Abstract
Tympanic membrane (TM) perforation is a global clinical dilemma. It occurs as a consequence of object penetration, blast trauma, barotrauma, and middle ear diseases. TM perforation may lead to otitis media, retraction pockets, cholesteatoma, and conductive deafness. Molecular therapies may not be suitable to treat perforation because there is no underlying tissue matrix to support epithelium bridging. Chronic perforations are usually reconstructed with autologous grafts via surgical myringoplasty. Surgical treatment is uncomfortable for the patients. The grafting materials are not perfect because they produce an opaque membrane, fail in up to 20% of cases, and are suboptimal to restore acoustic function. Millions of patients from developing parts of the world have not got access to surgical grafting due to operational complexities, lack of surgical resources, and high cost. These shortcomings emphasize bioengineering to improve placement options, healing rate, hearing outcomes, and minimize surgical procedures. This review highlights cellular, structural, pathophysiological, and perforation specific determinants that affect healing, acoustic and surgical outcomes; and integrates necessities relevant to bioengineered scaffolds. This study further summarizes scaffolding components, progress in scaffolding strategies and design, and engenders limitations and challenges for optimal bioengineering of chronic perforation.
Export citation and abstract BibTeX RIS
1. Introduction
Tympanic membrane (TM), usually known as eardrum, forms an interface between the ear canal (length, 25 mm) and the middle ear. It is roughly conical in cross-section (cone angle, 135°; cone depth, 1.7 mm), oval (vertical axis, 9 mm; horizontal axis, 8 mm) in shape, 85 mm2 in diameter, and heterogeneous in thickness (50–150 μm) [1]. TM makes an acute angle (55°) concerning the lower wall of the ear canal. TM has a greater surface area than the ear canal (diameter, 7 mm) because of its conical shape and angular orientation [2]. It is semi-translucent and possesses an impermeable structure. TM protects the middle ear from microbial invasion and foreign substances. The transparency, shape, mobility, and light reflection of TM often change in response to the middle ear pathologies [3]. The eardrum is a viscoelastic membrane and functions as the first part of the sound transduction mechanism. It vibrates in response to a range of frequencies (20 Hz to 20 kHz), and at the same time, withstands pressure fluctuation [4]. TM vibration pattern is complicated as different TM sections move in different phases in response to sound waves [5]. The acoustic vibrations drive the motion of middle ear bones (malleus, incus, and stapes). The auditory ossicles are arranged in a lever system and serve as an acoustic transformer between TM and the fluid-filled cochlea (figure 1(A)) [6].
Figure 1. (A) Schematic diagram shows the TM interface and associated parts. The conical configuration, angular placement, and umbo-manubrium interaction amplify the airborne sound. (B) The lateral surface photograph shows the morphological dynamics of human TM. The change in opacity, movement, reflex light, and ossicular hallmarks could represent pathophysiological changes in the middle ear. (C) The medial surface photograph of rat TM shows the interaction between pars tensa and manubrium of malleus. (photograph by the authors). Note: Inset of the figure represent the following hallmarks: 1, tympanic membrane; 2, ear canal; 3, tympanic cavity; 4, Eustachian tube; 5, malleus; 6, incus; 7, stapes; 8, annulus; 9, umbo; 10, handle of malleus; 11, lateral process of malleus; 12, anterior malleolar fold; 13, posterior malleolar fold; 14, cone of light; 15, long process of incus; 16, tympanic membrane perforation; 17, tympanic ring; 18, tympanic notch; PS, posterior-superior; AS, anterior-superior; PI, posterior-inferior; AI, anterior-inferior; PT, pars tensa; PF, pars flaccida.
Download figure:
Standard image High-resolution image1.1. Morphology and interface of TM
TM can be divided into pars tensa and pars flaccida regions. Pars tensa represents up to 90% area of TM. The periphery portion of pars tensa shown a fibrous ring-like structure called the annulus. The annulus is thoroughly inserted in the tympanic sulcus, a groove on the bony tympanic ring [7]. The medial-superior part of pars tensa is connected to the manubrium of the malleus. The manubrium tip produces a slight rounded outshoot in the TM central points, called the umbo [8]. The manubrium attachment is varying throughout its length, i.e. the umbo and lateral process of the malleus are firmly embedded within the mesenchymal layer of the pars tensa while the handle of the malleus is loosely bound to the medial surface of pars tensa [9]. The umbo is generally taken as a reference point to divide TM into posterior-superior, anterior-superior, posterior-inferior, and anterior-inferior quadrants [10]. Such division is helpful for surgical consideration, e.g. the ventilation tube is not to insert in the posterior-superior quadrant of TM due to the close association of the incus portion [11]. The cone of light is reflected at 5 o'clock and 7 o'clock position on the right and left TM, respectively [12].
Pars flaccida or Shrapnell's membrane forms an inverted triangular structure above the pars tensa. It represents a small fraction of TM and is loosely attached to the tympanic notch (Notch of Rivinus). Pars flaccida is more flexible than pars tensa and plays no prominent role in sound conduction [13]. It moves inward or outward following pressure fluctuation. Such a damping mechanism is essential to protect pars tensa from blast waves and rapid pressure fluctuation. The pars tensa and pars flaccida attachment point forms a thickened band called the malleolar fold (figures 1(B) and (C)) [14, 15].
1.2. TM perforation
TM perforation (TMP) represent a hole in the eardrum. It reduces the surface area for sound conduction and establishes communication between the middle and external environment for possible microbial infections. TMP could occur due to object penetration, physical trauma, blast trauma, barotrauma, loud noises, middle ear diseases, and drainage tubes [16–18]. Clinically, TMP can be graded based on perforation size (small, medium, subtotal, and total), perforation site (anterior, posterior, median, marginal, and attic), and healing duration (acute and chronic) [19].
Acute perforations have mild symptoms such as aural fullness, otalgia, residual tinnitus, and hearing difficulty. Up to 80% of perforation spontaneously heal within 3 months via precautions to avoid superimposed infection, water, and cold air [20, 21]. The remaining TMP cases could progress to a chronic stage and are unlikely to be self-heal. Chronic perforation could results in recurrent infection, chronic otitis media [22], retraction pockets, and cholesteatoma [23], discussed in detail in section 2.2. The perforation size, perforation site, and associated middle ear diseases contribute to conductive hearing loss (up to 40 dB) [24]. TMP following recurrent otitis media is a global clinical dilemma and affects the health, academic, psychosocial-life, and lifestyle practices (i.e. strenuous sports) of up to 360 million people worldwide [25].
1.3. Limitation in surgical grafting of TMP
The myringoplasty is the choice for sealing chronic perforations with autologous grafts (i.e. temporalis fascia, tragal cartilage, fat tissue, and perichondrium) to improve sound conduction and barrier function. These tissues are less than optimal and have many limitations, such as required a prolonged closure time, create an opaque membrane, and hide middle ear pathologies [26, 27]. The geometric and biological mismatch between the grafting tissue and perforated TM results in poor integration, i.e. initial grafts uptake rate fails up to 22% of cases. The tissue grafts may undergo structural defects following otitis media and pressure fluctuation [28, 29]. The cartilage holds good mechanical stability; nevertheless, their increased thickness, mass, and stiffness properties [30] affect the acoustic transfer characteristics. The hearing loss (>10 dB) could persist in up to 50% of patients. In particular, tissue grafts function to seal the perforations but are suboptimal to restore TM translucent and biomechanical properties [26, 31, 32].
The transcanal (across the ear), endaural (through the ear), and postaural (behind the ear) surgical approaches have been developed for myringoplasty to place grafting material in the underlay, overlay, and inlay of TMP remnant [33, 34]. The transcanal approach is non-invasive but faces hurdles to place the grafting material in anterior perforation due to multiples reasons, i.e. the ear's narrower surface, bending of the ear canal at medial side, and angular placement of TM [35]. On the other hand, the endaural and postaural are invasive approaches, necessitate experienced surgeons, and bring about limitations in anesthesia, morbidity, packing materials, long-term care, and financial burden. The inconsistency in surgical reconstruction underlines the need for sophisticated grafts that should fit into the defect with negligible overlap [3, 36, 37].
The intrinsic limitations in grafting tissues, lack of surgical resources, microsurgical complexities, and high treatment costs limit tissue grafting applications and underscore the need for bioengineered strategies to improve healing, hearing, and surgical outcomes. This review highlights cellular, structural, pathophysiological, and perforation specific determinants that affect healing, acoustic and surgical outcomes; and integrates necessities relevant to an ideal scaffold. This review further summarizes scaffolding components, progress in scaffolding strategies and design, and engenders limitations and challenges for optimal bioengineering of chronic perforations.
2. Factors affecting TMP healing, acoustic, and surgical reconstruction
2.1. Cellular and structural determinants
TM is a complex trilaminar structure composed of the outermost epidermal layer, intermediate lamina propria, and innermost mucosal epithelial layer [38].
The lateral surface (ectoderm) of TM consists of a keratinized stratified squamous epithelium. TM holds an unusual epidermal site, and unlike skin, it lacks hair follicles and sweat glands. The epithelial layers are non-uniform in structure [39]. The different sections of TM hold different epithelial thickness. The epithelium showed more thickness in the handle of the malleus (25 μm), umbo (20 μm), and annular (40 μm) region compared to the intermediate portion (5–10 μm) [40]. The keratinocytes around the umbo/manubrium migrate in the radial direction towards the annulus [41]. If a tracing dye spot is placed onto the centre of TM, the spot would break and move towards the ear canal. Such a distant migration pattern prevents the accumulation of cellular debris cerumen impaction [42, 43]. The different TM sections hold different biological properties, i.e. epithelium around the manubrium fold, umbo, and annulus showed increased keratinocyte migration and proliferation potential [44].
The intermediate lamina propria (mesoderm) contains collagen fibers, fibroblasts, elastin fibers, nerves, and extracellular matrix components. It can be distinguished into subepidermal, collagenous fibers, and submucosal layers [39, 45]. The composition, orientation, and arrangement of collagen fibers vary between pars tensa and pars flaccida. The collagen fibers (type I collagen) present in pars flaccida are loosely organized in a 3D form [46]. On the other hand, collagen fibers present in pars tensa reveal a structure similar to a spiderweb, i.e. orient onto radial fibers (type III collagen) and circumferential fibers (type II collagen) in the lateral and medial sides (figures 2(A) and (B)) [47]. Some short length parabolic, crescent, and oblique fibers are also associated with the radial/circumferential fibers. The radial fibers emerge from the umbo/manubrium, radiate out towards the annulus, and converge near the attachment site [48]. The circumferential fibers originate from the umbo/manubrium and run inferiorly in a circumferential arc and rejoin on their opposite side. These fibers become packed in the annulus and contribute to their high thickness [46, 47]. The unusual fibers orientation contributes to acoustic energy propagation, dissipation, and transmission to the auditory ossicles [49] (figure 2(C)).
Figure 2. Schematic trilaminar structure of TM. The collagen fibers are represented as a separate layer. (A) Collagen fibers of pars flaccida are loosely organized in the 3D structure (B) Collagen fibers of pars tensa are arranged into the outer radial and inner circumferential orientation. (C) The orientation of collagen fibers varies between pars flaccida and pars tensa. Collagen fibers of pars tensa reveal a structure similar to a spiderweb. Abbreviations: RCF, radial collagen fibers; CCF, circumferential collagen fibers; LCF, loose collagen fibers; PCF, parabolic collagen fibers
Download figure:
Standard image High-resolution imageThe inner surface (endoderm) of TM continues with the middle ear mucus lining. It consists of monolayer cuboidal mucosal epithelium (5–10 μm). The regenerative mechanism and functional role of mucosal epithelium remain unclear despite its importance in understanding middle ear illness [39].
2.1.1. Compromised epithelium and stem cell niches
The borders of chronic perforations are suspended in the air. There is no structural support that helps the epithelium margins to bridge the gap. The air interface on both perforation sites compromises regulatory biomolecules and cellular functioning. The insufficient structural support could result in medial folding of perforation boundaries [38, 45]. The interaction between keratinocytes and fibroblasts during the late-phase of healing could fail and result in a prolonged bilaminar structure. The bilaminar TM possesses suboptimal biomechanical properties due to compromised collagen structures [50, 51].
The traumatic perforation, recurrent infection, otitis media, retraction pockets, and age-related differences could reduce the migration and proliferation of cells. For example, the epithelium cells' migration rate is faster in children (⩾130 μm d−1) than adults (⩽105 μm d−1) [43]. The long-term placement of the ventilation tube could stress the TM and could compromise cellular and acoustic functioning. It has been observed that the tissue grafts hold suboptimal epithelium migration, which later affects self-healing and self-cleansing mechanisms. These factors indicate that compromised epithelium contribute to chronic perforation and pose hurdles in healing [52, 53].
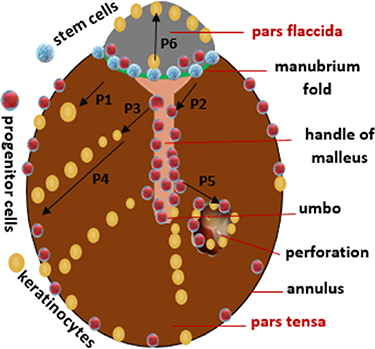
The manubrium fold is the proposed niche for the self-renewing stem cell population. The handle of the malleus, umbo, and annulus contains committed progenitor cells [40]. The significant migration and proliferation of keratinocytes are usually noticed around the traumatic perforation [44], suggesting that the migration mechanism is under paracrine control. The niche biochemical and biophysical components regulate the stem and progenitor cell differentiation and homeostasis [54]. The umbo grafts cells hold the potential to attach, migrate, and expand on the artificial scaffolds and could function as a stem organoid and assist in developing the TM equivalent model [55, 56].
The perforation at the umbo and manubrium region significantly alters the self-repairing mechanism because such perforation could compromise niche functioning [57]. The harmful substances in otitis media could influence stem cell differentiation and contribute to chronic perforation [58]. The air interphase on both sides of perforation could compromise stem cells' bioactivity and reduce the TMP healing potential. In particular, stem cells and progenitor cells are regulators of TMP healing and can serve as molecular targets for tissue engineering approaches [39, 59]. The progenitor could be isolated and expand from scarring patients' samples and lead to personalized treatment of complicated TMP (figure 3) [60].
Figure 3. The schematic diagram shows the proposed location and fate of stem cells, progenitor cells, and keratinocytes on TM outermost epidermal layer. (P1) and (P2) The malleolar fold is the proposed zone for the stem cell population. These cells undergo self-renewal and differentiate into committed progenitor cells and keratinocytes. (P3) The progenitor cells are differentiated into keratinocytes. (P4) The keratinocytes migrate in the radial direction from the umbo/manubrium to the annulus. (P5) The massive cellular response observed around perforation shows the existence of paracrine signaling. (P6) The pars flaccida has a limited capacity for keratinocyte proliferation and migration [40, 43, 44, 54, 59].
Download figure:
Standard image High-resolution image2.1.2. Disorganized collagen fibers
The unusual orientation of collagen fibers is crucial for sound conduction and biomechanical properties [61]. It presumably acts as guide to dictate adherence and migration of keratinocytes in the radial direction. The traumatic perforation, recurrent otitis media, and several unknown factors compromise cellular functioning and result in collagen fibers disorganization [39]. For example, circumferential collagen fibers are thinner than radial collagen fibers and are prone to break on exposure to the blast waves [62]. The fibrous structure restores much slower than the epidermal and mucosal layers during self-healing, and newly-formed TM may remain in the bilaminar configuration for several months [46]. The bilaminar membrane is similar to pars flaccida and lacks a tense collagen structure. The bilaminar membrane affects acoustic functioning and is susceptible to retraction and perforation even under mild barotrauma [63]. The autologous grafts are suboptimal for high-frequency sound conduction since they lack the complex orientation structures. In particular, disturbance in collagen fibers orientation affects TM acoustic, biomechanical, and cellular functioning [64].
2.2. Pathophysiological and associated determinants
The middle ear of children are often susceptible to microbial infection due to the anatomical predisposition of the Eustachian tube and compromised immune response [65]. The Eustachian tube is narrower, shorter, less rigid, and more horizontal in infants (length, 15 mm; horizontal angle, 10°) than adults (length, 33 mm; horizontal angle, 42°). Such anatomical predisposition enables respiratory infection, prevents mucus drainage, and obstructs gas exchange [66]. These factors promote middle ear inflammation, otitis media production, alters TM structure, and open window for microbial infections and biofilm development [17, 65, 66].
2.2.1. Retraction pockets and cholesteatoma
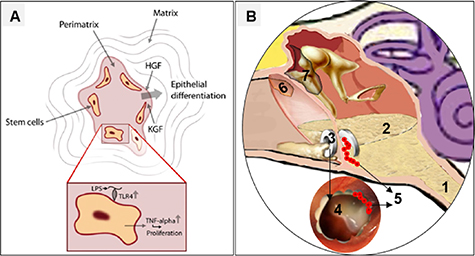
The retraction pocket is an inward invagination of the fragile part of TM. It occurs as a result of persistent negative pressure and adhesive otitis media in the tympanic cavity. The pars flaccida and posterior-superior quadrant of TM are retraction zones due to weak fibrous structures. The deep retraction pockets show a mild effect on sound conduction, affect cellular migration, and self-cleaning mechanism [67, 68]. The squamous debris could invade the middle ear and lead to an abnormal sac called cholesteatoma. It appears as a white mass and usually occurs behind the posterior-superior quadrant. Recent studies showed that cholesteatoma contains stem cells, and malleolar fold, which is the proposed niche for the stem cell population, may contribute to cholesteatoma formation. Cholesteatoma holds a suitable micro-environment that direct the differentiation of stem cells into keratinocyte resembles cells. It secretes proteolytic enzymes and contributes to the foul-smelling otitis media. Cholesteatoma is a 'benign' disease but can extend and damage the ossicular/intracranial structures (figure 4(A)) [69, 70].
Figure 4. (A) Cholesteatoma possesses stem cells and contains a suitable microenvironment for differentiation into keratinocyte resembles cells [70] (copyright © 2018, Springer Nature). (B) Schematic Illustration of pathophysiological and their associated factors that affect TM structure, biology, and physiology. Note: inset of the figure (B) represents the following hallmarks: 1, Eustachian tube dysfunction; 2, otitis media; 3, ventilation tube; 4, chronic perforation; 5, bacterial biofilm; 6, retraction pockets; 7, cholesteatoma.
Download figure:
Standard image High-resolution image2.2.2. Otitis media and bacterial biofilm
Otitis media is characterized by mucosal lining inflammation and persistent discharge of pus behind the perforated TM. It is a common disease of childhood and has a high recurrence rate, i.e. more than three episodes of otitis media have been reported among 50% of children [22, 71]. The harmful substances in otitis media could disturb the collagen composition and microstructure. The accumulation of otitis media in the tympanic cavity could restrict ossicular motion and bulge TM. The otitis media load and rapid change in pressure fluctuation could mediate hole at the delicate site TM [72, 73]. TMP following otitis media promotes inflammation, recurrent infection, and biofilm formation. These factors affect the acoustic, biomechanical, and self-healing properties of TM [74, 75].
Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, and Moraxella catarrhalis are common bacterial species that cause mucosal inflammation. The quorum-sensing mechanism among the bacterium encourages the development of biofilm on the mucosal surface. Biofilm is an organized bacterial community covered in an exopolysaccharide matrix. It adopts complex mechanisms to evade the immune responses. Biofilm contributes to recurrent infection and purulent otitis media. The bacterial biofilm could be 103 fold more resistant to antibiotic treatment [76, 77].
2.2.3. Ear drops and ventilation tube problems
Quinolones or aminoglycosides based ear drops are often prescribed to otitis externa and otitis media. The excessive applications of antibiotics could contribute to the development of multidrug-resistant strains. The superbugs infections necessitate higher concentrations of antibiotics in the middle ear, but most drugs cannot cross the TM barrier [78]. There is increasing evidence that available ear drops are cytotoxic to TM. It could disturb the collagen structure and composition, i.e. the antibiotic/dexamethasone combination could hinder TMP healing and could potentiate chronic perforations [79–82].
The ventilation tube placement in TM is the mainstay of maintaining an aerated middle ear in children and adults. It could relieve otalgia and improve hearing. It permits concentrated antibiotic drops to reach the tympanic cavity and obviates systemic antibiotics [83]. On the other hand, ventilation tubes could alter the middle ear flora and promote recurrent infections. It could contribute to biofilm development. Most importantly, the ventilation tubes could result in persistent perforation [18, 84]. In particular, the pathophysiological determinants and associated factors are more or less dependent on each other and affect TM structure, biology, and physiology (figure 4(B)).
2.3. Perforation specific and other determinants
The eardrum is non-uniform is structure, and it holds many peculiar properties in terms of TM-manubrium interaction [6], biomechanics of pars tensa, and pars flaccida [13], epidermal thickness [40], and confinement of stem/progenitor cells [59]. TM vibration pattern is complicated as different TM sections move in different phases in response to acoustic waves. These factors indirectly indicate that perforation in different regions of TM could pose different challenges [85]. This section discusses the impact of perforation size, perforation location, and perforation biomechanics on the healing, acoustic, and surgical outcomes.
2.3.1. Perforation size and location
Based on percentage (%) loss of drum surface area, TMP could be classified as small (⩽25%), medium (⩽50%), subtotal (⩽75%), and total perforation (⩾76%). The conduction disturbance increases with an increase in perforation size and perforation duration. The sub-total and total perforation are difficult to self-heal and possess less holding sites for grafting surgical materials. The spindle-shaped perforations more disturb the conduction mechanism than circular shaped perforation at high frequencies [19, 86].
Based on location, TMP could be classified as anterior, posterior, median, marginal, and attic perforation. The marginal and attic perforations are unsafe and can predispose to retraction and cholesteatoma than median perforation. The perforation at the umbo/manubrium region results in significant conduction disturbance and compromises the healing [57, 87, 88]. The anterior and marginal perforations have less holding sites for surgical materials placement. It is often challenging to reach anterior perforation via transcanal approach due to the narrower ear canal, TM angular placement, and ear canal bending at their medial side. These dimensional factors pose hurdles in surgical reconstruction, and invasive approaches (i.e. postaural and endaural) are often used to place the grafting materials in the perforation [3, 89].
2.3.2. Reduced biomechanics of TMP
The oriented collagen fibers and tight anchoring of most of the TM region in the tympanic sulcus are crucial for mechanical properties and optimal sound conduction [61]. The total and marginal perforation lost the tense structure of TM compared to small and central perforation. The perforated TM hold reduced mechanical properties [19] and pose significant challenges for the placement of surgical grafts and ventilation tubes. The mechanical and architectural mismatched between TM and grafting materials could reduce the acceptance rate and later abnormalities [18, 29]. The perforated boundaries of TM bulge in and bulge out following rapid change in atmospheric and middle ear pressure. The reduced biomechanics allocate complex necessities for desired grafting materials [1, 90].
3. Scaffolding components for TMP bioengineering
Bioengineering is an emerging multidisciplinary field towards assembling biological substitutes to reconstruct, maintain and restore damaged or lost tissue anatomy and physiology. Soluble biomolecules, stem cells, and polymeric biomaterials are the main scaffolding components. They can be used to tune the biophysical, biomechanical, and biochemical functionalities of scaffolds to influence biomaterial-tissue interaction. A significant effort has been made to produce functional scaffolds for the optimal reconstruction of perforations. This section discussed the prospect of growth factors, viable cells, and polymeric biomaterials toward chronic TMP reconstruction [91].
3.1. Biomolecules for TMP bioengineering
The topical ointments of basic fibroblast growth factors (bFGFs), platelet-rich plasma (PRP), epidermal growth factors (EGFs), and hyaluronic acid and have been investigated as regenerative medicine to accelerate the regeneration of perforated TM [92]. EGF mainly acts on epithelial layers and promotes keratinocyte migration. It induced a significant increase in collagen content. It has no apparent risk of ototoxicity but can promote bacterial infection. EGF holds a short half-life, and repeated applications could accelerate perforation healing [93, 94]. bFGF stimulates keratin migration, fibroblast proliferation, and better formation of collagen fibers. It can improve the closure rate and early restoration of the TM structure. The healing potential of a single application of bFGF is lower than that of multiple topical treatments. There are few pieces of healing evidence for bFGF in repairing chronic TMP [95].
Hyaluronic acid is biocompatible and offers numerous sites for optimal adhesion and migration of cells. It is safe for middle ear applications and often used as packing materials after myringoplasty. It can improve the healing of traumatic perforation and prevents dehydration of the perforation boundaries [96, 97]. PRP is the plasma fraction and is commonly extracted from umbilical cord blood. It contains a significant level of self-derived platelets (1.1 × 106 platelets μl−1), growth factors, and regulatory proteins. PRP could promote cellular proliferation and migration and has been used in TMP repair [98, 99]. The direct use of biomolecules has limited effectiveness due to a short half-life and uncertainty about appropriate dosing requirements. The multiple doses could diffuse into surrounded tissues, create excess moisture, promote infections, epithelial pearl formation, and open concerns related to cholesteatoma [100]. More clinical studies are warranted to evaluate receptor-binding specificity and healing mechanisms to restore TM structure. Incorporating the biomolecules into the scaffolds could improve shelf-life and control release for better drug-tissue interaction and extended applications [101].
3.2. Cell sources for TMP bioengineering
3.2.1. Embryonic and adult stem cells
Stem cells often undergo self-renewal and can transform into specialized mature cells under suitable extracellular matrix stimulation. Beyond proliferation and differentiation, stem cell applications exert beneficial effects on tissue regeneration through various mechanisms, i.e. releasing a range of trophic mediators and matrix proteins. Embryonic stem cells (ESCs) and bone-marrow-derived mesenchymal stem cells (BM-MSCs) have been used in TMP reconstruction [102].
ESCs are commonly collected from the mammalian blastocyst's inner cell mass and can be maintained and expanded in vitro for long periods. In 2007, Rahman et al investigated the potential of mouse-derived ESC on repairing acute perforation in Sprague-Dawley (SD) (n= 16) rats. In brief, ESC was seeded onto the gelatin patch's surface and applied to the perforated membrane. Gelatin based scaffold provides conductive sites for the optimal migration and proliferation of cells. The result demonstrated that ESCs shortened the closure rate and improved TM structural configuration [103]. BM-MSCs hold limited differentiation potential and are the gold standard in cell-based therapy. In 2008, Rahman et al studied that the BM-MSCs accelerate the healing of perforation and strengthen the TM structure [104]. In 2016, Goncalves et al spread the BM- MSCs on a porous scaffolding, and it forms a layer on the scaffolding surface. In another study, stem cells were embedded within a hydrogel network to minimize uncontrolled stem cells' infiltration. The in vivo results of both studies demonstrated that BM- MSCs hold the potential to accelerate closure of acute perforation rate and complete restoration of TM structure of C57BL/6 mice [105, 106].
3.2.2. TM-derived progenitor cells
The organoids are in vitro 3D self-organized stem cell construct. It is often grown under an appropriate extracellular matrix microenvironment to recapitulates the multicellular tissue composition. The organoid technology has been studied for pharmacokinetics screening, niche component analysis, disease modelling, regenerative medicine, and personalized tissue engineering [107]. In terms of TMP bioengineering, the progenitor cells around the umbo show enhanced cellular proliferation than progenitor cells in the annulus. The umbo grafts cells hold the potential to attach, migrate, and expand on the artificial scaffolds. These organized cells can function as a stem organoid and TM equivalent model. TM cell-based models could be promising alternatives to animal testing and can help to studies mechanisms involved in tissue regeneration [55, 56]. The progenitor cells have been isolated from scarring samples of patients and expanded via in vitro culturing. The clinical isolation and expansion of TM derived cells can generate substantial benefits over extrinsic stem cells and lead to personalized treatment of complicated TMP [60].
3.2.3. Other cell types
Many differentiated cells (i.e. fibroblasts and keratinocytes) have been isolated, expanded, and seeded onto a matrix to treat TMP reconstruction and restore normal tissue homeostasis [108]. These co-cultures of fibroblasts and keratinocytes have been spread onto the scaffolding surface and represent an epithelial-equivalent like tissue. The cell-seeded scaffolds showed rapid closure of acute perforation in SD rats [109]. The decellularized AlloDerm was seeded with human dermal fibroblasts and investigated to repair chronic TMP in the guinea pig. The results supported that recellularization improves the integration of scaffold into the perforated membrane [110].
In particular, stem cells hold several concerns, such as immunological rejection, high osteogenic potential, angiogenesis, and tumor formation. The transplanted cells could lose their biological activity (due to air interface) and infiltrate into the tympanic cavity to form cholesteatoma [70, 111]. One of the critical steps of stem cell usage for regenerative application is to control the differentiation into desired tissue lineages. TM-derived cells and growth factors could meet regulatory requirements [55, 112]. However, stem and progenitor cells' confinement into specific TM regions could pose significant hurdles in clinical applications [70]. There is a need to consider autologous cell sources, tissue-equivalent model, encapsulated platform, and regulator biomolecules for the optimal TMP reconstruction [59, 102].
3.3. Polymers for TMP bioengineering
The polymeric biomaterials serve as the structural frameworks of scaffolds. It could perform a crucial role in promoting cell-biomaterial interaction and could provide optimal mechanical guides and support for optimal tissue regeneration. Natural, synthetic, and natural/synthetic composite based materials have been used in TMP regeneration applications [113].
3.3.1. Natural biomaterials
Natural biomaterials such as collagen, gelatin, silk fibroin, chitosan, bacterial cellulose, and alginate have been used in TMP reconstruction. Collagen is predominant in the extracellular matrix of many tissues and plays a crucial structural and functional role. It is biocompatible with surrounding tissues and comprises a peptide sequence (arginine-glycine-aspartate) for cell adhesion. The exogenous collagen applications are suitable for bioengineering because they could resemble TM fibrous compositions [114]. Gelatin is commonly produced via partial denaturation of collagen. It is biocompatible, could provide proper hemostasis, and has been used in many bioengineering applications. The gelatin-based sponge (Gelfoam) has been applied as bioresorbable packing materials in the middle ear. Gelatin can be modified to produce gelatin methacrylate (GelMA), which exposes more integrin-binding domains for cell adhesion [115].
Silk is biocompatible, presents many sites for adhesion/migration of cells, and can adopt several structural forms. It has been used in controlled drug delivery applications, skin regeneration, contact lenses, and TMP reconstruction due to its tunable degradation, biomimetic, and mechanical properties [116, 117]. Chitosan is a positively charged polysaccharide formed by chemical deacetylation of chitin. It is biodegradable into non-cytotoxic components. The degradation properties and chemical reactivity depend on the amount of protonated NH2 group. Chitosan holds adhesive and antibacterial properties. The charged groups of chitosan are attractive for electrostatic interactions, sustained drug release, and many bioengineering applications [118]. Bacterial cellulose is an unbranched polysaccharide. Compared to plant cellulose, it is highly porous and can be extracted in pure form. Bacterial cellulose exhibits a high degree of polymerization and could aggregate to form nanometer wide fibrils. It has been used in sustained drug release and many bioengineering applications [119]. Alginate is an organic biopolymer derived from seaweed. It is an anionic unbranched copolymer arranged consecutive or in alternative units of mannuronic acid and guluronic acid. The guluronic acid unit participates in intermolecular interaction. Their gelation potential could form flexible scaffolds for cell transplantation applications [120].
3.3.2. Synthetic biomaterials
Many synthetic biomaterials such as poly(glycerol sebacate) (PGS), poly(lactic-co-glycolic) acid (PLGA), polylactic acid (PLA), poly(ethylene oxide terephthalate) (PEOT), poly(butylene terephthalate) (PBT), and polycaprolactone (PCL) have been used in TMP reconstruction. PGS is transparent, semi-crystalline, inexpensive, and holds thermoset elastomeric properties. It is elastic and can mold into different forms. The byproducts of PGS expresses minimal inflammatory response. The tunable degradation and mechanical properties make them attractive for soft tissue engineering, i.e. retina, cardiac muscle, nerve tissue, and TMP [121]. PCL is a biocompatible and biodegradable semi-crystalline polyester. It holds a low glass-transition temperature, high elastic properties, and are soluble in various organic solvents. It has been used in medical sutures and prosthetics applications due to its slower degradation rate. The surface and degradation properties of PCL can be modified via blending with natural biomaterials. The PCL based biocomposite scaffolds have been used for sustained drug release and soft tissue bioengineering [122]. PLA is a polyester of lactic acid monomer prepared via ring-opening polymerization and poly-condensation. It is biocompatible and degraded into a metabolic byproduct of L-lactic acid and D-lactic acid monomers. PLA holds a long degradation time, suitable physical properties, and has been used in many biomedical applications [123]. PLGA is made from lactic acid and glycolic acid units. It is amorphous and soluble in various organic solvents. PLGA has gained attention in bioengineering applications due to its biocompatibility, tunable biodegradation, and interface properties [124].
In particular, natural biomaterials degradation, mechanical, structural, and scalable properties are challenging to tune according to the desired application. On the other hand, synthetic biomaterials are non-adhesive to biological surfaces, lack appropriate sites for cell adhesion/migration, and their degradation products are not conducive to cell growth. Natural and synthetic biomaterials' composite gains considerable attention in tissue engineering to tone biomimetic, mechanical, structural, and degradation properties of scaffolds for a particular application [113, 125].
4. Scaffolding techniques and designs for TMP bioengineering
Three dimensional (3D) scaffolds are structural, functional, mechanical, biological, and supporting biomaterial that could guide cellular attachment, proliferation, migration, and differentiation for optimal regeneration of damaged or lost tissues. Scaffold-based bioengineering attracts significant attention because it can incorporate chemical, physical, biological, and biochemical stimuli to influence host tissue regeneration and thus could act as a template for model tissue fabrication [126, 127]. Decellularized tissues, films, sponges, hydrogels, nanofibers, and 3D printed based scaffolds have been studied to reconstruct perforation in human and animal models (figure 5). Up to 40 research studies have focused on developing TMP constructs, with 28 of these examining in vivo potential of scaffolding to reconstruct perforation. This section discusses the progress, design, potential success, and limitation in each scaffolding strategy towards TMP reconstruction (table 1).
Figure 5. The proportion of publications (selected studies: 28) on different scaffolding approaches that investigate in vivo methodologies to reconstruct TMP: (A) Decellularized scaffolds. (B) Melt-molding scaffolds. (C) Film, sponge, and other patches. (D) Hydrogel scaffolds. (E) Fibrous scaffolds. (F) Bioprinted scaffolds.
Download figure:
Standard image High-resolution imageTable 1. Summary of scaffolding strategies for preclinical and clinical reconstruction of TMP.
| Year | Scaffolding composition | Perforation condition and control group | Assessment method | Closure % | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biomaterial + growth factors + (cells) | Animal model | TMP type | TMP size | Follow-up time | Control treatment | OMS | HIS | IHC | ABR | Control group | Test group | ||
| 3D bioprinted scaffold | |||||||||||||
| 2018 | GelMA + EGF + fibronectin | Chinchilla | Acute | Subtotal | 03 week | Prototype | ✓ | ✓ | × | ✗ | 75% | 100% | [128] |
| 2017 | PCL/Collagen/Alg + (MSCs) | SD rat | Acute | Subtotal | 03 week | Prototype | ✓ | ✓ | × | ✓ | 57% | 100% | [129] |
| 2013 | Collagen + UCS | Pig | Acute | Subtotal | 04 week | Patch | ✓ | ✓ | × | ✓ | 43% | 100% | [130] |
| Fibrous scaffolds | |||||||||||||
| 2018 | PCL + EGF | SD rat | Chronic | Medium | 08 week | Untreated | ✓ | ✓ | ✓ | ✗ | 12% | 37% | [131] |
| 2017 | PLA/PLGA + (co-culture) | SD rat | Acute | Medium | 04 week | Prototype | ✓ | ✓ | ✓ | ✗ | 46% | 100% | [109] |
| 2014 | PCL/SF + UCS | Pig | Acute | Subtotal | 03 week | Patch | ✓ | × | × | ✓ | 41% | 100% | [132] |
| Hydrogel scaffolds | |||||||||||||
| 2018 | Hyaluronic acid/chitosan | Chinchilla | Chronic | Medium | 14 week | EpiDisc | ✓ | ✓ | × | ✗ | 50% | 93% | [133] |
| 2014 | Gelatin + bFGF | Pig | Acute | Small | 04 week | Prototype | ✓ | ✓ | × | ✗ | 37% | 100% | [134] |
| Film, sponge and other patches | |||||||||||||
| 2019 | Chitosan + IGFBP2 | SD rat | Chronic | Medium | 10 week | Untreated | ✓ | ✓ | × | ✗ | 16% | 44% | [135] |
| 2017 | Ducks feet collagen | SD rat | Acute | Small | 02 week | Patch | ✓ | ✓ | × | ✗ | 100% | 100% | [136] |
| 2016 | Collagen + FGF | SD rat | Acute | Medium | 03 week | Prototype | ✓ | ✓ | ✓ | ✓ | 81% | 100% | [137] |
| 2015 | Silk patch | Human | Acute | Medium | 12 week | Patch | ✓ | × | × | ✓ | 85% | 93% | [138] |
| 2014 | Silk fibroin and Collagen | SD rat | Chronic | Small | 12 week | Patch | ✓ | ✓ | ✓ | ✗ | 66% | 100% | [139] |
| 2013 | Silk fibroin and Collagen | SD rat | Acute | Small | 04 week | Gelfoam | ✓ | ✓ | ✗ | ✗ | 20% | 100% | [140] |
| 2013 | Silk fibroin and Collagen | Pig | Acute | Medium | 04 week | Patch | ✓ | ✓ | × | ✓ | 66% | 100% | [141] |
| 2013 | Bacterial cellulose | SD rat | Acute | Medium | 02 week | Untreated | ✓ | ✓ | × | ✓ | 100% | 100% | [142] |
| 2013 | Chitosan + EGF | SD rat | Chronic | MEDIUM | 10 week | Untreated | ✓ | ✓ | × | ✗ | 20% | 56% | [143] |
| 2011 | Chitosan | SD rat | Acute | Medium | 02 week | Patch | ✓ | ✓ | × | ✗ | 100% | 100% | [144] |
| 2011 | Gelatin + bFGF | Human | Chronic | Mixed | 03 week | Untreated | ✓ | × | × | ✗ | 10% | 98% | [145] |
| 2010 | Silk fibroin | SD rat | Acute | Small | 02 week | Untreated | ✓ | ✓ | × | ✗ | 20% | 93% | [146] |
| Melt-molding based scaffolds | |||||||||||||
| 2012 | Poly(glycerol sebacate) | Chinchilla | Chronic | Subtotal | 16 week | Gelfilm | ✓ | ✓ | × | ✗ | 75% | 90% | [147] |
| 2010 | Poly(glycerol sebacate) | Chinchilla | Chronic | Subtotal | 06 week | Gelfilm | ✓ | ✓ | × | ✗ | 75% | 90% | [148] |
| 2006 | Calcium alginate | Chinchilla | Chronic | Medium | 10 week | Patch | ✓ | ✓ | × | ✓ | 18% | 82% | [149] |
| Decellularized scaffolds | |||||||||||||
| 2019 | AlloDerm | Human | Chronic | Small | 6 month | Cartilage | ✓ | × | ✗ | ✓ | 82% | 89% | [150] |
| 2011 | AlloDerm | Human | Chronic | Medium | 18 month | Fascia | ✓ | × | ✗ | ✓ | 100% | 100% | [151] |
| 2009 | Urinary bladder matrix | Chinchilla | Chronic | Subtotal | 12 week | Untreated | ✓ | ✓ | × | ✗ | 64% | 100% | [152] |
| 2005 | Intestines submucosa | Chinchillas | Chronic | Medium | 06 week | Cartilage | ✓ | ✓ | × | ✗ | 60% | 100% | [153] |
| 2009 | AlloDerm + fibroblast | Pigs | Chronic | Medium | 07 week | Dura mate | ✓ | ✓ | × | ✗ | 86% | 94% | [110] |
Note and abbreviations: Prototype means scaffolding without the bioactive agents. Closure % is based on the statistical difference between control and experimental groups during the follow-up time. OMS, otomicroscopy; HIS, histology; IHC, Immunohistochemistry; ABR, auditory brainstem response.
4.1. Decellularized scaffolds
Decellularized scaffolds are obtained after removing cellular and genetic components from the allograft and xenograft via chemical, mechanical, thermal, and enzymatic methods. Decellularized tissue should contain less than 50 ng mg−1 and no longer than 200 base pairs of nucleic acid contents to avoid host antigenic response. The decellularization process preserves biochemical, biophysical, and microstructural properties of the basement membrane. Decellularized scaffolds hold the limited risk of host rejection and could provide conductive cues required for cellular homeostasis. Unlike autologous tissues, decellularized scaffolds can avoid donor site morbidity and reduce post-operative time due to commercial recognition [154].
Decellularized small intestine submucosa has been studied to reconstruct chronic perforation in chinchilla (n= 5). The results showed that acellular scaffolds increase graft uptake rate (5/5) and better structural organization of TM compared to cartilage graft (3/5) [153]. The urinary bladder matrix provided an inductive template for tissue regeneration and has been used to repair chronic TMP in the chinchilla (n= 11). The acellular matrix successfully integrated into TM remnant and disintegrate after tissue remodeling. The newly healed TM appeared more translucent and showed a better-organized structure [152]. The skin-derived acellular dermis (AlloDerm) is commercially available (thickness; 300 nm) and has been studied to reconstruct the medium-sized (n= 21) and small-sized chronic perforation (n= 27) in humans. The morphological healing outcomes are similar to temporalis fascia and better than cartilage [150, 151].
The basement membrane of decellularized scaffolds could be in vitro recellularized with appropriate living cells. Such a process could enhance the adhesion between the scaffolds and host tissues [155]. The decellularized AlloDerm is seeded with human dermal fibroblasts and investigated to repair a chronic perforation in the guinea pig (n = 50). The recellularization improves the scaffold's integration into the TMP remnant, and the recellularized scaffold could partially absorb and restore the TM structures [110]. There are some limitations in decellularized scaffolds, i.e. decellularization protocols could disturb the basement membrane matrix composition and textural properties. It could delay the recellularization mechanism. It underwent the same surgical procedure and associate concerns as autologous tissues. Decellularized scaffolds are suboptimal to replicate the biomechanical and micro-anatomical properties of TM. The clinical application of decellularized scaffolds may be challenging due to ethical reasons for the source of extraction from animals or cadavers [156].
4.2. Melt-molding scaffolds
The bioengineering of tissue depends on the structural and functional design scaffolds. Melt-molding is a simple technique to fabricate complex 3D scaffolds with specific dimensional properties. A porogen compound is loaded into the mold and annealed over the glass-transition temperature of the biomaterials. The leaching of porogen can introduce porosity in the molded scaffold that is beneficial for cellular penetration and proliferation [157].
Calcium alginate has been molded into the desired shape via computer-aided design based injection molding. The resultant plug improves chondrocyte's survival. It has been investigated to repair chronic TMP in a chinchilla model (n = 11). The resulting scaffold holds better potential to heal chronic perforation than the paper patch [149, 158]. PGS based spool-shaped engineered plugs have been investigated to reconstruct chronic perforation in a chinchilla model (n = 11). The moist nature, elastic properties, and sophisticated design permit the plug to settled into the defect. The PGS plug (90.9%) efficiently seals the perforation than the film patch (75%). The cellular layer has formed around both grafts, and plug size decreased due to surface erosion of scaffolding materials. The biodegradation rate of the plug has been evaluated during the reconstruction of chronic perforation in the chinchilla. The result demonstrated that the plug could remain stable for up to 16 months after implantation and create a regeneration problem. There is a need to tune the degradation rate that matches the tissue formation rate [147, 148].
4.3. Hydrogels scaffolds
Hydrogels are porous 3D scaffolds capable of absorbing and retaining a high amount of fluid in their microstructure. It has been formed via chemical, enzymatic, thermal, light-induced, or radical based cross-linking between macromolecules [159]. Hydrogels can undergo conformational changes under particular stimulus (i.e. temperature, light, ionic strength pH, ultrasound, magnetic field), thus engineered for controlled delivery [160].
Gelatin based hydrogels impregnated with bFGF have been studied to reconstruct acute perforation in guinea pigs (n = 8). The results depicted that the controlled release of bFGF from the scaffolding could accelerate perforation closure compared to gelatin hydrogel [134]. A photocurable hyaluronic acid and chitosan-based composite construct could be safe for otological applications as the scaffolds do not damage cochlear cells. The hydrogel is attractive to reconstruct chronic perforation in the chinchilla model (n = 26) [133]. The reduced mechanical properties, uncontrolled swelling, and inappropriate hydrogels design are concerns for practical applications. The future advancement in nanocomposite and double network hydrogels could tune mechanical and structural properties for the optimal reconstruction of damaged tissues [161].
4.4. Films, sponge, and other patches
The biomaterial, viable cells, and biomolecules could combine via simple approaches into films, sponge, and porous scaffolds. The collagen patch loaded with bFGF encouraged fibroblasts' proliferation and accelerated healing rate of acute perforation in the SD rats (n = 16) than paper patch treatment [137]. The bacterial cellulose-based fibrillary scaffolds exhibit a transparent nanostructure, and It holds the potential to accelerate the regeneration of acute TMP [142]. The duck feet derived collagen film is biocompatible, biodegradable, transparent, and showed better healing of acute perforation (SD rats; n = 16) than the paper patch [136].
Silk fibroin is a transparent patch and holds the potential to shorten the closure time of perforation. The scaffolding application showed better restoration of the TM structure (SD rat; n = 40) compared to paper patch treatment (n = 40) [146]. Silk fibroin based patch can speed up the healing rate in treating acute perforation in humans (n = 24) compared to paper patch [138]. Silk fibroin and acellular collagen-based scaffolds are biocompatible and have been investigated to repair sub-acute TMP in SD rats (n = 9 + 9/group), acute TMP in guinea pigs (n = 18/group), and acute TMP in SD rats (n = 30/group). The silk fibroin and acellular collagen scaffold underwent faster closure and complete restoration of TM structure than control treatment [139–141].
The chitosan patch holds better healing of acute perforation (SD rats; n = 20) than paper patch treatment [144]. The chitosan/EGF patch controlled released the growth factors and showed adequate healing capacity (56.5%) against chronic perforation (SD rats; n = 23) compared to the untreated perforations (20.8%) [143]. The chitosan patch loaded with insulin-like growth factor-binding protein showed control release of entrapped drug from scaffolds. The bioactive patch has the potential to improve cellular adhesion, spreading, and survival. The biocomposite patch holds a better healing rate (43.8%) than spontaneous healing (20.8%) of perforation in SD rats [135].
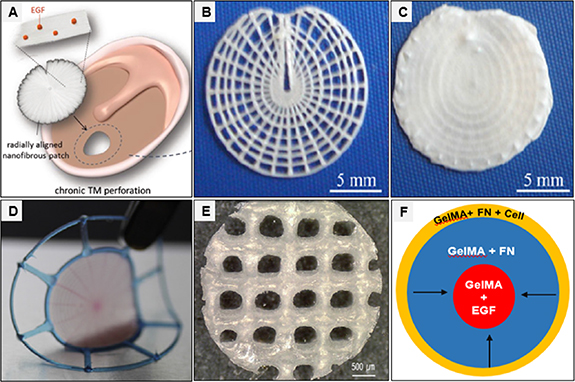
EGF and EGF receptor gene have been encapsulated in the polyethyleneimine (PEI)/chitosan based composite patch. The PEI provides a positive charge to improve cellular adhesion and migration. The sustained release of growth factor and receptor gene could provide biological cues to dictate cell response (figure 6(A)) [162]. Gelatin sponge dipped in bFGF ointment was applied over the chronic TMP (n = 53) in human patients. The patch was sealed with gelatin glue. bFGF immersed gelatin sponge results in better closure of TMP (52/53) compared to gelatin sponge treatment (1/10), and it also improved hearing. The gelatin sponge is cost-effective, has minimal invasiveness, and can be used for outpatient treatment [145]. In particular, the polymer-based patch could be transparent and can provide opportunities to see healing progress and middle ear pathologies. These scaffolds provide physical support to bridge the perforation gab and accelerate cellular response [136, 142, 146].
Figure 6. (A) EGF-control releasing radially oriented electrospun nanofibrous-based patches are developed for optimal adhesion and radial migration of cells to accelerate TMP regeneration [131] (copyright © 2018, Wiley-VCH). (B) The radial and circumferential microfiber layers are fabricated as a single piece via additive manufacturing technique. (C) The electrospun meshes are coated over circumferential and radial patterned microfibers [163] (copyright © 2015, IOP Publishing Ltd.). (D) Multilayered circumferential and radial bioprinted filaments are infilled with fibrin/collagen hydrogel matrix [164] (copyright © 2016, Elsevier). (E) A layer of MSC-laden alginate on the MSCs-laden collagen-coated PCL fibrous structure [129] (copyright © 2017, Elsevier). (F) The bioprinted cell-laden and EGF functionalize GelMA constructs could ease the host cells to invade from the scaffolding periphery to the centre and promote the active proliferation of cells [128] (copyright © 2018, Marry Ann Liebert, Inc.).
Download figure:
Standard image High-resolution image4.5. Fibrous scaffolds
Electrospinning is a convenient, inexpensive, and versatile manufacturing approach for producing nanoscale fibrous structures from polymeric solutions using a strong electric field. The electrospun nanofibers hold several unique properties, i.e. high surface to volume ratio, mechanical stability, tunable porous structure, nanoscale architect, and controlled drug delivery potential. These properties make nanofibers suitable for biomedical applications. The diversity in selecting the range of biomaterial and alteration in the electrospinning collector type could provide opportunities to develop scaffolds with tunable structure and function [165].
The gelatin nanofibers and chitosan/PVA nanofibers were separately cross-linked via glutaraldehyde and genipin, respectively. Both studies demonstrated that the cross-linking agent enhanced the mechanical and water resistance kinetic of nanofibers. The resulting scaffolds showed an improved survival rate of different cells and are biocompatible for tissue engineering of TMP [166, 167]. The PEOT/PBT nanofibers hold superior structural and mechanical properties. It could improve the adherence and survival rate of MSCs and keratinocytes on their porous structure [168]. The PLA/PLGA nanofibers facilitate the adhesion and spreading of co-cultures (fibroblasts and keratinocytes). The bioscaffold represents epithelial-equivalent like tissue. The biocomposite scaffolds demonstrated the rapid closure of acute perforation in SD rats (n = 5) compared to the non-cultured scaffolds [109].
The composite nanofibers of PCL/silk fibroin combined with human umbilical cord serum have been investigated to regenerate acute TMP in the guinea pigs (n = 10). The bioactive nanofibers provide physical reinforcement to stimulate cellular proliferation. The animal studied confirms the better regenerative potential of bioscaffold compared to paper patch [132]. Electrospun PCL nanofibers loaded with EGF are fabricated in a radial-aligned direction. Such oriented scaffolds promote and guide cell migration and proliferation. The sustained discharge of regulatory molecules from scaffolding could maximize the regenerative potential. The radial aligned fiber demonstrates better healing potential of chronic perforation in the SD rats (n = 38) than randomly aligned scaffolds [131]. The electrospinning is not under computer-controlled and holds limitations in the spatial design of fibers network and inaccurate distribution of molecules cues to dictate cellular organization and spreading [169].
4.5.1. 3D bioprinted scaffolds
3D bioprinting is an additive fabrication process in which computer-aided design, mathematical modeling, and image scanner data guide the instrument to fabricate complex microstructural scaffolds using bioink materials. The inkjet, extrusion, stereolithography, laser, and melt-electrowritting are common additive manufacturing approaches allowing the fabrication of scaffolds with high spatiotemporal control. The selection and development of proper bioink is a challenging parameter in the bioprinting to control optimal crosslinking, resolution, structural architect, mechanical and biological properties of scaffolds [170, 171].
The 3D porous collagen bioprinted scaffold (diameter, 236 ± 51 μm) loaded with human umbilical cord serum has been studied to reconstruct chronic perforation in guinea pigs (n = 10). The bioscaffold accelerates the healing rate (100%) and complete restoration of the TM structure compared to the paper patch treatment (43%) [130]. A triple-layered scaffold has been studied to recapitulate human TM properties using electrospinning and additive manufacturing approaches. The radial and circumferential bioprinted constructs were coated with random electrospun mesh. The oriented scaffold permits the adhesion/survival of MSCs and tunes the mechanical and vibrational properties (figures 6(B) and (C)) [163]. PLA, PDMS, and PCL based composite bioink materials were bioprinted in the radial and circumferential configuration. The filament spaces were filled with fibrin/collagen-based hydrogel. The resultant scaffold (thickness; 604 ± 12 μm) hold sound-induced motion and better mechanical properties than temporalis fascia (figure 6(D)) [164].
The BM-MSCs laden hybrid bioprinted scaffold (PLC/collagen/alginate) has been studied to repair an acute perforation in SD rats (n = 7). The bio-hybrid scaffold restores the structure of TM (figure 6(E)) [129]. A different combination of GelMA, EGF, and fibronectin scaffolds has been used as a bioink for 3D bioprinting. The scaffold (thickness, 400 μm) is designed in a butterfly-shaped to fit into perforation. The 3D bioprinted scaffolds hold reasonable stability and acoustic transfer function. The bioprinted cell-laden and EGF functionalize GelMA constructs could ease the host cells to invade from the scaffolding margins to the centre. The bioprinted scaffolds are optimal to heal perforation acute perforation in the chinchilla (n = 6) (figure 6(F)) [128].
3D bioprinting could provide opportunities to recapitulate collagen fibers orientation, improve acoustic transfer characteristics, and optimize radial migration of cells from perforation boundaries to the scaffolding centre. It can be customized in hospital settings and could fabricate perforation-specifics scaffolds via the defect's medical images [172]. The co-assembly of hydrogels/bioprinting [164] and electrospinning/bioprinting has been recognized for the complicated design of scaffolds better recapitulate TM properties [129, 163]. To date, the balance between materials properties and patterned scaffold surfaces is a significant concern because many biomimetic materials are challenging to bioprinted into bioscaffolds [173]. The bioprinting strategies are progressing dramatically, and there is a need to improve nanoscale resolution, the biomimetic composition of bioink, biomechanical properties, and the sophisticated design of the scaffolds for practical applications [170, 171].
5. Necessities, challenges, and prospects for TMP bioengineering
5.1. Preclinical consideration
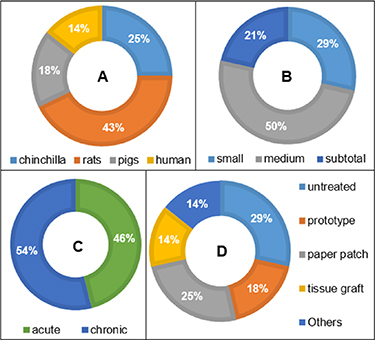
Most studies agree that the perforation persisting for 8–12 weeks is considered chronic; however, the true definition of chronic perforation could be varying among different species because different animals (pigs, gerbil, rats, and chinchilla) (figure 7(A)) hold different self-healing rates [38, 43]. The infolding, chemical, thermal, re-myringotomy, chemical, traumatic, and genetic modification have been introduced to create chronic perforation. Each method has its own merits and demerits to create similar and reproducible perforation, but the point of concern is that most of the animal models are less prone to recurrent infection and cholesteatoma. The current animal models could hide potential concerns associated with growth factor and cell-based scaffolds [174, 175].
Figure 7. (A) Percentage of animal species used in scaffolding based preclinical studies. (B) The proportion of consideration of perforation size during scaffolding-based preclinical studies. (C) The proportion of consideration of perforation complication during scaffolding-based preclinical studies. (D) The proportion of consideration of controls treatment during scaffolding-based preclinical studies.
Download figure:
Standard image High-resolution imageThe subtotal/total perforation and marginal perforation possess less holding sites for grafting materials integration, complicate the surgical procedure, and necessitate additional packing materials to hold the materials [3, 37]. Most of the scaffolding-based preclinical studies reconstruct small/medium perforations (which often self-heal) instead of subtotal and total perforation. The investigated studies have paid less attention to how scaffolds fit into the perforations to bridge the gap (figure 7(B)). Acute perforation could self-heal and require precautions to avoid superimposed infection; however, most of the constructs have been used to treat uncomplicated perforation. This indicates that scaffolding strategies are at an early stage to treat complicated perforation (figure 7(C)).
The different kinds of controls (paper patch, prototype, tissue graft, and untreated) have been used, but except for tissue graft, none of the control holds the potential to reconstruct chronic perforation (figure 7(D)). Few studies investigated the impact of scaffolding treatment on hearing performance (via auditory brainstem response) and collagen contents (via Immunohistochemistry). The collagen fibers orientation is often neglected despite its importance in acoustic/mechanical properties (table 1), and there is a need for a reliable method to detect collagen orientation. The ototoxicity, cytotoxicity, and genotoxicity of scaffolds need detailed investigation because biomaterials can enter the middle ear can cause adverse effects to the cochlear and vestibular apparatus [176, 177].
5.2. Biological consideration
The compromised epithelium migration, stem niches, and disorganized collagen fibers [38, 39] necessitate the scaffolding based application of exogenous cells and regulator biomolecules. The radial oriented scaffolds could dictate cellular adhesion and migration to bridge the perforation gap until complete TM regeneration [46]. The differentiation of stem cells and progenitor cells depends on their interactions with niche components; however, the null therapeutic benefit about the mechanism of action and detailed understanding of niche biology remains incomplete [59, 178]. There are considerable gaps in mechanism involved in the orientation of collagen fibers, clues that dictate cellular migration, and how TM cells migrate from perforation boundaries to the scaffolding surface. The future advancement in organoid culturing [55], encapsulated technologies, tissue-equivalent scaffolds, and nano-patterning surface cues could fill the scientific gap and open opportunities to dictate cellular response to reconstruct complicated perforations [179–181].
5.3. Biomechanical consideration
TM primes the biomechanical and sound conduction role due to the radial/circumferential orientation of collagen. It could be desirable to recapitulate TM collagen orientation to improve scaffolding acoustic, mechanical, and cellular compatible properties [1, 61]. Among scaffolding approaches, electrospun nanofibrous and 3D printed based scaffolds presents opportunities to recapitulate collagen fibers orientation. The electrospinning is not under computer control, and various solution parameters and alterations in the collector setup can fabricate radial aligned fibers [131]. On the other side, natural biomaterials (i.e. collagen) are challenging to print into the desired architect [170]. The synthetic materials based bioink could print into radial/circular and proposed construct design, but their degradation rate, acoustic properties, potential to integrate into TMP remnant, and antifouling properties against bacterial biofilm need detailed studies for clinical applications [163, 164]. Recent findings showed that collagenous structures play a crucial role in energy dissipation and wave propagation. The additive manufacturing and nano-patterning techniques are needed to improve the orientation of scaffolds using biomimetic biomaterials [49].
5.4. Dimensional and other consideration
The dimensional confinement of TM (angular placement, conical arrangement, and high thickness), ear canal (small diameter and curved surface area), variation in perforation (size, shape, and site of perforation), manubrium-TM interaction, and reduced mechanical properties of perforated membrane pose significant hurdles to suture the grafting materials [3, 29, 89]. TMP often occurs in conjunction with middle ear pathologies, and complete closure of perforated TM cannot cure a disease in patients prone to otitis media, chronic infection, bacterial biofilm, and Eustachian dysfunction [66, 76].
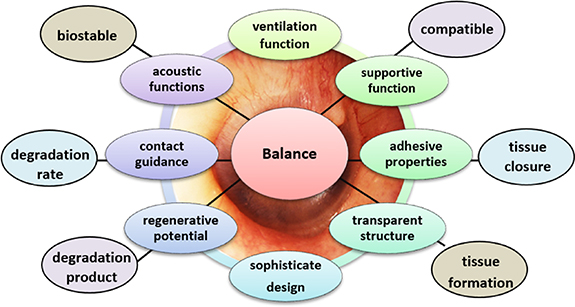
These dimensional and pathophysiological factors necessitate sophisticated designed scaffolding that should fit into perforation with negligible overlap and minimum invasiveness. The scaffolds should be semi-translucent to light on middle ear pathologies. The biomechanical properties should be kept close to TM properties for optimal interface interaction. For otitis prone patients, the scaffold scaffolds should be robust for surgical use and possess an engineered space for trans-tympanic drug delivery, maintain an aerated tympanic cavity, and protect TM from the effect of middle ear diseases [182, 183]. Melt-molding and 3D printing have presented the concept of perforation-specific constructs. Such designed scaffolds could minimize the need for packing materials and open office-based treatment opportunities. The current scaffolds hold several limitations in thickness, biomimetic design, and mechanical properties. The selection of optimal biomimetic materials, controlling degradation rate, and resolution of scaffolds at the nanoscale need close attention [158, 164]. In particular, factors that affect TMP healing, acoustic, and surgical outcomes plus current limitation in bioengineering approaches underscores the sophisticated design of scaffolds, which should present a balance between acoustic, regenerative, translucent, supportive, adhesive, and degradation properties (figure 8).
Figure 8. Proposed necessities for the bioengineered scaffolds to improve healing rate, hearing outcomes, and surgical reconstructions of chronic TMP.
Download figure:
Standard image High-resolution image6. Summary and concluding remarks
TMP bioengineering has dramatically progressed since the last decade with advancements in understanding disease contributing factors, stem cell niches, cell sources, biomaterials, and scaffolding technologies. Decellularized, melt molding, hydrogels, films, sponges, nanofibers, bioprinting, hydrogels/bioprinting, and nanofibers/bioprinting based scaffolds have been recognized for TMP reconstruction. Each scaffolding approach has its own merits and demerits. The preclinical studies showed that scaffolding based applications could accelerate the closure of acute perforations and offer advantages in ready availability, handling, reduced cost, and treatment in office settings. Unfortunately, none of the scaffolding approaches holds the potential to replace surgical tissue grafting and is suboptimal to reconstruct chronic perforation in clinical settings.
Our study recognized that TM presents a delicate balance between acoustic, mechanical, dimensional, self-healing, and self-cleaning mechanism due to its non-uniform structure. Important peculiar properties include radial/circumferential collagen fibers orientation, confinement of progenitor cells into specific regions, radial migration of keratinocytes, different biomechanics between pars tensa and pars flaccida, precise eardrum-manubrium interaction, and varying thickness across different sections. The irregularities of the TM make it an outstanding functional tissue, but on the other hand, such factors hinder in TMP reconstruction because perforations of different sizes, shapes, and locations offer different complications.
There are apparent biological and technological difficulties to recapitulate TM-equivalent properties for optimal TMP reconstruction. Much progress is required to control biomaterials at nanoscale resolution, tune the degradation rate, replicate biomechanical properties, biomimetic composition, spatial cellular arrangement, and translucent and perforation specific scaffolding design. Future advancements in chronic animal models, tissue-equivalent models, additive manufacturing, patterning techniques, and high design research studies could bridge the scientific gap. It is plausible that bioengineering strategies are in the right direction, and future innovation in 3D bioscaffolds could improve sound conduction, deliver drugs in the tympanic cavity, and open opportunities to reconstruct chronic TMP in office-based settings.