible with the 3D printing process [46, 47]. The materials chosen for 3D printing in neural tissue engineering play a crucial role in determining the physical, chemical, and biological properties of the scaffold. To achieve optimal results, it is essential to select materials that possess characteristics similar to native tissue, such as biodegradability, electroconductivity, biocompatibility, and mechanical strength, and are also compatible with the 3D printing process. Polymers such as PCL, PLA, and PEG are commonly used in 3D printing due to their mechanical properties, biocompatibility, and ease of processing [48]. By employing these materials, 3D scaffolds can be produced with high resolution and controlled porosity, facilitating neural tissue regeneration. Hydrogels, such as alginate, gelatin, and HA, are also used in 3D printing and can create scaffolds that simulate the soft and hydrated environment of the native neural tissue. These hydrogels can provide mechanical support and promote cell proliferation and differentiation [49]. In this regard, Ma et al in 2020 designed and fabricated a 3D printed scaffolds for the stimulation of brain microenvironment in vitro. They used HA and normal glial cells (HEBs) for the creation of Ha-based HEBs laden scaffold for stimulation of biological and mechanical features of human brain microenvironment. They investigated various factors such as concentration of gelatin, formulation of bioinks, and optimization of bioprinting process for providing suitable scaffold which mimic natural brain microenvironment [50].
The development and refinement of appropriate materials for brain tissue engineering are instrumental in advancing therapies for brain cancers and neurological disorders, paving the way for personalized treatments and improved patients outcomes. Three-dimensional printing plays a significant role in brain tissue engineering, particularly in the context of brain cancers. For instance, creation of implantable constructs such as scaffolds can be utilized to support the regeneration and growth of brain tissue after tumor resection. Furthermore, 3D printing can be used to create personalized drug delivery systems for brain cancers treatment. Incorporation of drugs or therapeutic agents with 3D-printed structures leads to the controlled and localized drug release which enhances the effectiveness of treatment while minimize the side effects. Development of drug delivery into the CNS has remained a challenge due to the blood–brain barrier (BBB), the complexity of the brain microenvironment, and heterogeneity of brain tumors [51]. Therefore, the selection of materials for creation of 3D-printed objects for brain tissue should be conducted considering these challenges. When considering materials for 3D printing in CNS, it is important to select materials that possess specific characteristics to facilitate successful integration with brain tissue while respecting the BBB unique properties. Synthetic biocompatible polymers such as poly (lactic-co-glycolic acid) PLGA, poly lactic acid (PLA), and PCL have been extensively used in brain tissue engineering. In addition, natural biomaterials including collagen, gelatin, and HA by mimicking the native brain tissue environment can promote the integration of engineered constructs with brain tissue and facilitate appropriate cellular responses [52–55].
4. Printing techniques
The novel technique of 4D printing allows for the creation of 3D structures that can change shape or properties over time in response to external stimuli, such as temperature or humidity. In the field of neural tissue engineering, 4D printing has the advantage of producing dynamic scaffolds that can imitate the intricate mechanical and biochemical cues of natural neural tissue. This type of environment can enhance cell growth, differentiation, and regeneration, leading to improved outcomes in neural tissue engineering [49]. Below are some examples of 4D printing techniques. The use of shape-memory polymers (SMPs) in 4D printing has led to the creation of neural implants that can change their shape or properties over time. For instance, implants made from SMPs can be designed to alter their shape to better fit the surrounding neural tissue, thus minimizing the possibility of tissue damage and enhancing the stability of the implant [56]. A research conducted a technique for 4D printing of shape memory scaffolds with poly (propylene fumarate) (PPF) star polymers. The characteristics of the scaffolds, such as glass transition temperatures and Young's moduli, can be modified while keeping the same polymer formulation and stoichiometry. The rate and extent of shape recovery can be regulated by modifying the strut design, post-curing time, and temperature used for recovery [57]. In addition, hydrogels have been utilized in 4D printing to develop scaffolds for neural tissue engineering. The hydrogel scaffold can be designed to alter its shape, stiffness, or porosity over time, enabling it to replicate the dynamic characteristics of native neural tissue [49]. In 4D printing, another option is to use programmable materials that can be configured to alter their properties, such as shape, stiffness, or color, in response to particular stimuli. These types of materials are frequently employed in the development of smart materials or devices [58]. The potential applications of 4D bioprinting in tissue engineering and regenerative medicine have been recognized [59]. Nevertheless, this technology is in its nascent stage and requires further research to refine the materials, printing techniques, and stimuli employed for 4D printing in neural tissue engineering [58].
5. Applications of 3D and 4D printing technology in the field of neuroscience
5.1. Scaffold fabrication
In the field of neuroscience, one of the prominent applications of 3D and 4D printing technology is scaffold fabrication. Scaffolds are 3D structures that provide mechanical support and guidance to developing cells, facilitating tissue regeneration. By utilizing 3D and 4D printing techniques, it becomes feasible to create scaffolds with intricate and precise geometries that closely resemble the natural structure of neural tissue [49]. These printed scaffolds offer several advantages in neuroscience research and applications including:
5.1.1. Peripheral nervous system (PNS)
Three-dimensional and 4D printing technologies provide precise control during the manufacturing of nerve conduits, enabling the creation of customized designs that specifically cater to the requirements of peripheral nerve repair. These printing techniques make it possible to utilize bioactive and biocompatible materials that enhance cellular adhesion, proliferation, and differentiation, thereby promoting the regeneration of nerves. By engineering conduits with intricate patterns and channels, the growth of regenerating nerve fibers can be guided along desired paths, minimizing the formation of scar tissue and facilitating the recovery of normal function [60]. In this regard, Vijayavenkataraman et al [61] in a research study explored the use of neural guide conduits as a substitute treatment for peripheral nerve injuries, highlighting the disadvantages of using nerve autografts and the benefits of reduced graphene oxide (rGO), which includes its ability to conduct electricity and high surface area. To create a 3D environment for neural differentiation, PCL/rGO scaffolds were manufactured using electrohydrodynamic (EHD)-jet 3D printing. The addition of rGO led to the production of softer scaffolds that stimulate the expression of genes associated with neural differentiation (β3-tubulin, NF-H, and GAP43) as well as the expression of NF200 and β3-tubulin during in vitro tests utilizing PC12 cells [61].
Rao et al prepared [62] a decellularized nerve matrix hydrogel derived from porcine sciatic nerve, which showed promising results. They introduced longitudinally oriented microchannel structures into the bioscaffold through controlled freeze-drying. These microchannels effectively directed and promoted neurite extension and Schwann cell migration. In vivo studies using nerve guidance conduits containing the modified bioscaffold demonstrated significant improvements in axonal extension, myelination, and functional recovery in rat sciatic nerve defects. The incorporation of nerve growth factor further enhanced the overall performance of the implanted scaffold. The findings suggest that this bioactive bioscaffold, with controlled release of neurotrophic factors and topological cues provided by the microchannels, holds potential for clinical treatments of peripheral nerve injuries [62].
5.1.2. Spinal cord
Three-dimensional printing of scaffolds demonstrates immense potential in the realm of spinal cord regeneration. These scaffolds can be tailored to meet the specific requirements of individual patients, considering factors such as the location and size of the injury. They serve as guides and provide mechanical support for the regrowth of nerve fibers, effectively bridging the gap caused by SCIs [27]. The incorporation of biomimetic materials into 3D printed scaffolds promotes the adhesion, proliferation, and differentiation of cells, facilitating the regeneration of damaged neural tissue [63]. Chen et al [64] discussed the difficulties associated with using mesenchymal stromal cells (MSCs) for treating SCIs, such as poor survival rates, tumorigenicity, and ethical concerns. They suggested an alternative approach of using MSCs-derived secretome instead, which was found to be more stable and practical for clinical use. The researchers discovered that implanting 3D-printed scaffolds made of collagen/silk fibroin carrying MSCs secretome resulted in improved locomotor function, nerve fiber regeneration, remyelination, and synaptic connection establishment at the injury site. Therefore, it is believed that implanting these scaffolds has the potential to treat SCIs [64].
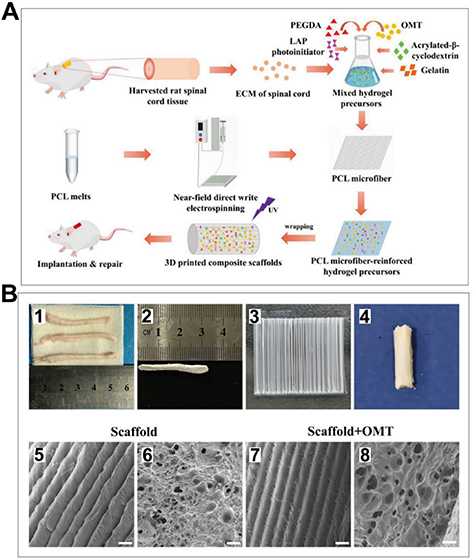
In another study, Song et al [65] used nearfield direct write electrospinning to create a 3D-printed spinal cord extracellular matrix hydrogel scaffold reinforced with fiber bundles and loaded with oxymatrine (OMT) (figure 3(I)). This scaffold (figure 3(II)), which was a combination of materials, was found to have multiple beneficial effects on the healing of SCIs in rats. These benefits included promoting the formation of neurons from NSCs, discouraging the formation of astrocytes, attracting NSCs from the host tissue, aiding in the growth of neurons and their axons at the injury site, preventing the formation of glial scars, and ultimately improving the rats' ability to move [65].
Figure 3. I: Schematic of creating spinal cord extracellular matrix (ECM) hydrogel microfiber scaffolds reinforced with oxymatrine (OMT) using 3D bioprinting technology and then implanting them. II: characterization of scaffolds used in neural tissue engineering (A) shows a normal spinal cord tissue, whereas (B) shows a spinal cord decellularized scaffold. Figure (C) shows a PCL microfiber structure, and (D) general morphology of the 3D-bioprinted composite (E)–(H) microstructure of scaffolds which reveal parallel microfibers of the same thickness and hydrogels attached to the fibers. The scale for all images is 100 μm. Reproduced from [65]. CC BY 4.0.
Download figure:
Standard image High-resolution image5.1.3. Brain
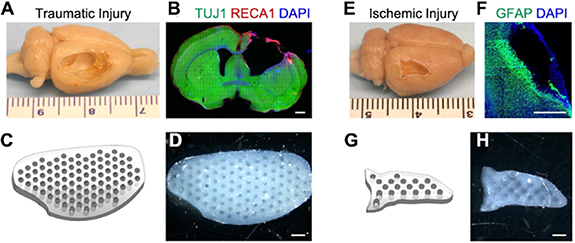
Three-dimensional printed cortical scaffolds offer immense potential in facilitating neural tissue growth within the brain. These scaffolds are specifically engineered to replicate the intricate structure of cortical tissue, providing an ideal framework for cellular attachment and proliferation. By closely mimicking the complex architecture and organization of the brain's cortical tissue, these scaffolds create an environment that closely resembles natural tissue, promoting the growth and regeneration of neural cells [39]. In a study by Li et al [66], after a period of 4 weeks following the controlled cortical impact, the cortical tissue located at the point of impact was nearly entirely destroyed, causing a cavity to form with approximate dimensions of 10 mm × 6 mm × 2 mm (as depicted in figures 4(A) and (B)). Hydrogel scaffolds were created using 3D printing technology with precise shapes (as seen in figures 4(C) and (D)), aligned microchannels, and tunable mechanical properties. The hydrogel scaffold was successfully created with a design that precisely corresponds to the location of the ischemic lesion, and the printed structure is depicted in figures 4(G) and (H). After a period of 4 weeks following the middle cerebral artery occlusion, a cavity with approximate dimensions of 6 mm × 3 mm × 1 mm was observed due to the presence of infarcted tissue (as depicted in figures 4(E) and (F)). These scaffolds were found to be effective in promoting the viability, proliferation, and migration of neural stem cells (NSCs). The 3D printed hydrogels were tailored to match the size and mechanical properties of brain tissue lesions and could be used as stem cell carriers for personalized treatment of patients with ischemic or TBIs [66].
Figure 4. Hydrogel scaffolds were 3D printed with high accuracy to correspond with specific sites of brain lesions. (A) The traumatic injury was induced using a controlled cortical impact model, (E) the ischemic injury was induced using a middle cerebral artery occlusion model (the site of the lesion was indicated by a line consisting of dashes.), (B) and (F) coronal brain sections were subjected to immunostaining to determine the size of the lesion, (B) neurons were labeled with TUJ1, depicted in green, while endothelial cells were labeled with RECA1. (F) Astrocytes were visualized using GFAP staining, which appeared green in color. Nuclei were stained with DAPI, appearing as blue. (C) Scaffold designs that matched the shape of the lesion were created for both traumatic injury, (G) ischemic injury, and they contained 400 μm microchannels that were properly aligned. (D) Lesion shape-matching hydrogel scaffolds were 3D printed for both traumatic injury, and (H) ischemic injury, and they contained 400 μm microchannels that were correctly aligned. A scale bar of 1 mm was used to visualize the resulting structures. Reprinted with permission from [66]. Copyright (2021) American Chemical Society.
Download figure:
Standard image High-resolution imageLiu et al [67] conducted a study to evaluate scaffolds made of collagen, chitosan, and exosomes derived from human umbilical cord MSCs using 3D printing technology. These scaffolds were referred to as 3D-CC-BMExos and exhibited exceptional mechanical properties and biocompatibility. In vivo experiments on rats demonstrated that 3D-CC-BMExos therapy improved neuromotor and cognitive function in TBI models, facilitated the regeneration of nerve fibers, synaptic connections, and myelin sheaths in TBI lesions [67].
Liu et al [68] conducted research on a novel 3D-printed scaffold made of collagen and chitosan, which was filled with exosomes derived from NSCs that had been treated with insulin-like growth factor-1 (INExos), to treat TBI in rats. The scaffold was able to release INExos for a duration of 2 weeks and significantly enhanced the motor and cognitive functions in the rats that received it. The nerve tissue was also analyzed using immunofluorescence staining and transmission electron microscopy (TEM), indicating that the scaffold aided in the repair of the injured nerve tissue [68].
5.2. Bioprinting of neural tissue constructs for transplantation
Bioprinting can create 3D neural tissue constructs for transplantation by depositing living cells and biomaterials layer-by-layer. This approach has several potential applications in neural tissue engineering by overcoming the limitations of traditional tissue engineering methods, including poor cell survival and limited control over scaffold architecture. The bioprinting process involves several steps, including cell source selection, biomaterial selection, printing process optimization, and post-printing processing. Hydrogels like alginate and collagen are common biomaterials, and NSCs, induced pluripotent stem cells (iPSCs), and primary neural cells are common cell sources. The bioprinted tissue must be cultured and vascularized before transplantation [69].
5.2.1. Cell-based therapies
Bioprinted neural tissue constructs can be used for transplantation in the treatment of neurological diseases and injuries [69]. Li et al [70] conducted a study to compare three different treatment strategies for promoting peripheral nerve regeneration. The first strategy involved transplanting Schwann cell progenitors that were induced from purified neural crest stem cells. The second strategy involved implanting a multiscale scaffold that was created using 3D printing. The third strategy involved implanting the same multiscale scaffold, but with Schwann cell progenitors loaded onto it. The results showed that the third strategy, which involved using the scaffold with preloaded Schwann cell progenitors, was the most effective in promoting peripheral nerve regeneration. Song et al [71] utilized 3D bioprinting to produce a conductive composite hydrogel (CCH) scaffold that delivered NSCs for the repair of SCIs. The researchers developed a CCH and combined it with a photocrosslinkable gelatin/PEG matrix to generate a composite bioink. The NSCs-laden CCH scaffold was 3D printed with high accuracy and properties similar to those of natural spinal cord tissue. In vivo experiments showed that the NSCs in the scaffold established connections with neighboring cells and the conductive matrix and predominantly differentiated into neurons, leading to the elimination of glial scar tissue and the regeneration of nerve fibers. This method demonstrated potential for the stem cell-based treatment of SCIs [71].
5.2.2. Disease modeling
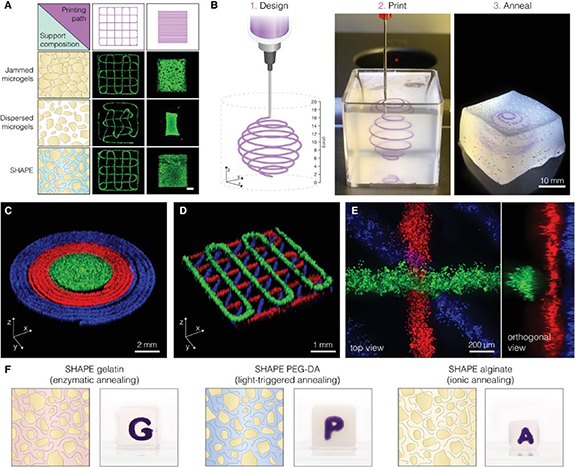
Neural tissue constructs that are created through bioprinting have the potential to serve as in vitro models for studying the pathophysiology of neurological disorders and for developing novel therapies [72]. Kajtez and colleagues introduced a novel method for engineering neuronal networks by using 3D printing technology to pattern human stem cells within self-healing annealable particle-extracellular matrix (SHAPE) composites (figure 5(a)). The SHAPE composites are made of soft microgels in extracellular matrix solution (figure 5(b)), enabling precise patterning of human stem cells and resulting in the development and maintenance of subtype-specific neurons extending within the support volume. The approach also allows for the deposition of multiple inks, monitoring of oxygen levels, and creation of vascular channels. The authors suggested that this SHAPE biomanufacturing platform could be used for modeling not only mechanically sensitive neural constructs but also a range of biomaterials with different crosslinking mechanisms to replicate complex architectural features and topological cues necessary for modeling tissues and diseases [73].
Figure 5. Embedding 3D printing into a shape hydrogel support. (A) The accuracy of the programmed path for embedded printing of hNSCs can be observed through fluorescent images labeled with Calcein-AM. The left side of the image (shown in blue) depicts varying packing densities and the composition of the continuous phase. The scale bar represents 1 mm. (B) A printing procedure that has the ability to print in all directions. (C) Three hNSC inks were used to print concentric rings. (D) A crosshatch structure made from three hNSC inks was printed. (E) A closer look at the intersection of a stacked crosshatch structure. The different hNSC inks were produced by staining live cells with Calcein-AM (in green), Calcein-Red-AM (in red), and Hoechst (in blue). (F) Different formulations of SHAPE were used to create various annealing mechanisms. The granular component of the SHAPE was kept constant while the contents of the continuous phase were altered: 10% gelatin (on the left), 30% PEG-DA (in the middle), and 1% alginate (on the right). Reproduced from [73]. CC BY 4.0. © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Download figure:
Standard image High-resolution image5.2.3. Drug screening
The use of bioprinted neural tissue constructs is not limited to modeling neurological diseases, but they can also be applied in drug screening and toxicity testing, reducing the need for animal experimentation. However, additional research is required to enhance the bioprinting process and enhance the quality and functionality of the bioprinted tissue constructs [74]. Li et al [75] developed a bioink suitable for 3D printing of human dopaminergic brain tissue with integrated optical dopamine (DA) sensors that employ tetrapodal-shaped-ZnO microparticles (t-ZnO). After printing, the neurons with t-ZnO demonstrated high viability, and within a week, the cells developed extensive neural networks. The t-ZnO sensor was capable of detecting DA in the 3D printed neural network with a limit of detection of 0.137 μM [75]. Researchers have developed a new model for glioblastoma (GBM) using a 3D printing technique that involves fibrin and an Aspect Biosystems RX1 bioprinter equipped with a microfluidic print head. This technology enables the printing of delicate neural tissue with minimal shear stress, resulting in high cell viability of the printed GBM structures for 12 d. The GBM spheroids produced in this model contained increased levels of CD133 and DCX proteins, which are associated with cancer stem cells and metastatic invasiveness. These findings indicate that the 3D printed GBM model may provide a more accurate representation of the in vivo response to drug treatment [76].
5.3. Neural drug delivery
The use of 3D and 4D printing techniques has been investigated for drug delivery in the field of neuroscience. These techniques provide a means to design personalized drug delivery devices with intricate and accurate shapes, capable of targeting particular neural tissues and cells, and controlling the release of drugs in a sustained and controlled manner. The possible applications of 3D and 4D printing in neural drug delivery include [77].
5.3.1. Implantable devices
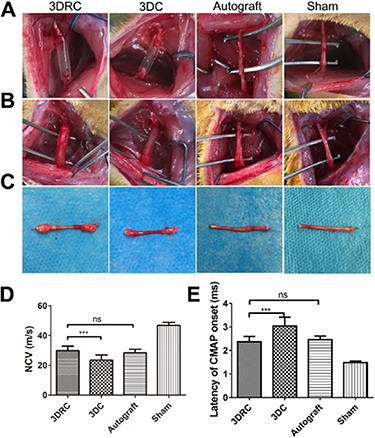
The utilization of 3D and 4D printing to produce implantable devices, such as drug-eluting stents, for the treatment of neurological disorders can be considered. These devices can be designed to release drugs at specific rates and time intervals, providing a more targeted and effective treatment [78]. In this content, Xu et al [79] utilized DLP-based 3D printing (the conduit was incorporated polymeric nanoparticles that release RGFP966, which activates the PI3K-AKT-ERK signal pathway to stimulate Schwann cell remyelination.) to create a nerve conduit to link a 10 mm injury in the sciatic nerve (figure 6(A)), and three months later, the regenerated nerves were surgically exposed (figure 6(B)). In the experimental groups, the distal and proximal sites were successfully reconnected after a 3 month period (Figure 6(C)). The functional restoration was significantly higher in the 3DRC group, where the nerve conducting velocity (NCV) was measured at 29.83 m s−1, compared to the 3D printed conduit group, which had an NCV of 23.49 m s−1. This suggests that RGFP966 was effective in promoting functional restoration (figure 6(D)). Furthermore, the study also assessed the latency of compound motor action potential (CMAP) after the surgery and found similar results as shown in figure 6(E). The study suggests that 3D-printed hydrogel nerve conduits that locally release RGFP966 could be a promising treatment for nerve injuries and may pave the way for future functional nerve conduits [79].
Figure 6. The effectiveness of the 3D-printed conduits was assessed in vivo. (A) The sciatic nerve defects model of each group was presented. (B) The regenerated sciatic nerves were surgically exposed after 3 months. (C) Representative dissection photographs of regenerated sciatic nerve in different groups after 3 months were displayed. (D) Nerve conduction velocity (NCV) and (E) compound muscle action potential (CMAP) were measured to quantitatively analyze the functional regeneration. The data are expressed as the mean ± standard deviation (SD), and statistical significance was determined using *** to indicate p < 0.005, while ns indicated no significant difference. Reprinted from [79], Copyright (2019), with permission from Elsevier.
Download figure:
Standard image High-resolution image5.3.2. Micro and nanoparticles
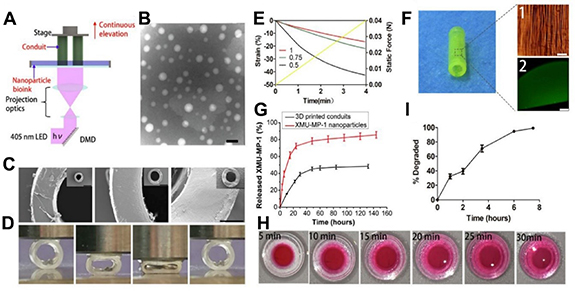
The utilization of 3D and 4D printing is not limited to creating macro structures, it can also be utilized in creating micro and nanoparticles that are applicable in drug delivery systems. By attaching targeting ligands, these particles can specifically target neural tissues and cells. In addition, they can also be loaded with various drugs such as proteins, small molecules, and nucleic acids [80]. In this regard, Lee et al [81] developed a 3D printed scaffold that mimics biological structures, containing core–shell nanoparticles created using electrospraying techniques to provide a slow and continuous release of neurogenic factors. The scaffold's porous structure was modified, with larger pores enhancing cell adhesion. The incorporation of nanoparticles increased cell proliferation and neurite length, especially in scaffolds with nerve growth factor nanoparticles, as evidenced by confocal microscopy [81]. The researchers in another study utilized GelMA hydrogels and drug-loaded nanoparticles (figures 7(A) and (B)) to create a functional nerve conduit (figures 7(C) and (F)) that promotes the regeneration of peripheral nerves. Through 3D printing, they produced a customized conduit that provided a physical microenvironment for axonal elongation (figures 7(D) and (E)). Within the conduit, nanoparticles were incorporated to sustain the release of a drug (figure 7(G)), which improved the proliferation and migration of Schwann cells and up-regulated genes associated with neurotrophic factors. In vivo experiments demonstrated that the nerve conduit was successful in inducing recovery from sciatic injuries in terms of morphology, histopathology, and function, showing potential for clinical application in peripheral nerve repair [82].
Figure 7. Preparation and characterization of the nerve conduit. (A) The DLP 3D printing technique used to produce the hydrogel conduit. (B) TEM image of XMUMP-1 nanoparticles with a scale bar of 100 nm. (C) SEM images of 3D-printed conduits with scale bar of 1 mm and a high magnification scale bar of 200 μm. (D) The compression of the conduits with wall thicknesses of 0.5 mm, 0.75 mm, and 1 mm and quantitative analysis (E). (F) The microstructure (imaged by a digital camera with a scale bar of 40 μm) and nanoparticles distribution of the conduit (imaged by confocal microscopy with a scale bar of 500 μm). (G) The in vitro release of XMU-MP-1 from XMU-MP-1 nanoparticles and nanoparticle-enhanced conduits. (H) The diffusion of small molecules in the conduits. (I) The degradation of the conduits in a collagenase solution. Reprinted from [82], Copyright (2019), with permission from © 2019 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Download figure:
Standard image High-resolution image5.3.3. 4D printed responsive devices
Responsive drug delivery devices that can adapt their shape or release properties based on various stimuli, such as temperature, pH, or light, can be created using 4D printing technology. The potential benefits of such devices include improved drug delivery efficacy and reduced side effects [83]. For instance, Zhang et al [84] presented a method for 4D printing using zein plant protein gel and a specialized layered-Carbopol support bath with varying water concentrations. This printing process altered constructs' functions over time through changes to hydrophobic and hydrogen bonding. When printed in support baths with higher water content, the constructs showed increased drug loading, quicker drug release, and degradation. The study also evaluated the biomedical efficacy of these constructs as drug-delivery systems and nerve conduits, which have been shown to be effective as drug-eluting urethral stents in a porcine model [84]. Moreover, a multiresponsive structure is created using 4D bioprinting based on SLA, which employs stress-induced shape transformation to achieve 4D reprogramming. This structure has the capability to autonomously and reversibly alter its programmed configuration after being printed, thanks to light-induced graded internal stress followed by solvent-induced relaxation. It possesses shape memory and is capable of undergoing multiple shape transformations. An intelligent nerve guidance conduit made from a graphene hybrid 4D structure was produced to demonstrate the technology, which provides multifunctional features for nerve regeneration [85]. The use of 3D and 4D printing in the delivery of drugs to the nervous system has the potential to enhance the effectiveness of treatments for neurological disorders. These printing methods offer a more accurate and regulated drug delivery system that can enhance drug efficacy, diminish side effects, and improve patient outcomes. However, additional research is necessary to refine drug delivery devices and verify their effectiveness and safety in clinical trials [86].
5.4. Nerve guides, and neural implants
The use of 3D and 4D printing technology can enhance the precision and customization of nerve guides and neural implants. The 3D-printed nerve guides can guide nerve growth in a specific direction, which can improve nerve regeneration speed and accuracy. Similarly, 3D-printed neural implants can be customized to fit the patient's specific needs, which can improve the implant's performance and reduce the risk of complications. Four-dimensional printing takes this further by allowing the implant to change its shape or properties over time, which could help reduce the risk of damage or inflammation [4]. Lee et al [87] explored how 3D printing can be utilized to create neural probes that are both cost-effective and highly customizable. This technique can produce neural interfacing devices that are biocompatible and durable, which may potentially replace the need for costly cleanroom processes. Although their research was centered on developing 3D printed optogenetic probes for the brain, their methodology can be expanded to produce other bioelectronic devices intended for diverse organs. By integrating 3D printing with advanced materials and packaging techniques, it is possible to produce economical implantable and wearable electronics that have significant applications in the fields of biomedical sciences, healthcare, and medicine [87].
Scientists have designed a tiny neuromodulatory system for mice that is completely implantable and wirelessly controlled. The system is equipped with a bidirectional wireless interface that enables the simultaneous monitoring of several physiological signals and control of stimulation parameters. The system has a rechargeable battery that can be charged wirelessly and lasts up to 5 d on a single charge. When implanted in mice to deliver vagus nerve stimulation, the device displayed a functional neural interface that persisted for more than three weeks. The system employs commercially available electrical components and is 3D-printed to facilitate the adoption and implementation of future bioelectronic therapies [88]. Zurita et al [89] investigated the potential of interfacing with the PNS to diagnose and treat drug-resistant epilepsy, depression, and similar diseases. They stressed the importance of targeting large nerves such as the vagus and hypoglossal nerves, which convey multiple nerve fibers and require complex stimulation strategies. However, they also acknowledged that targeting small nerves can provide better fiber selectivity. To address the challenges associated with electrode fabrication and implantation for small nerves, the scientists developed a cuff electrode utilizing two-photon SLA and 3D inkjet printing methods. The electrode is simple to implant and can be customized to fit specific nerve dimensions. They demonstrated its ability to record and selectively stimulate nerves by focusing on the hind leg nerve of a locust [89]. In another study, the implantation of brain–computer interfaces (BCIs) in the brain, employing electrodes to record and stimulate neural signals, was discussed. This research specifically highlighted the progress in printed electronics for these interfaces, analyzing the advantages and disadvantages of using printed electrodes, and exploring the potential of printing technology in optogenetic BCIs [90].
6. Advantages and limitations of 3D and 4D printing in neural tissue engineering
Over the past decade, bioprinting has made significant progress in tissue engineering and holds enormous potential for neural regeneration. The incorporation of 3D and 4D printing technologies has transformed neural tissue engineering. It enables the precise control of cells and biomaterials distributed in space to better address the intricate nature of neural tissue. By adhering, sorting, and fusing cells, 3D printing cells are expected to swiftly form and assemble tissues, synthesize extracellular matrices, and maintain desired geometrical shapes and mechanical properties in the tissue. Three-dimensional printing allows the creation of patient-specific scaffolds. Thus, a highly controllable microenvironment can be engineered, and the scaffold can be tailored to the individual's needs. Also, it can deliver cell-laden materials in space with high precision. As a result, cells can be placed in specific locations within the scaffold, which is important for neural tissue engineering, where the scaffold structure influences neural cell behavior and function [91]. The stimuli-responsive nature of 4D bioprinting opens a new avenue in tissue regeneration. Four-dimensional printing also considers how living tissues develop gradually after being printed. Fabricating scaffolds using smart materials mimics the dynamic nature of tissues to a large extent. It means that neural tissue engineering scaffolds can respond to specific stimuli such as temperature, pH, and mechanical forces. Also, growth factors can be released from the scaffold when triggered by certain stimuli, promoting neural cell growth [49, 92], 4D printing is regarded as an innovative, cutting-edge technology resulting from the convergence of 3D printing, smart materials, and programming [93].
While 3D and 4D printing technologies have significantly advanced in neural tissue engineering, some problems, including long production times, complex software, postprocessing requirements, material limitations, and a controlled environment, remain unresolved. The main drawback of 3D printing is that it considers the target to be steady and insensitive. Once the objects are printed, they will always retain their geometrical shape. Therefore, 4D bioprinting has developed as a solution in which 'time' is combined with 3D additive printing [94]. Through 4D printing, it is possible to create dynamic shapes morphing from static parts and enhances 3D printing applicability. Despite its advantages, it has some weaknesses, such as the need for expensive materials and hardware. Currently, there have been materials that respond to a variety of stimuli, but they are limited. Researchers continue developing 3D and 4D printing methods to produce new biomaterials and biomedical devices. Consequently, the development of multi-sensory materials is still a challenge for enhancing devices' dynamic capabilities. Several laboratories and prototyping facilities are experimenting with 4D technology in their early stages. A 4D printer cannot be purchased at the moment. As of 2017, the development of 4D printing technology was primarily led by MIT's Self-Assembly Lab, Stratasys, and Autodesk [95]. In recent times, 5D printing has brought additional advantages to 3D printed objects, including improved strength and better design, making it possible to create personalized medicines. The use of 5D printing may help 3D printing to transition from laboratory research to practical medical applications, achieving the status of translational science [96]. iPSCs have garnered significant attention in the field of regenerative medicine and are widely employed in various research studies, including clinical trials [97]. Unlike embryonic stem cells (ESCs), iPSCs are derived from mature somatic cells using reprogramming techniques, eliminating the need for embryos or fetuses. This eliminates the ethical and legal dilemmas associated with ESCs, making iPSCs a more ethically viable option for biofabrication of human tissues and organs [98]. Incorporating a discussion on iPSCs would allow the researchers to highlight the advancements and potential of this alternative approach in bioprinting. It can be explored how iPSCs offer a practical solution to the ethical concerns tied to ESCs, presenting a more comprehensive viewpoint on the subject. Additionally, emphasizing the current progress and clinical applications of iPSCs in regenerative medicine would be beneficial. The researchers should consider successful studies and clinical trials that have utilized iPSCs for tissue regeneration, underscoring the feasibility and potential of iPSCs within the realm of bioprinting technology [99]. Addressing the utilization of iPSCs in bioprinting and elucidating their advantages and applications would provide a more thorough comprehension of the ethical considerations and alternatives involved in this field, thereby strengthening the overall discussion on ethical concerns related to bioprinting [100].
The entire bioprinting process is currently unregulated in regenerative medicine and tissue engineering. Advanced therapy, medicinal products regulations may apply to different stages of 3D bioprinting production [101]. Bioprinting management requires regulation of risks and quality control. In related to the possible legal model for bioprinting technologies, Russia addressed the legal issues related to the creation and use of bioprinted human organs. However, there is no Russian legislation governing 3D bioprinted human organs, which could impede the development of these technologies [102]. As a result, 3D and 4D printing in neural tissue engineering raise ethical and legal issues that must be resolved before they can be applied to clinical applications.
7. 5D and 6D printing technologies
Theoretical extensions of 3D printing, known as 5D and six-dimensional (6D) printing, involve adding dimensions to the printing process beyond the conventional three spatial dimensions (x, y, and z). Although these technologies are still in the research phase and have not been widely adopted, they have the potential to significantly transform the way we design and produce intricate objects, including biological tissues [18]. In 5D printing, the additional dimensions are time and material properties. This means that the printed object can change over time or in response to different stimuli, such as temperature, humidity, or light [103]. Precisely controlling printing parameters such as temperature, pressure, and speed is crucial to ensure optimal cell viability and functionality in 5D printing. Using bioink with appropriate characteristics is also essential for biomedical applications in tissue engineering and regenerative medicine. Various natural and synthetic materials, such as hydrogels, alginate, collagen, gelatin, and fibrin, have been studied as possible bioinks. These materials can be customized with bioactive molecules such as peptides or growth factors to improve cell attachment, proliferation, and differentiation [104].
The process of the production of objects using a 5D printer is similar to that of a 3D printer, but the resulting structures are of much higher quality due to the formation of curved layers by the 5D printer. Curved layer creation in 5D printing leads to the production of objects that are more durable than those made with a 3D printer. For example, a scaffold created with a 5D printer can withstand pressure four times better than one made with a 3D printer. This makes 5D printers particularly useful for producing complex and robust structures, such as those needed for bone tissue engineering [104].
The implementation of 5D printing in neuroscience and neural tissue engineering has the capability to transform the industry by allowing the creation of intricate neural circuits and interfaces that emulate the form and function of natural tissues [105]. Even though the concept of 6D printing is new, the emphasis is on using the more established 4D and 5D printing techniques to create biomaterials. It is possible in theory to achieve 6D printing by combining 4D and 5D methods in a coordinated way, but it is not yet feasible in practice due to a lack of expertise and fabrication capabilities. The biggest challenge is to identify or develop the most suitable material that can be both rigid and responsive to one or more stimuli. Six-dimensional printing can enable the fabrication of structures with additional dimensions such as topology and texture, which can enhance the mechanical, biological, and physical properties of the printed tissues [104]. Overall, the application of 5D printing in neural tissue engineering and neuroscience holds great promise for the development of new therapies and technologies for treating neurological disorders and injuries, as well as for advancing our understanding of the brain and its functions. However, continued research is needed to optimize bio-ink formulations and printing parameters, as well as to validate the safety and efficacy of 5D printed structures for biomedical applications.
8. Future directions and conclusion
Biomedical engineering and regenerative medicine encompass a range of disciplines for the regeneration of damaged neural tissue, better understanding tumor behavior, providing targeted drug delivery, and etc. Notably, significant progress has been made in bioprinting, allowing for the creation of intricate bionic structures [106]. To address the complex nature of neuronal tissue, precise control over spatially distributed cells and biomaterials is crucial. Three-dimensional bioprinting has emerged as a promising method for generating patient-specific neural grafts, leveraging the synergistic incorporation of various bioactive factors and cells to promote neural regeneration [95]. Table 1 provides an overview of the diverse applications of 3D printing in neural tissue engineering and neuroscience.
Table 1. A brief overview of the various ways in which 3D printing is used in the fields of neural tissue engineering and neuroscience.
| Applications | Materials | Manufacturing | Design | References |
|---|---|---|---|---|
| Peripheral nerve injuries | PCL/rGO | EHD | Scaffold | [61] |
| Brain injuries | Hyaluronic acid gelatin-based hydrogel | DLP -based 3D printing | Scaffold | [66] |
| Spinal cord injuries | Collagen/silk fibroin | Low temperature 3D printing | Scaffold | [64] |
| Traumatic brain injury | Collagen/chitosan loaded with INExos | Low-temperature 3D-printing | Scaffold | [68] |
| Spinal cord injuries | Hydrogel reinforced with fiber bundles and loaded with OMT | Nearfield direct write electrospinning | Scaffold | [65] |
| Spinal cord injuries | CCH | Bioink 3D bioprinting | Scaffold | [71] |
| Controlled release of biologically active molecules to enhance nerve regeneration | Poly(ethylene glycol) diacrylate | SL-based 3D printing | Scaffold with core–shell nanoparticles | [81] |
| Tunable drug loading | Zein gel | 4D printing using a tailor-made supporting bath | Nerve conduit and drug delivery system | [84] |
| 3D printing of human dopaminergic brain tissue | SHAPE-composite | Bioink 3D printing | Sensor | [73] |
| Drug screening | Fibrin-based printed hydrogels | Bioink 3D printing | Microfluidic | [76] |
| Drug release for promoting nerve regeneration | Hydrogel functionalized with RGFP966 loaded nanoparticles | DLP based continuous 3D-printing | Nerve conduits | [79] |
| Repairing nerve defect | Functional conduit embedded with XMU-MP-1 | DLP-printer | Nerve conduits | [82] |
| Nerve regeneration | Soybean oil epoxidized acrylate | SL-based 4D bioprinting | Nerve guidance conduit | [89] |
| Neuroscience | Stereolithography | Optogenetic probes | [90] |
a Electrohydrodynamic jet. b Insulin-like growth factor-1. c Oxymatrine. d Conductive composite hydrogel. e XMU-MP-1 (4-((5,10-dimethyl-6-oxo-6,10-dihydro-5Hpyrimido[5,4-b]thieno[3,2 e][1, 4]diazepin-2-yl)amino) benzenesulfonamide).
Beyond 3D bioprinting, emerging technologies such as 4D and 5D bioprinting hold immense potential by integrating biomaterials, cellular microenvironments, time, and different geometrical dimensions. In 4D bioprinting, specific stimuli can induce dynamic morphological changes that benefit cell function, while stimuli-responsive printing further enhances tissue regeneration. The advantages of bioprinting, such as faster and more sterile fabrication of neural tissues compared to manual methods, highlight its transformative potential in neural tissue engineering.
To fully harness the capabilities of bioprinting, several factors must be considered, including securing sufficient funding, ensuring long-term sustainability, fostering interdisciplinary collaborations, and establishing centers of excellence at national or transnational levels. These centers would conduct multidisciplinary research on cell and material requirements, tissue maturation, and function. Encouragingly, well-funded multidisciplinary efforts and competition among emerging bioprinting companies are paving the way for the transplantation of functional and vascularized human tissues and organs [107]. Consequently, the commercialization of bioprinting technology and the development of a profitable bioprinting industry are becoming feasible with the next generation of bioprinters [108]. However, it is important to recognize that many traditional biomaterials lack the biomimetic properties necessary for neural tissue regeneration. Therefore, the integration of bioprinting with biologically inspired materials holds the potential to usher in the next generation of neural tissue regeneration and repair. In conclusion, while advancements in bioprinting have opened up new possibilities in neural tissue engineering and neuroscience, further refinement is required to address the remaining challenges and provide a comprehensive perspective on potential solutions. Moreover, 3D bioprinting holds immense potential for revolutionizing the field of brain cancers research. With the ability to fabricate complex 3D structures using living cells, 3D bioprinting enables the precise engineering of brain tumor models that closely mimic the intricate structures and cellular interactions found in real tumors. This breakthrough technology paves the way for enhanced drug screening, personalized medicine, and improved treatment strategies. Three-dimensional bioprinted models, by accurately replicating the heterogeneity and complexity of brain cancers, provide a platform for studying tumor behavior, drug response, and potential therapeutic targets. Furthermore, the ability to create patient specific brain tumor models can lead to tailored treatment plans, optimizing outcomes and minimizing side effects. As 3D bioprinting continues to advance, it offers tremendous promise in the fight against brain cancers, bringing us closer to more effective diagnostics, treatments, and ultimately improved patient outcomes. The prospects of 3D to 6D printing present exciting opportunities for advancing the field and realizing the full potential of bioprinting technology [109].
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Author contributions
E A and P S reviewed literature, and drafted the manuscript, writing. B S designed the schematic figure, reviewed the manuscript and performed language polishing. S J reviewed the manuscript. M K conceived the design of the study, supervision, writing, review and confirmation the final manuscript. Elahe Amiri and Mehrdad Khakbiz contributed equally to this work.
Conflict of interest
The authors declare no conflict of interest.