Abstract
Heterogeneous photocatalysis premised on advanced oxidation processes has witnessed a broad application perspective, including water purification and environmental remediation. In particular, the graphitic carbon nitride (g-C3N4), an earth-abundant metal-free conjugated polymer, has acquired extensive application scope and interdisciplinary consideration owing to its outstanding structural and physicochemical properties. However, several issues such as the high recombination rate of the photo-generated electron–hole pairs, smaller specific surface area, and lower electrical conductivity curtail the catalytic efficacy of bulk g-C3N4. Another challenging task is separating the catalyst from the reaction medium, limiting their reusability and practical applications. Therefore, several methodologies are adopted strategically to tackle these issues. Attention is being paid, especially to the magnetic nanocomposites (NCs) based catalysts to enhance efficiency and proficient reusability property. This review summarizes the latest progress related to the design and development of magnetic g-C3N4-based NCs and their utilization in photocatalytic systems. The usefulness of the semiconductor heterojunctions on the catalytic activity, working mechanism, and degradation of pollutants are discussed in detail. The major challenges and prospects of using magnetic g-C3N4-based NCs for photocatalytic applications are highlighted in this report.
Export citation and abstract BibTeX RIS
1. Introduction
Environmental pollution has been increasing at an alarming rate because of the rapid consumption of non-renewable fossil fuels to cope with the energy demands arising from industrialization and growing civilization [1, 2]. In addition, the wastewater derived from various chemical industries also contains a high concentration of organic pollutants, dyes, and heavy metals, creating severe threats to the ecological systems [3, 4]. Moreover, many synthetic dyes are extensively used in various sectors like printing, food, plastic, cosmetics, pharmaceuticals, and textile industries which discharge plenty of harmful pollutants in the water reservoir, carcinogenic for aquatic life as well as human beings or living organism [5, 6]. According to World Health Organization statistics, approximately 485 000 diarrheal deaths occur every year due to major outbreaks of waterborne diseases, which necessitates removing the dyes from wastewaters [7]. Therefore, considering water as the essential basis for all living beings, it is thus necessary to conserve and recycle it through scientific techniques for sustainable growth and development [8]. Till now, various conventional techniques are used for the water purification such as coagulation [9], flocculation [10], precipitation [11], adsorption [12], filtration, sedimentation, reverse osmosis [12], adsorption on activated carbon, chlorination [13], biological treatment [14], etc. However, these remedial techniques are expensive, time-consuming, and produce heavy sludge and toxic by-products. Also, the outcome of these conventional methods is inefficient for a good number of contumacious pollutants, e.g. pesticides, pharmaceuticals, organic solvents, household chemicals [15], etc. Therefore, an additional proficient treatment step is requisite [16], like the advanced oxidation processes (AOPs), to remove dispersed chemicals or low biodegradable compounds effectively and efficiently. The mentioned process represents a promising technology for the destruction of highly refractory compounds to produce water (H2O), Carbon dioxide (CO2), and respective inorganic ions through the generation of highly reactive organic species (ROS), mainly the hydroxyl radicals (·OH) [17]. The hydroxyl radical is considered as the second strongest oxidant after fluorine due to large oxidation potential (E◦ (·OH/H2O) = 2.80 V/SHE) and rate constants (106–1010 l mol−1 s−1). Furthermore, these radicals rapidly vanish from the reaction vessel because of the shorter lifespan (few nanoseconds) in H2O [18].
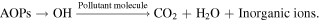
The most frequently employed AOPs derived from a combination of technologies [14, 19] are as follows: (a) H2O2 under irradiation of UV light (H2O2/UV light), (b) ozone and ozone-hybrid mechanism (O3, O3/UV light, O3/H2O2, and O3/H2O2/UV light), (c) photocatalysis (Semiconductor/UV light and Semiconductor/H2O2/UV light) and (d) Fenton reactions (Fe2+/H2O2), (e) photo-Fenton (Fe2+/H2O2/UV light), and (f) Sono-Fenton (Fe2+/H2O2/Ultrasonication). The classification of AOPs is tedious as it includes numerous processes, broadly classified as homogenous and heterogeneous processes [13, 14]. Despite their applicability in eradicating pollutants from wastewater, many significant hindrances associated with these processes restrict their practical applications on an industrial scale. The major limitations are identified as requisite of sophisticated equipment [18, 20], higher energy demands, additional chemicals as oxidants, ozone's diminutive half-life time [13], partial mineralization of toxic waste [13], high operating costs [18], limited UV light absorption [13], formation of highly toxic bi-products [18], etc. Alternatively, the semiconductor-mediated photocatalysts show great attention and the most promising AOPs [21] in the present state-of-the-art. Following green synthesis technology, researchers have attempted to completely degrade a wide range of pollutants, employing natural energy (for environmental restoration) under ambient reaction conditions [22]. These processes leave almost negligible post-treatment sludge, which consequently restricts the formation of secondary pollutants. Furthermore, the non-consumable property of the photocatalyst during the process makes them recyclable and re-usable even after many successive runs, which showcase their chemical steadiness and inertness [23]. Apart from the convenient aspects discussed above, semiconductor-based photocatalysts suffer from several drawbacks which curb their practical applications. The frequently used wide bandgap semiconductor photocatalysts, particularly TiO2 and ZnO (bandgap ∼ 3.2 eV), use UV light which covers about 3%–4% of the entire solar spectrum [24]. On the other hand, visible and near-infrared light covers approximately 97% of the solar spectrum [25]. Therefore, visible-light-active (VLA) photocatalysts are in high demand due to their remarkable performance in the visible regime. Another shortcoming of the semiconductor-based photocatalysts is the quick recombination of photo-generated electron/hole (e−/h+) pairs, which reduces their activities [26]. Hence, different design strategies have been exploited, which include band-gap engineering, semiconductor hetero-junction construction [27], porous structure tailoring [28], surface sensitization, active site controlling, element doping, carbon material incorporation [27], etc. Similarly, the successive filtration and centrifugation process causes a significant loss of photocatalysts during the recovery and segregation from the reaction medium. Therefore, different separation strategies are followed to avoid the difficulties faced during the recovery of catalysts on other support systems. Several approaches ease the segregation of photocatalyst but significantly reduce the specific surface area (SSA) and overall effectiveness. In recent years, magnetic photocatalysts have become very promising that offer a practical pathway towards separating nanosized photocatalysts from their suspension. The magnetically separable nanoparticles (NPs) grafted with non-magnetic g-C3N4 photocatalysts can tackle the major issues related to catalysts segregation [29] and carrier recombination [30]. Further, the formation of the built-in electric field at the heterojunction interface promotes effective migration of photo-excited charge carriers and lowers the electron–hole pairs (EHP) recombination rates [29]. To date, various magnetically separable semiconductor NCs have been studied such as, transition metal oxides [31], metal sulfides [32], carbon nitrides [33], carbides [34], etc. Among them, it is found that the g-C3N4-based magnetic photocatalyst shows more potential towards the degradation of numerous organic pollutants owing to its excellent electronic, optical properties and thermal stability in various aqueous mediums. The magnetic behavior of g-C3N4-based magnetic photocatalysts is merely offered by embedded magnetic materials, for example, different iron oxide derivatives. Incorporation of these magnetic materials not only provides the advantage of easy recovery and recycling of the photocatalyst, but it also offers high SSA along with large visible light absorption ability. However, many other factors such as improper band edge positions, high recombination rate, ineffective charge carrier generation, and separation need to be studied further for commercialization. Thus, in the field of pollutant degradation, the search for highly VLA magnetic photocatalysts continues, which are cost-effective and made from earth-abundant elements [35]. In this report, the major challenges and prospects of using magnetic NCs as photocatalysts have been reviewed and explored their practical applicability in the degradation of organic pollutants and antibiotics.
1.1. Magnetic materials as potential visible light photocatalyst
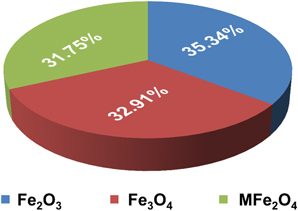
A good photocatalytic material must possess low bandgap energy, the appropriate positioning of the conduction band (CB)/valance band (VB), suitable redox potentials, and enhanced visible-light activity [36]. In recent times, magnetic NPs based materials have been more effective towards the photocatalysis application. The benefits of using magnetic NPs over non-magnetic NPs are as follows: high photocatalytic efficiency [37], reproducibility [38], sustainability [39], and simple preparation procedure [40]. Other benefits of using magnetic NPs include separation of the catalysts from the post-reaction liquid using an external magnetic field [40]. The steps followed in the entire process have led to low cost, low time consumption, and better reusability property of the catalyst. Among the magnetic NPs, iron oxide nanoparticles (IONPs) have received considerable attention in the domain of photocatalysis. Few notable features of such NPs are highlighted as follows: paramagnetic/ferromagnetic in nature [41], possesses high chemical and structural stabilities, high saturation magnetization (Ms), narrow bandgap energy [42], high visible light activity, and excellent transport properties [43]. Different iron oxide phases are synthesized and employed in catalytic applications based on their characteristics and attributes. The percentage distribution of published papers based on the type of iron-oxide magnetic materials is shown in figure 1.
Figure 1. Percentage distribution of published papers based on the type of iron-oxide magnetic materials (Found in the Web of Science database; dated on 5th August 2021).
Download figure:
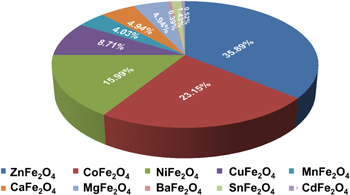
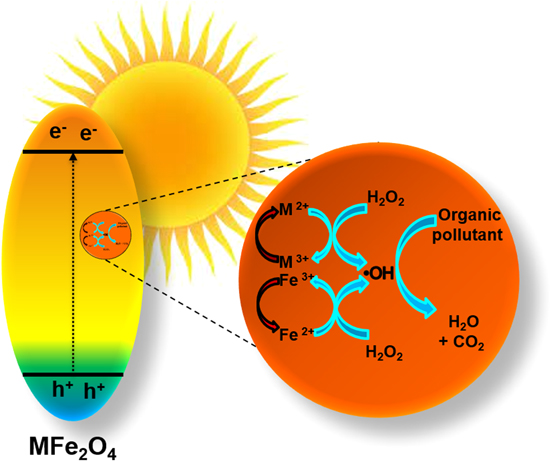
Standard image High-resolution imageAmong them, Fe3O4, α-Fe2O3, β-Fe2O3, γ-Fe2O3, and MFe2O4 (M = Mn, Fe, Zn, Ni, Co) are very popular magnetic NPs used as photocatalysts, shown in figure 2. The α-Fe2O3 (hematite) and γ-Fe2O3 (Maghemite) are stable iron (III) oxides [44, 45], out of which α-Fe2O3 absorbs yellow light in the visible region, possesses high saturation magnetization value [46], and low bandgap energy [47]. Therefore, hematite-based NPs show considerable photocatalytic activity [48]. The Fe3O4 and γ-Fe2O3 have similar crystal structures; however, the Fe3O4 unit cell constitutes both Fe2+ and Fe3+ions [49]. Fe3O4 and γ-Fe2O3 both have high Ms values at room temperature and offer low bandgap energy [50, 51]. The value of permanent magnetization and coercivity depends on the magnetic nanomaterial's shape and size [52, 53]. The Fe3O4 has low resistivity because of the speedy exchange of electrons between Fe2+ and Fe3+ in the octahedral sites [54]. The generation of intermediate bandgap due to shifting electrons from VB to the CB results in enhanced visible light activity. In addition, they show a high surface-to-volume ratio, which enhances the degradation due to its particle size. The MFe2O4 are spinal ferrite NPs [55], where M is a divalent metal ion such as Fe2+, Zn2+, Mn2+, Co2+, Ni2+, and Mg2+ [56]. They display superparamagnetic behavior and possess a low EHP recombination rate [57]. The presence of metallic ions enhances their characteristics and attributes. It shows high colloidal stability, and the magnetic core enhances the reusability properties [58]. Even after a significant number of cycles, the photodegradation rates for the magnetic materials are quite high.
Figure 2. Percentage distribution of published papers on photocatalysis using different types of ferrite (Found in the Web of Science database; dated on 5th August 2021).
Download figure:
Standard image High-resolution imageHowever, the IONPs display photocatalytic activity under UV light [59], and the metal-based sulfides/ or nitrides are also not stable under an aqueous environment [60]. The fast recombination rate of EHP is the primary concern. Hence, the combination of IONPs with semiconductors judiciously paves the way towards developing photocatalysts to overcome the limitations mentioned above. The iron oxide-based semiconductor NPs must possess the following attributes.
- I.The NPs must have enhanced visible-light photocatalytic activity, high stability, and high efficiency.
- II.The preparation of the material should be straightforward and cost-effective.
- III.The catalysts must have a low recombination rate of electrons and holes.
- IV.The catalysts should be effortlessly recycled, employing a simple external magnet.
- V.The efficiency must not decrease drastically even after a repeated number of cycles.
Various IONPs have been developed and used in the photocatalytic degradation of wastewater [61]. The choice of a semiconductor depends upon the positioning of VB and CB. The positive redox potential of the VB enhances the production of ·OH radicals [62], and the CB is negative enough to enhance the superoxide radical production (·O2 −) [63]. The semiconductor material absorbs the incident photons when the energy is greater than the bandgap. Consequently, the electron is excited from the VB to CB, leaving a hole in the VB that causes the formation of EHP. For effective separation of EHP, heterojunction-based semiconducting materials are realized that facilitate the transfer of electrons and holes based on the relative energy band positions [64].
1.2. Graphitic carbon nitride (g-C3N4)
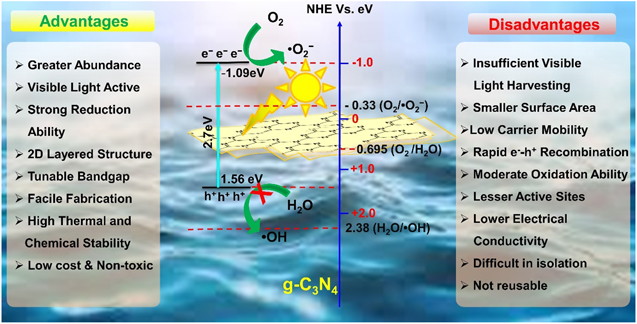
The graphitic carbon nitride (g-C3N4) has become very promising over the years due to commercial availability, high visible-light quantum efficiency, biocompatibility, non-toxicity, robustness, and cost-effectiveness [65]. The g-C3N4 consists of earth-profuse elements Carbon (C) and Nitrogen (N) in the company of some content of Hydrogen (H) [66]. It is a metal-free polymeric n-type semiconductor [67] and is considered a 'golden' photocatalyst due to its variety of attractive features. The unique feature determining the photocatalytic performance of g-C3N4 is the tunability of the energy band-gap (lowest energy gap ∼2.7 eV), with the CB and VB potentials located at −1.09 and +1.56 eV, respectively [68–70]. Therefore, the researchers have explored g-C3N4 extensively as potential photocatalysts to degrade waterborne contaminants and pathogen inactivation. The photocatalytic effectiveness of bulk g-C3N4 is limited on account of high photo-generated EHP recombination [71], smaller surface area [72], and low electrical conductivity [73]. Several strategies have been explored, such as noble metal deposition [74], proto-nation [75], construction of heterojunction with another semiconductor [76], energy band engineering, designing of well-defined g-C3N4 nanostructure [28, 77], coupling with other semiconductors [78], forming hybrid materials [76], etc. All these strategies significantly improve the performance as well as unfold its properties and reliable applications. The integration of non-metal dopants such as phosphorus (P), Boron (B), and Sulphur (S) onto the surface of g-C3N4 induce more reactive sites with an increased SSA, reduce the energy bandgap, and enhance the visible light absorption [76, 79]. However, it has never been unlocked the door of commercialization due to poor recovery and recyclability of the non-metal doped photocatalysts [80]. Numerous separation strategies are followed, such as immobilizing the photocatalysts on different support systems, i.e. sand, zeolites, ceramics, etc. However, the SSA of the composites is reduced in most instances. The advantages and limitations of the g-C3N4 photocatalysts, including the charge transfer mechanism, are presented in figure 3. Thus, the development of highly active, easily recoverable and recyclable g-C3N4-based photocatalysts is still a major challenge. The magnetically separable photocatalysts are optimized by embedding magnetic material on the g-C3N4 framework. Different strategies to prepare magnetically separable g-C3N4-based photocatalysts are discussed in detail in the following sections.
Figure 3. The advantage and limitations of the g-C3N4 photocatalyst including charge transfer mechanism. Reproduced with permission from [14] Copyright 2018, Springer Nature.
Download figure:
Standard image High-resolution image2. Magnetic material supported g-C3N4: potential strategy for enhanced photocatalytic activity and separation of photocatalyst
2.1. Metal/non-metal doped g-C3N4 photocatalysts
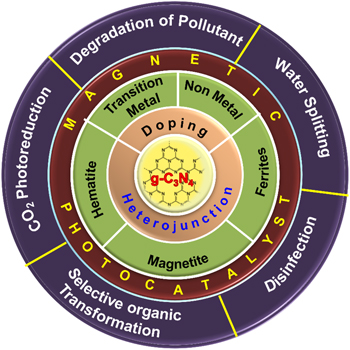
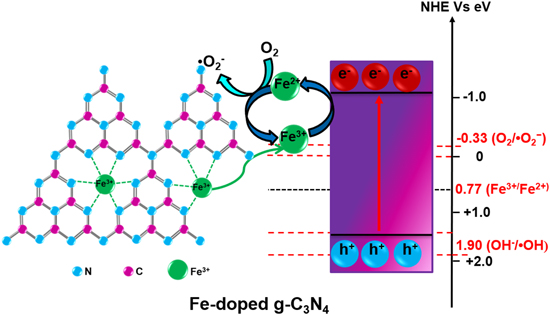
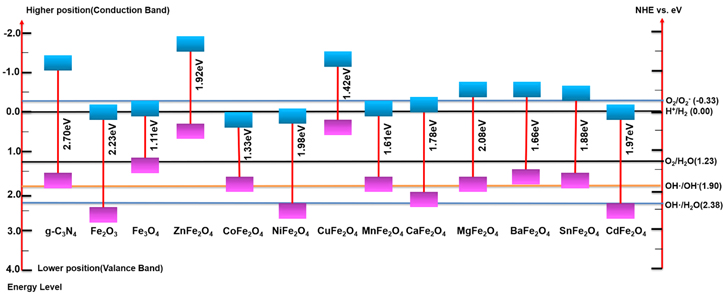
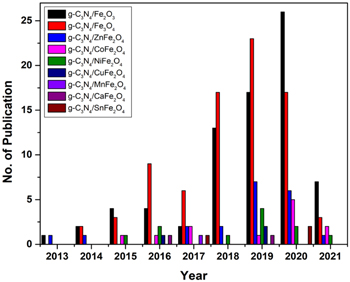
Various design strategies have been followed to date to synthesize magnetic photocatalysts to degrade pollutants, reduce CO2, water splitting, selective organic transformation, etc. Transition-metal/or non-metal doping, including constructing heterojunction with g-C3N4 using transition metal oxides (figure 4), has become very popular nowadays. Pristine g-C3N4 does not possess any intrinsic spin polarization, which prompted researchers to induce magnetic moment through suitable elements doping. The spin-polarized transition metals such as V, Cr, Mn, Fe, Co, Ni, Cu, and Zn -doped g-C3N4 are incorporated to harness the induced spin moments [81–83] of the layered composites. It is to mention here that the transition metal atoms are more stable in the cavity of the g-C3N4 system than that of their bulk phase. These atoms tend to segregate and form metal clusters in the cavity. Thus, single transition metal atoms doping on the g-C3N4 sheets are reasonably stable and prevent the segregation on the cavity, which is in full agreement with the theoretical observations [84]. Depending on the ionic radius, the atoms with a larger radius (V, Cr, Mn, and Fe) are mostly symmetrically placed in the g-C3N4 cavity. Whereas, the Co, Ni, Cu and Zn (relatively smaller radius) are asymmetrically coordinated with g-C3N4 [84]. Interestingly, the transition metal atoms (V, Cr, and Fe-doped g-C3N4) interact ferromagnetically, resulting in a ferromagnetic ground state whereas Mn couples antiferromagnetically. On the other hand, the Cu and Zn doped g-C3N4 induce structural distortion in the sheets, and the composites become nonmagnetic [84]. But, Co and Ni-doped g-C3N4 does not possess well-ordered magnetic orientation [84]. However, the modified g-C3N4 sheets display prominent absorption at low energy, confirming their enhanced visible light absorption capacity due to reduced bandgap energy [85]. For example, Liu et al [86] have synthesized Fe-doped g-C3N4 via a facile and cost-effective approach and showed that the Fe atom doping significantly influences the electronic structure and catalytic properties of g-C3N4. As shown in figure 5, the doped Fe3+ acts as electron capture sites to facilitate the separation of photo-induced charge carriers [87, 88]. The captured electrons then react to reduce O2 to ·O2 − species. Meanwhile, Fe3+ can act as a hole mediator to oxidize OH− or H2O molecules to ·OH species. Moreover, Fe-doped g-C3N4 offers superior photo degradation efficiency for RhB under visible light irradiation. Van et al [87] have designed a new-style heat stirring method to prepare Fe-doped g-C3N4 nanosheets using Fe(NO3)3 · 9H2O and Melamine as the starting raw materials. The introduction of single Fe atom doping on the exfoliated g-C3N4 nanosheets enhances the SSA (up to 132 m2 g−1). The large SSA not only absorbs light but also excites more EHPs. As a result, it shows complete degradation of RhB within 30 min of visible light irradiation. Many other transition metals (Co, Ni, Zn, Sc, Ti, V, Cr) have also been doped with g-C3N4 to improve the photocatalytic activity. Therefore, the doping of transition metal ions on the g-C3N4 framework can increase the SSA and the recombination of photo-generated carriers is suppressed by forming intermediate energy levels. Subsequently, the non-metal doping (S, P, B, N, O, F, etc) also shows interesting results due to the bandgap tunability of the g-C3N4 framework. It is evident from the theoretical study that only Boron-doped g-C3N4 nanosheets show ferromagnetism and half-metallicity [89]. However, the MS value of transition metal or non-metal doped g-C3N4 composites is not high enough to separate it simply using an external magnet. Therefore, the recovery and recycling of doped g-C3N4 still remain a challenge. The relative position of the valence and conduction bands (with respect to eV versus NHE) of g-C3N4 and various magnetic materials [90–101] is displayed in figure 6. Considering the above-mentioned challenges, the magnetic materials, e.g. Fe3O4, Fe2O3, MFe2O4, and so forth, are grafted onto the surface of g-C3N4 to prepare magnetically separable photocatalyst. The catalysts can be easily separated from the treated suspension using an external magnetic field, which eases the recovery and reusability of the same. It is the most rational and feasible solution for separating magnetic photocatalysts from the suspension [102]. As per the web of science database (August 2021), the number of publications during 2013–2021 on the g-C3N4-based magnetic photocatalysts is presented in figure 7. In the following section, the magnetically separable g-C3N4 –based NCs are thoroughly discussed.
Figure 4. Schematic illustration of g-C3N4-based magnetic photocatalyst and their application in different disciplines.
Download figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageDownload figure:
Standard image High-resolution imageFigure 7. No. of publications on g-C3N4–based magnetic photocatalysts up to 2021 (Found in the Web of Science database; dated on 5th August 2021).
Download figure:
Standard image High-resolution image2.2. Fe2O3/g-C3N4-based photocatalyst
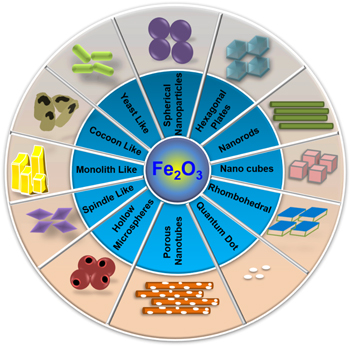
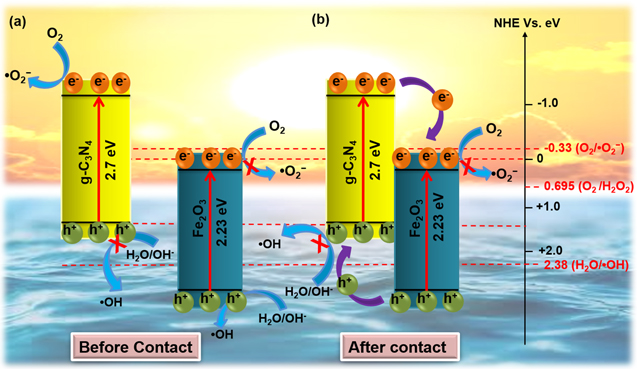
Hematite (Fe2O3), an n-type semiconductor [103], is considered as one of the most promising visible-light-driven photocatalyst owing to narrow bandgap ∼2.2 eV [104], excellent stability [105], corrosion-resistant [106], non-toxicity [107], and wider pH range [108]. Being earth-abundant elements and environmentally benign, iron-oxide's strong photo-oxidation abilities [109] have been exploited for contaminant degradation. However, the weak photo-reduction ability, shorter lifetime, and small diffusion length (in the range from 2 to 4 nm) of the photo-generated charge carriers limit their extensive application. Therefore, the Fe2O3 in their pure form cannot separate the photo-generated EHPs effectively [105, 106] and, hence couples with suitable semiconducting materials to form efficient photocatalytic system. In an effort, the Fe2O3 (strong oxidation ability) is combined with g-C3N4 (strong reduction ability) to construct a heterojunction system [109], where both g-C3N4 and Fe2O3 produce EHPs under visible-light irradiation. It is found that the different morphological (figure 8) features of Fe2O3 such as spherical nanoparticles [110–113], nanorods [114, 115], nanocubes [116], quantum dots [117, 118], porous nanotubes [119], hollow microspheres [120], rhombohedral shape [121], hexagonal plate-like [122, 123], spindle-like [124, 125], monolith-like [126], cocoon-like [127], yeast-like [128] etc. affects the charge transfer mechanism and photocatalytic activity. Therefore, various methods are followed to synthesize Fe2O3/g-C3N4 NCs such as calcinations [103], wet impregnation [129], hydrothermal [106, 107, 130], thermal treatment [131], microwave-assisted [132], NaCl assisted templating [133], etc. Using the Fe2O3/g-C3N4 composites, photocatalytic degradation of various organic pollutants and antibiotics such as Rhodamine B (RhB), Direct Red 81, Methyl Orange (MO), Cr (VI), dinitrogen, Methylene Blue (MB), Phenol, Tetracycline (TC), Ciprofloxacin, etc. have been attempted. The salient feature of the composites responsible towards superior photocatalytic performances is the formation of heterojunction interface (Type II and Z scheme) between Fe2O3 and g-C3N4 [106, 134].
Download figure:
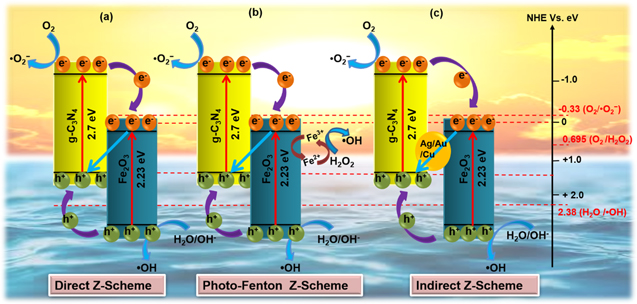
Standard image High-resolution imageIn the case of Type II heterojunction, as shown in figure 9, the photo-generated electrons can migrate from the CB of g-C3N4 into the CB of Fe2O3 during the reduction reaction [135, 136]. On the other hand, the holes migrate from VB of Fe2O3 into the VB of g-C3N4 for oxidation reaction as the CB and VB edge potentials of g-C3N4 are more negative than Fe2O3. But as the CB edge of Fe2O3 is more positive than the redox potential of O2/O2 −(−0.33e V versus NHE), the electrons on CB of Fe2O3 cannot reduce O2 to ·O2 −. Hence, the poor reduction ability of electrons and higher recombination rate of photogenerated EHPs in Fe2O3 reduce the photocatalytic activity of such a system. Whereas in the case of Z-scheme heterojunction [137, 138], as shown in figure 10, the photo-generated electrons from the CB of Fe2O3 migrates into the VB of g-C3N4 and recombines with holes. This process leaves behind photo-generated charges in the VB of Fe2O3 and CB of g-C3N4 for redox reactions [106, 134, 139]. Hence a direct Z-scheme structure, instead of Type II heterojunction, is recommended to form the g-C3N4/Fe2O3 hybrid. As a result of such systems, the rate of EHP recombination decreases, and the charge separation efficiency increases, leading to enhanced photoactivity [129]. Recently, Rajiv et al have reported that the hydrothermally synthesized Fe2O3 NPs decorated g-C3N4 display a high SSA (416.3 m2 g−1), and more active sites are expected to improve photocatalytic activity [110]. In contrast, Hou et al [140] have synthesized similar composite via calcination method and have reported relatively lower SSA with higher photocatalytic activity. This superior performance in activity is attributed to the highly suppressed charge carrier recombination via the Z-scheme charge transfer mechanism compared to the Type II system. Similarly, numerous Fe2O3 decorated g-C3N4 NCs have been synthesized, and their charge transfer mechanisms are thoroughly studied by varying morphologies. It can be concluded that Fe2O3 NPs decorated g-C3N4 nanocomposites offer relatively higher SSA/active sites compared to other morphologies. Therefore, the photocatalytic activity of the composites depends not only on the number of active sites but also on the types of the charge transfer mechanism. The charge transfer mechanism of the Z-scheme system exhibits superior photocatalytic performance than a typical Type II heterojunction structure [141]. Moreover, many metallic and semi-metallic elements such as Ag, Cu, Au, and carbon (Graphene derivatives) are deposited at the g-C3N4/Fe2O3 composite interface, which delivers routes for the fast charge transfer process. Therefore, many strong charge carriers are left to participate in the indirect Z scheme mechanism [142].
Figure 9. Schematic diagram of photo-generated charges transfers (a) before contact (g-C3N4, Fe2O3) and (b) after contact (g-C3N4/Fe2O3) (Typical Type II hetero-junction) [110, 119, 122, 135, 136].
Download figure:
Standard image High-resolution imageFigure 10. Schematic diagram of photo-generated charge transfer mechanism in g-C3N4/Fe2O3 composite system (a) direct Z-scheme (b) photo Fenton and (c) indirect Z-scheme [119, 126, 137, 138].
Download figure:
Standard image High-resolution imageMoreover, the content of Fe2O3 significantly affects the photocatalytic activity of Fe2O3/g-C3N4 composites. The significant enhancement in the photocatalytic activity is achieved close to the optimal value by increasing the content of Fe2O3. Recently, Huang et al have synthesized α-Fe2O3/g-C3N4 NCs by calcinations [103]. Under the visible-light irradiation, the NCs shows photocatalytic degradation efficiency ∼87% for RhB at about 3 h. The possibilities of agglomeration and non-uniform dispersion property of α-Fe2O3 in the α-Fe2O3/g-C3N4 NCs reduce the contact surface area between g-C3N4 and α-Fe2O3. As a result, the photocatalytic activity is retarded considerably. However, Xu et al have reported relatively higher degradation efficiency by increasing SSA for α-Fe2O3/g-C3N4 NCs prepared through solvothermal calcination technique (a two-step synthesis method) [105]. In another study, α-Fe2O3/g-C3N4 composite (∼0.5 wt%) is prepared by a facile hydrothermal method which displays higher photocatalytic activity for the reduction of Cr(VI) than pure g-C3N4 and α-Fe2O3[130]. The α-Fe2O3 NPs are dispersed on the surface of g-C3N4, and no aggregation is observed by Xiao et al Table 1 summarizes [143–159] several preparation methods of α-Fe2O3/g-C3N4 composites and their uses as photocatalysts.
Table 1. Photocatalytic performancesg of Fe2O3/g-C3N4-based magnetic photo-catalyst.
| Photo-catalyst | Method of preparation | Saturation magnetization (emu g−1) | Reactive species | Specific surface area (m2 g−1) | Pore diameter (nm) and pore volume (cm3 g−1) | Targeted pollutant | Light source | Degradation rate time(min)−1 | Runs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| (8.6%) α-Fe2O3/g-C3N4 | Calcination | — | — | — | — | RhB | 300 W Xenon lamp | 87%/180 min | — | [103] |
| (2%) α-Fe2O3/g-C3N4 | Wet impregnation | — | — | — | — | Direct red 81 | 100 W Tungsten halogen lamp | — | — | [129] |
| (10%) Fe2O3/g-C3N4 | Solvothermal and calcination | 70.1 | ·O2 −, ·OH | — | — | RhB | 300 W Xenon arc lamp | 96.7%/240 min | 5 | [105] |
| α-Fe2O3/g-C3N4 | Hydrothermal | — | — | 77.58 | — | Cr(VI) | 300 W Xenon lamp | 98%/150 min | 4 | [112] |
| Au/Fe2O3/g-C3N4 | Sonication | — | — | 46.5 | 12/ | RhB | Halogen lamp | ∼100%/50 min | 5 | [121] |
| Fe2O3/Ag/porous-g-C3N4 | Chemical adsorption | — | ·O2 −, h+ | 54.05 | 22.65/0.34 | RhB | 500 W Xenon lamp | ∼100%/50 min | 4 | [143] |
| (3%) α-Fe2O3/g-C3N4 | Hydrothermal | — | ·O2 −, h+ | 21.7 | 7.8/0.0107 | MO | 350 W Xenon lamp | 52.7/120 min | — | [107] |
| Fe2O3/g-C3N4 + H2 O2 | Solvothermal | — | — | — | — | MO | 1000 W Tungsten halogen lamp | 90%/90 min | 4 | [144] |
| (0.38%) α-Fe2O3/g-C3N4 + H2 O2 | Calcination | — | ·OH, h+ | — | — | RhB | 500 W Tungsten halogen lamp | ∼100%/40 min | 5 | [122] |
| Fe2O3/g-C3N4 | Two step hydrothermal | 9.9 | — | — | — | RhB | 300 W Xenon lamp | 97.9%/210 min | — | [127] |
| 0.5 wt%Fe2O3/g-C3N4 + H2 O2 | Calcination-Wet impregnation | — | ·OH | 28.4 | 2–3/ | 4-NP | 300 W Xenon lamp | 90%/40 min | 3 | [145] |
| (0.2wt%) α-Fe2O3/g-C3N4 | Hydrothermal and in situ growth | — | ·O2 −, h+ | 69 | — | RhB TC | 300 W Xenon lamp | 99%/80 min 76%/80 min | — | [106] |
| Fe3O4/α-Fe2O3/g-C3N4+H2 O2 | Hydrothermal | 17.98 | ·O2 −, ·OH, h+ | 88.37 | —/0.55 | Orange II | 500 W Xenon lamp | 96%/60 min | 5 | [108] |
| Fe2O3 Nanorod /ultra-thin g-C3N4(0.75g) | Solvothermal | — | ·O2 − | — | — | 4-nitrophenol | 300 W Xenon arc lamp | 100%/360 min | 6 | [114] |
| (0.5%) 0D/2D Fe2O3 QDs/2D-C3N4 | Ultrasonic dispersion-low temperature calcination | — | ·O2 − | — | — | MB MO RhB | 300 W Xenon lamp | 75%/180 min | 4 | [117] |
| 2D(5wt%)α-Fe2O3/g-C3N4 | Ultrasonicate-assisted self assembly | — | — | — | — | RhB | 500 W halogen lamp | 90%/120 min | 4 | [123] |
| α-Fe2O3/g-C3N4 + H2 O2 | Co-calcination | — | ·OH | 6.22 | — | TC | 100 W LED lamp | 92%/60 min | 5 | [124] |
| (0.5%) α-Fe2O3/g-C3N4 | Hydrothermal | — | ·OH | 77.58 | — | Cr (VI) | 300 W Xenon lamp | 98%/150 min | 4 | [130] |
| γ-Fe2O3/g-C3N4 | — | — | ·O2 −, ·OH | 124.09 | — | Tetracycline hydrochloride (TC-HCl) | 500 W Xenon lamp | 73.8%/120 min | [138] | |
| Fe2O3-xSx/S-doped g-C3N4 | Thermal condensation | — | ·O2 −, ·OH | 33.5 | 25/0.195 | MB | 300 W Xenon lamp | 82%/150 min | — | [146] |
| Fe2O3/g-C3N4 | Thermal treatment | — | — | 46 | 39/0.26 | Dinitrogen | — | 47%/240 min | 3 | [131] |
| α-Fe2O3/g-C3N4 | Hydrothermal | — | ·O2 − | 98 | 28 | CR MG | — | 87%/100 min 95%/100 min | 5 | [134] |
| Fe2O3/nitrogen deficient g-C3N4−X | One step synthesis | — | — | 59.27 | 4.48/— | RhB | 200 W high pressure sodium lamp | 92%/60 min | 3 | [147] |
| g-C3N4/ZnO@ α-Fe2O3 | Direct pyrolysis @ sol-gel | — | ·O2 − | — | 22/ | Tartrazine (Acid yellow 23) | 350 W Murcury-Xenon lamp | 99.35%/35 min | 3 | [148] |
| Fe2O3/g-C3N4 | One step carbonizating | — | — | — | — | RhB | 300 W Xenon lamp | 80%–90%/80 min | — | [149] |
| Fe2O3/g-C3N4 | Calcination | 0.177 | ·O2 −, h+ | 14.9 | 28.7/0.107 | MO Textile effluents (TE) | Sunlight | 99%/90 min 97%/60 min | 5 | [150] |
| 2D/3D/2D (3%)rGO/(4%)Fe2O3/g-C3N4 | Hydrothermal | — | ·OH | — | — | TC CIP | 500 W halogen lamp | ∼100%//40 min ∼100%//40 min | 5 | [151] |
| α-Fe2O3/g-C3N4@3D Graphene aerogel | Hydrothermal | — | h+, ·O2 − | 119.6 | 2–80 | MB | 300 W Xenon lamp | 76.5%/180 min | 5 | [152] |
| Mesoporous α-Fe2O3/g-C3N4 | — | — | ·O2 − | 147 | — | Hg (II) | 300 W Xenon lamp | 90%/60 min | 5 | [113] |
| α-Fe2O3/g-C3N4 + H2 O2 | Hydrothermal and chemical vapor deposition | — | ·OH | — | — | MB | UV | ∼100%//75 min | — | [115] |
| Fe2O3/g-C3N4 monoliths | Nanocasting and vacuum impregnation | 0.39 | ·O2 − | 175 | <50/0.89 | RhB | 65 W CFL lamp | 94.7%/140 min | — | [126] |
| Fe2O3/g-C3N4@ CoFe2O4 | Hydrothermal and calcination | — | ·OH, ·SO4 − | — | — | TC BPA SMX DFC IBP OFX | 500 W Xenon lamp | 99.7%/80 min 98.1%/80 min 94.8%/80 min 97%/80 min 96.1%/80 min 96.5%/80 min | 5 | [128] |
| (1.0%) Fe2O3/g-C3N4 | Microwave assisted | — | ·OH | 45.4 | — | MB | 50 W LED lamp | 70%/90 min | 4 | [132] |
| Fe2+ self-doped Fe2O3/g-C3N4 + H2 O2 | NaCl assisted templating | — | ·OH | 38.3 | 3.5 | Phenol | 500 W Halide lamp | 93%/— | — | [133] |
| (0.1%) Fe2O3/g-C3N4 | Co-precipitation and calcination | — | e−, h+ | 27 | — | RhB | 300 W Iodine tungsten lamp | 97%–98%/120 min | 6 | [135] |
| (2.95%)α-Fe2O3/Few-layer g-C3N4 | Two-step calcination | — | ·O2 −, ·OH | 168.3 | 10–100/ | RhB | 300 W Xenon lamp | 98%/40 min | 5 | [140] |
| α-Fe2O3/Cu/g-C3N4 | Chemical reduction-calcination | — | ·O2 −, ·OH | 20.3 | 5.4/0.079 | Air ethylbenzene | 50 W LED 4 | 37%/— | 6 | [142] |
| g-C3N4/Fe2O3@N-TiO2 nanotube arrays | Electrochemical anodization and calcination | — | ·OH | — | — | Bisphenol A (BPA) | Sunlight | ∼100%//40 min | — | [153] |
| α-Fe2O3/Ag/g-C3N4 | Hydrothermal, chemical reduction and sonication impregnation | Ethylbenzene | 50 W White LED lamp at air atmosphere | 37.6%/ 15 ml min−1 air flow | 5 | [154] | ||||
| g-C3N4/γ-Fe2O3/TiO2 | Sol-gel | — | h+, ·O2 − | 66.33 | 8.66/0.14 | Cefixime trihydrate (CEF) | 9W Visible Blue light | 98.09%/90 min | 7 | [155] |
| Fe2O3/g-C3N4 | Hydrothermal | — | h+,·OH | 416.3 | /0.534 | Ciprofloxacin(CIP) TOC | 36 W Mercury lamp | 100%/60 min 93.86%/60 min | — | [110] |
| (6%) α-Fe2O3/g-C3N4 | Ball-Milling | — | h+, ·SO4 − | 19.5 | 10.2/10.1 | RhB | 300 W Xenon lamp | 92%/120 min | [111] | |
| 3D/0D porous g-C3N4 /Fe2O3 QDs + H2 O2 | Self-assembly | — | ·OH | 143.27 | 2–5/0.875 | Phenol | 500 W Metal halide lamp | 96.8%/240 min | 5 | [118] |
| Porous-Fe2O3@SiO2/g- C3N4 | Electrospinning | — | ·O2 −, ·OH | 54.9 | — | 2-chlorophenol (2-CP) | 150 W Xenon lamp | 3.77 times higher than Fe2O3 | 4 | [119] |
| α-Fe2O3/g-C3N4hollow microsphere | Hydrothermal-Calcination | — | ·O2 −, ·OH | — | — | TC | 300 W Xenon lamp | 67%/120 min | 3 | [120] |
| spindle-like Fe2O3/g-C3N4 | Hydrothermal | — | ·O2 − | 29.04 | — | Fuchsin | 300 W Xenon lamp | 92%/210 min | 5 | [125] |
| 2D/2D (7%) α-Fe2O3/g-C3N4 | Impregnation- Hydrothermal | — | e−, h+, ·O2 − | 67.05 | — | NO | 300 W Xenon lamp | 60.8%/60 min | 5 | [137] |
| Fe2O3/EuVO4/g-C3N4 | Sonochemical | 18.99 | — | 23.357 | 26.07/0.152 | RhB | 400 W Mercury lamp | 80.06%/120 min | 4 | [156] |
| Fe2O3/PrFeO3/g-C3N4 | Wet chemical | — | ·O2 −, ·OH, h+ | — | — | Acid violet 7 | 513 W CFL | 99%/20 min | 4 | [157] |
| Fe3N/Fe2O3/g-C3N4 | Thermal pyrolysis | — | ·OH | 34.6 | — | RhB | 300 W Xenon lamp | >98%/30 min | 5 | [158] |
| K4Nb6O17/Fe3N/Fe2O3/g-C3N4 | Thermal pyrolysis | 12 | ·OH | — | — | Acetamiprid | 300 W Xenon lamp | 76%/180 min | 5 | [159] |
2.3. Fe3O4/g-C3N4-based photocatalyst
Recently, the magnetite (Fe3O4) NPs have shown considerable interest on account of their superparamagnetic and high saturation magnetization [160]. The multifunctional properties observe interdisciplinary applications, such as waste-water treatment [161], drug delivery, ultrahigh density magnetic recording [162], magnetic fluid [162], sensors [160], solid-phase extraction [163], lithium storage capacity, bio-nanotechnology and protein separation [164], etc. Alongside, Fe3O4 NPs are considered as one of the widely utilized iron oxide compounds for several advantages such as low cost [20], large SSA, low toxicity [164], etc. Moreover, the easy separation techniques, efficient recovery, and reasonable recyclability [16, 132] make them an ideal candidate for photocatalytic application. However, without any surface modification, the Fe3O4 NPs have a greater tendency to deform and aggregate during catalyst recovery, which is a vital issue from the application perspective [14, 161, 164]. Therefore, the Fe3O4 NPs are incorporated with photoactive materials (g-C3N4 nanosheets) to retrieve desirable photocatalytic performance, as demonstrated in numerous studies.
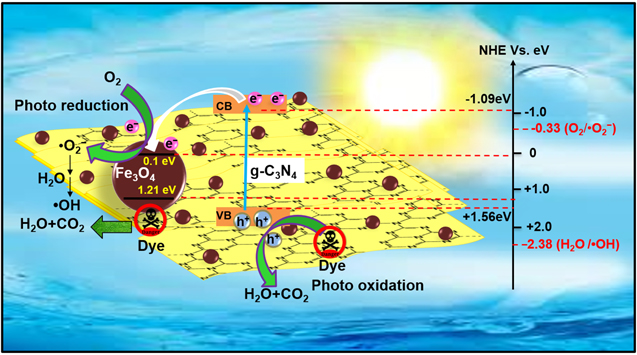
The advancement in the photocatalytic performance is attributed to the energy matching band structure observed in the g-C3N4/Fe3O4 NCs, as shown in figure 11. Upon visible light illumination, the photo-excited electrons move from the CB of g-C3N4 to the CB of Fe3O4 since the CB level of Fe3O4 is slightly lower than that of g-C3N4. Furthermore, the negligible bandgap energy (∼0.1 eV) and high conductivity (1.9 × 106 Sm−1) [163, 164] of the Fe3O4 NPs decline the chance of direct recombination of photo-generated excitons and facilitates the fast carrier transportation in the Fe3O4/g-C3N4 NCs. Thus, the available electrons in the CB of Fe3O4 are great reductants that competently reduce the absorbed O2 on the catalyst surface into various ROS to produce water, CO2, etc, through the generation of highly ROS ·OH (as shown in figure 11). The production of radicals is expressed by reactions as follows: [162]
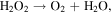
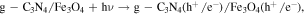
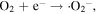
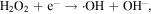
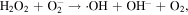
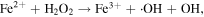
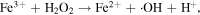
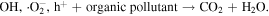
Figure 11. Schematic illustration of the mechanism for photo-induced charge carrier transfers in the g-C3N4/Fe3O4 photocatalyst under visible-light irradiation. Reproduced with permission from [161] Copyright 2013, American Chemical Society.
Download figure:
Standard image High-resolution imageIn a report, Kumar et al [161] have successfully fabricated g-C3N4/Fe3O4 NCs via a facile and efficient in situ growth mechanism. In contrast, Jia et al [164] have fabricated the same nanocomposites via a simple hydrothermal route. In both cases, g-C3N4 nanosheets are decorated with Fe3O4 nanoparticles with a diameter size in the range of ∼6–8 nm to prevent the possibility of nanoparticle agglomeration. Interestingly, compared to in situ growth, a large SSA of about 200.83 m2 g−1 and 115.1 m2 g−1 of g-C3N4/Fe3O4 are observed by Jia et al and Yang et al employing the hydrothermal route and calcination-coprecipitation method, respectively [163]. The materials preparation method significantly contributes to improving the photocatalytic effectiveness for degrading RhB under visible-light irradiation [163, 164]. Liu et al have reported photocatalytic performance of hydrothermally prepared Fe3O4/g-C3N4 NCs under photo Fenton eradication of RhB using H2O2 oxidizing agent [162]. Further, the NCs are recovered successfully and reused even after 5–6 successive cycles without any loss of photocatalytic activity. As per the literature, different preparation techniques are followed to synthesize NCs, and various parameters are tabulated below in table 2.
Table 2. Photocatalytic performances of Fe3O4/g-C3N4-based magnetic photocatalyst.
| Photocatalyst | Method of preparation | Saturation magnetization (emu g−1) | Reactive species | Specific surface area (m2 g−1) | Pore diameter (nm) and pore volume (cm3 g−1) | Targeted pollutant | Light source | Degradation rate time (min)−1 | Runs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4/g-C3N4 | In situ growth | — | ·O2 −, ·OH | 72.73 | 8/0.25 | RhB | 300 W Xenon lamp | — | 6 | [89] |
| Fe3O4/g-C3N4 | Hydrothermal | 47.6 | — | 200.82 | 7 nm/— | RhB | 500 W Xenon lamp | 95.5%/60 min | 5 | [92] |
| Fe3O4/g-C3N4 | Calcination and Co-precipitation | 80.49 | — | 115.1 | 10–13 nm/— | 2,4,6-trichlorophenol | 500 W Xenon lamp | 95.7%/100 min | 5 | [91] |
| Flower-like-flake Fe3O4/g-C3N4 | In situ growth | — | ·O2 −, ·OH, h+ | — | — | RhB | 300 W Xenon lamp | 97.83%/140 min | 5 | [88] |
| Fe3O4@ g-C3N4 + H2 O2 | Hydrothermal | 5 | ·O2 −, ·OH, h+ | — | — | RhB MB | Visible light | 100%/120 min 100%/180 min | 4 | [90] |
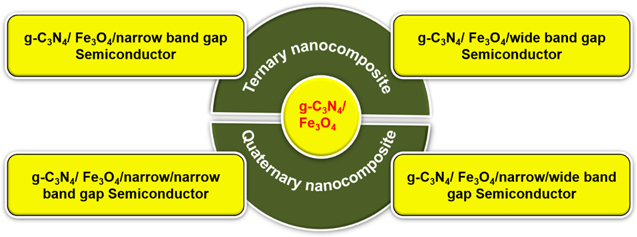
The coupling of Fe3O4 with g-C3N4 can resolve several problems like the separation and recycling of magnetic catalysts. However, the commercialization aspect of g-C3N4 is restricted because of the increasing recombination rate of the photo-generated EHP and lower visible-light absorption ability. Accordingly, many semiconductors with suitable band energies are combined to enhance the visible-light absorption capacity as well as to prolong the lifetime of photo-induced EHPs. Consequently, the g-C3N4-based ternary and quaternary NCs have attracted significant attention from researchers to get the collective advantages of the combined systems. These NCs are further sub-divided into four categories based on the band energies, as shown in figure 12.
Figure 12. Classification of g-C3N4/Fe3O4 based photocatalyst.
Download figure:
Standard image High-resolution image2.3.1. Fe3O4/g-C3N4-based ternary NCs
Various narrow and wide bandgap semiconductors such as Ag3VO4, AgBr, AgI, Ag3PO4, Ag2CrO4, BiOI, NiWO4, MnWO4, CoWO4, CuWO4, CoMoO4, MoO3, CdS, Bi2S3, TiO2, ZnO, etc, are combined with g-C3N4 to form heterojunction structure and boost the photoactivity. Combining a narrow bandgap semiconductor with pure g-C3N4 enhances visible-light absorption. At the same time, a combination of wide bandgap semiconductors with an appropriate band edge decreases the rate of photo-generated EHP recombination. Hence, the trade-off can be addressed by selecting semiconducting materials with suitable energy band edges. The photocatalytic activity is well explained by producing and characterizing several magnetically separable ternary NCs, as displayed in figure 13.
Figure 13. Various g-C3N4/Fe3O4-based ternary NCs for the application in dye degradation. Reproduced with permission from [14] Copyright 2018, Springer Nature.
Download figure:
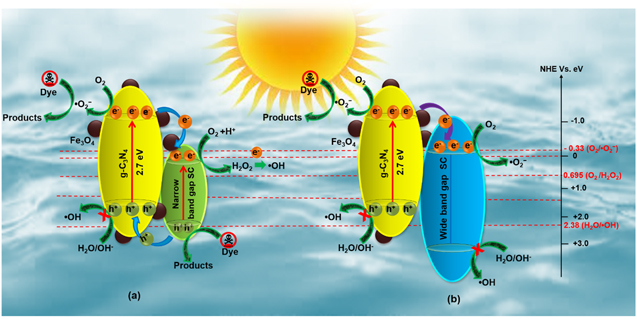
Standard image High-resolution imageThe coupling of noble metal NPs with semiconductor surfaces exhibits encouraging results caused by the surface plasmon resonance (SPR) effect [165]. The plasmonic effects of the noble metal NPs boost both the visible light absorption ability and broaden the spectral response range. Consequently, the Ag NPs, which are in close proximity with g-C3N4, favor the separation of charge carriers as the Fermi level of Ag is lower than the CB of g-C3N4 [166]. For example, Zhu et al have prepared a well-dispersed Ag/Fe3O4/g-C3N4 NCs photocatalyst to boost the photocatalytic activity by retaining the magnetic property. This improvement is related to the trapping of electrons by Fe3O4 nanoclusters and transfers to Ag, leading to superior separation efficiency of EHPs and optimal light harvesting [165]. Further, a series of Fe3O4/g-C3N4/Ag3VO4 and Fe3O4/g-C3N4/Ag2CrO4 NCs are prepared following a facile refluxing method [22, 167]. The residual magnetizations of the composites are high enough to make them separate with the help of a magnet from the treated solution in successive runs. The NCs of Fe3O4/g-C3N4/Ag3VO4 (60%) and Fe3O4/g-C3N4/Ag2CrO4 (20%) exhibit broad absorption covering the whole visible region as compared to the absorption edge (470 nm) of pure g-C3N4. Superior photocatalytic activities for the degradation of RhB are found, which is many folds higher than pure g-C3N4 and g-C3N4/Fe3O4. The enhancement of photocatalytic activity is related to the effective separation of charge carriers through a transfer of photo-generated electrons and holes, schematically shown in figure 14(a) [90, 162–172]. The energy bandgap of g-C3N4, Ag3VO4, and Ag2CrO4 are 2.7, 2.2, and 1.8 eV, respectively, which boosts the visible-light absorption and generates EHP. The CB and VB of g-C3N4 are more negative than Ag3VO4/Ag2CrO4. As a result, the photo-generated electrons quickly transfer from g-C3N4 to Ag3VO4/Ag2CrO4, and holes migrate in the reverse direction. Therefore, the effective separation of photo-generated charge carriers occurs, leading to a decrease in the recombination rate. Among various narrow bandgap semiconductor NCs mentioned in the below table, it is observed that g-C3N4/Fe3O4/Bi2S3 shows relatively higher photocatalytic performance than others [16]. This enhancement in performance can be understood by the effective charge transfer mechanism within the system. The main ROS (·O2 − and ·OH) suggests that the composite has substantial reduction and oxidation ability which leads to complete degradation of organic pollutants within 40 min of visible light irradiation [16]. On the other hand, wideband gap semiconductor NCs, such as g-C3N4/Fe3O4/AgCl (40%), prepared by a facile method, show 7.7 times higher photocatalytic activity than pure g-C3N4. Moreover, the NCs hold a maximum magnetization of 9.5 emu per gram, which is indeed an excellent feature to exploit potentially advantageous magnetic separation techniques. The energy band-gap of AgCl (3.25 eV) is higher than Ag3VO4/Ag2CrO4. Upon visible-light illumination, the EHPs are generated merely on the g-C3N4 counterpart of the nanocomposite. The VB and CB energies of AgCl are more positive than those of g-C3N4. Hence, the photo-induced electrons effortlessly migrate from the CB of g-C3N4 to that of CB of AgCl [168]. Therefore, the recombination rate decreases due to the effective separation of electrons and holes from one to another (figure 14(b)).
Figure 14. Schematic illustration of proposed degradation mechanism of organic dye over the (a) g-C3N4/Fe3O4/narrow band gap semiconductor and (b) Fe3O4/g-C3N4/Wide band gap semiconductor NCs [90, 167–172]. Reproduced from [167, 168], Copyright © 2015 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution imageIn other studies, a series of ternary composites (g-C3N4/Fe3O4/MWO4, where M = Cu, Co, Mn, Ni) are synthesized using a facile refluxing-calcination method [90, 169–171]. The ternary photocatalyst (M = Cu, Co, Mn, Ni) shows improved photocatalytic performance about 10.5, 12.5, 7, and 12 times higher for RhB; 17, 27.5, 10, and 30 times higher for MB; 12.5, 45.7, 25, and 52 times higher for MO; and 42.5, 102, 31 and 100 times higher for Fuchsine relative to pristine g-C3N4. These ternary photocatalysts also possess a large BET SSA and excellent absorption ability of visible light. It is revealed that the ·O2 − is the leading active species responsible for enhancing photodegradation reaction for ternary composites. Whereas, both ·O2 − and ·OH are the main ROS in the case of g-C3N4/Fe3O4/ZnO NCs as reported by Raha et al [172] and Yang et al [173]. Compared to different wideband gap semiconductor NCs (table 3), g-C3N4/Fe3O4/ZnO nanocomposite shows relatively higher degradation efficiency. This enhancement is attributed to the efficient separation of charge carriers by the composite counterparts, which consequently suppress the rate of EHPs recombination. Moreover, incorporating narrow and wide bandgap semiconductors with g-C3N4/Fe3O4 reduces overall bandgap energy and increases the visible light absorbance capacity. Therefore, for ternary NCs, the main ROS ·O2 − is responsible for the efficient separation of photogenerated charge carriers and the strong reduction ability of the CB electrons. However, the photocatalytic performances of ternary NCs are moderate due to the absence of sufficient active sites (i.e. low SSA). Additionally, the Ms value of the composites depends on the content of Fe3O4 and reduces significantly with increasing the counterparts. Hence, the content of Fe3O4 has to be optimized while preparing NCs in order to achieve better photocatalytic performances.
Table 3. Photocatalytic performances of Fe3O4/g-C3N4-based ternary and quaternary magnetic photocatalyst.
| Photocatalyst | Method of preparation | Saturation magnetization (emu g−1) | Reactive species | Specific surface area (m2 g−1) | Pore diameter (nm) and pore volume (cm3 g−1) | Targeted pollutant | Light source | Degradation rate/ time(min) | Runs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4/g-C3N4/AgBr | Chemical Co-precipitation | 19.5 | ·O2 − | — | — | RhB | 50 W LED | 98.3%/420 min | 5 | [20] |
| Fe3O4/g-C3N4/Ag3VO4 (60%) | Refluxing | 5.1 | ·O2 − | — | — | RhB | 50 W LED | 98%/240 min | 5 | [167] |
| Fe3O4/g-C3N4/AgCl (40%) | In situ growth | 9.5 | ·O2 − | — | — | RhB | 50 W LED | 98.3%/360 min | 5 | [168] |
| Fe3O4/g-C3N4/Ag2CrO4 (20%) | Refluxing | 12.9 | ·O2 − | — | — | RhB | 50 W LED | 95%/300 min | 5 | [22] |
| Ag(3wt%) /Fe3O4/g-C3N4 | Photo-deposition | 12.68 | ·O2 −, h+ | — | — | Tetracycline (TC) | 300 W Xenon lamp | 88%/90 min | — | [165] |
| Fe3O4/g-C3N4/AgI (20%) | Refluxing | 16.9 | ·O2 − | — | — | RhB | 50 W LED | 98%/270 min | 5 | [174] |
| Fe3O4/g-C3N4/BiOI (20%) | Refluxing | 8.7 | ·O2 −, h+ | 27.1 | — | RhB | 50 W LED | ∼100%/180 min | 5 | [175] |
| Fe3O4/g-C3N4/WO3 | Hydrothermal | 20.41 | ·O2 −, ·OH | 87.6 | — | TC | 300 W Xenon lamp | 89%/120 min | — | [176] |
| Fe3O4/g-C3N4/Bi2S3 | Refluxing | 8.12 | ·O2 −, ·OH | 23.1 | 19.59/0.1539 | RhB | 50 W LED | 100%/40 min | 4 | [16] |
| Fe3O4/g-C3N4/CuWO4 (30%) | Refluxing-Calcinations | 21.2 | ·O2 − | 38.5 | 15.63/0.152 | RhB | 50 W LED | 100%/270 min | 4 | [169] |
| Fe3O4/g-C3N4/CoWO4 (10%) | Refluxing-Calcinations | 7.04 | ·O2 − | — | — | RhB | 50 W LED | 100%/150 min | 4 | [170] |
| Fe3O4/g-C3N4/MnWO4 (10%) | Refluxing-Calcinations | ·O2 − | 45.04 | — | RhB | 50 W LED | 100%/360 min | [171] | ||
| Fe3O4/g-C3N4/NiWO4 (10%) | Refluxing-Calcinations | 6 | ·O2 − | 38.50 | 15.44/0.149 | RhB | 50 W LED | 100%/240 min | 4 | [90] |
| Fe3O4/g-C3N4/TiO2 | Sol-gel | 12.1 | — | — | — | Dinitro butyl phenol (DNBP) and MB | 500 W Xenon lamp | — | 6 | [177] |
| Fe3O4(10%)/g-C3N4/CdS | Heat treatment | 20.15 | ·OH | 84.9 | TC, Ciprofloxacin, Gatifloxacin, Tetracycline hydrochloride, Enrofloxacin hydrochloride | 300 W Xenon lamp | 100%/15 min 92% 91% 90% 89% | 5 | [178] | |
| petal-like Fe3O4-ZnO@g-C3N4 | Hydrothermal–in situ-calcination | 2.7 | ·O2 −, h+ | 18.4 | — | SMX | 10 W UVC lamp | 95%/90 min | 5 | [179] |
| Fe3O4-C3N4-Ag2MoO4 | Refluxing-Calcinations | 1.44 | ·OH | — | — | 4-Chlorophenol (4-CP) | Visible light | 80%/240 min | 4 | [180] |
| Fe3O4/g-C3N4/rGO | Hydrothermal | 4.7 | ·O2 −, h+ | 33.215 | — | TC | Visible light | 90%/120 min | 5 | [181] |
| Zn doped Fe3O4/g-C3N4 | Polythermal | — | — | — | — | Cephalexin | 350 W Xenon lamp | 91%/300 min | 6 | [182] |
| Fe3O4/g-C3N4/TiO2 | Solid combustion, hydrothermal and wetness impregnation | 0.1 | — | 66.2 | — | MB | 30 W (UV-C) Mercury lamp | 96%/210 min | — | [183] |
| Fe3O4/g-C3N4(50%)/ZnO | — | ∼5 | ·OH, ·O2 − | — | — | MO AYR OG | 500 W Xenon lamp | 97.8%/150 min 98.05%/150 min 83.35%/150 min | 5 | [184] |
| Fe3O4/Pd/mpg-C3N4 | Three step solution | — | ·O2 −, ·OH | — | — | E. coli S. Aureus | Solar irradiation | 99.9%/120 min 99.8%/120 min | 4 | [185] |
| Fe3O4/g-C3N4/CoMoO4 (30%) | Refluxing-Calcinations | 20.1 | ·O2 − | 41.69 | — | — | 50 W LED | — | 4 | [186] |
| Zn doped Fe3O4/g-C3N4(20%) | Polyol thermal | — | ·OH | — | — | Cephalexin (CEX) | 350 W Xenon lamp | 91%/300 min | 6 | [182] |
| Fe3O4/GO/g-C3N4 + H2 O2 | Sonicated-hydrothermal | — | ·O2 −, h+ | 23.1 | 37.1/0.084 | Phenol | — | ∼100%/120 min | 3 | [187] |
| Ag/Fe3O4/g-C3N4 | Hydrothermal | — | ·OH | — | — | Diazinon (DZN) | UV light Vissible light | 100%/60 min 58.4%/60 min | — | [188] |
| Fe3O4/g-C3N4/TiO2 | Hydrothermal | — | ·O2 − | 65.74 | 19.8/0.377 | RhB MO | 500 W Xenon lamp | 96.4%/80 min 90%/120 min | 4 | [189] |
| Fe3O4-QDs/Bi2O4/g-C3N4 | Calcination and hydrothermal | 0.77 | ·OH | 78.1 | 15.7/— | RhB | 250 W Xenon lamp | 78.4/160 min | 4 | [190] |
| Fe3O4/ZnO@ g-C3N4 | Facile chemical route | 16.16 | ·O2 −, ·OH | 23.117 | — | Pantoprazole | 23 W white LED | 97.09%/90 min | 4 | [172] |
| g-C3N4/ZnO@Fe3O4 | — | ·O2 −, ·OH | 26.53 | 16.32/ | RhB | Sunlight | 100%/180 min | [173] | ||
| Fe3O4@Bi2O3/g-C3N4 | Solvothermal-ultrasonicated-stirring | 4.53 | ·O2 −, ·OH, h+ | — | 393/— | Indigo carmine (IC) | 400 W Hg lamp | 94.9%/120 min | 4 | [191] |
| Fe3O4/g-C3N4/ZnO | Disperse-magnetic stirring | ∼8 | ·O2 −, ·OH | — | — | RhB | 250 W Xenon lamp | 100%/150 min | 3 | [192] |
| Fe3O4/g-C3N4/CdS | Chemical liquid phase | >54.98 | — | 54.7 | >20/— | Ciprofloxacin (CPFX) RhB | 250 W Xenon lamp | 81%/330 min 77%/330 min | 3 | [193] |
| Fe3O4/porous Titanosilicate/g-C3N4 | In-situ | 9 | ·O2 −, ·OH, h+ | 480 | 3–3.5 | RhB | 400 W Hg lamp and 65 W Phillips (42 W m−2) and sunlight | ∼100%/90 min ∼100%/50 min ∼100%/30 min | 5 | [194] |
| Fe3O4/g-C3N4/MoO3 (30%) | Calcination | 26.3 | ·O2 − | 72.7 | 13.85/0.25 | Tetracycline (TC) | 1000 W Xenon lamp | 94%/120 min | — | [195] |
| Fe3O4/C/g-C3N4 | Sonication and in-situ precipitation | — | ·O2 −, h+ | 125.1 | 2.5/0.078 | TC | 500 W Xenon lamp | ∼100%/120 min | 5 | [196] |
| Fe3O4/g-C3N4/diatomite + H2O2 | Electrostatic self-assembly | 4 | ·O2 −, ·OH | — | 203/— | RhB | 500 W Xenon lamp | 98%/40 min | 5 | [197] |
| Fe3O4/Graphene/S- g-C3N4 | Thermal polymerization, Impregnation & co precipitation | 5–10 | ·O2 −, ·OH, h+ | 57.11 | — | Ranitidine by product of N-nitrosodimethylamine | 300 W Xenon lamp | 100%/60 min | 4 | [198] |
| Fe3O4/g-C3N4/AgI/Bi2S3 (30%) | Refluxing | 7.13 | ·O2 − | — | — | RhB | 50 W LED | 99%/60 min | 4 | [24] |
| Fe3O4/g-C3N4/Ag3VO4/Ag2CrO4 (20%) +H2 O2 | Refluxing | 6.12 | ·O2 − | — | — | RhB | 50 W LED | ∼100%/180 min | — | [199] |
| Fe3O4/g-C3N4/Ag3PO4/AgCl (30%) | Ultrasonic-irradiation | 8.78 | h+ | — | — | RhB | 50 W LED | 100%/150 min | 4 | [200] |
| Fe3O4/g-C3N4/AgI/Ag2CrO4 (20%) | Chemical Co-precipitation | 8.5 | ·O2 − | — | — | RhB | 50 W LED | 99.4%/150 min | 4 | [201] |
| Fe3O4/g-C3N4/Ag3PO/Co3O4 (20%) | Impregnation | 13.5 | ·O2 − | — | — | RhB | 50 W LED | ∼100%/150 min | [202] | |
| Fe3O4/ g-C3N4/Ag2WO4/AgBr (30%) | Refluxing | 9.61 | ·O2 − | —— | —— | RhB | 50 W LED | 100%/150 min | 4 | [203] |
| Fe3O4@SiO2@Bi2WO6@g-C3N4 | — | — | ·OH | 36.56 | /0.19 | RhB | 350 W high stress Xenon lamp | ∼85%/70 min | 5 | [207] |
| Fe3O4/BiOCl/g-C3N4/Cu2O | Co-precipitation | 26 | ·O2 −, ·OH | 65 | 2–11/0.032 | Sulfamethoxazole (SME) | 800 W Xenon lamp and Sun light | 99.5%/60 min and 92.1%/120 min | 5 | [204] |
| Fe3O4@SiO2@MoS2/g-C3N4 | — | 5.31 | ·OH | 46.71 | 21.67/0.196 | RhB | 350 W Xenon lamp | 97%–98%/60 min | 6 | [205] |
| Ultrathin g-C3N4/TiO2//Fe3O4@SiO2 | Sol-gel | 8 | ·OH | — | — | Ibuprofen real sewage | 330 W Xenon lamp | 97%/15 min | 3 | [206] |
| rGo/Fe3O4/g-C3N4/TiO2 +H2 O2 | Co-precipitation and hydrolysis | — | — | — | — | MB | UV light | 97%/20 min | 10 | [207] |
| Fe3O4@SiO2@g-C3N4/TiO2 core–shell | Hydrothermal | — | ·O2 −, ·OH | — | — | RhB MO | Sunlight | ∼100%/180 min ∼100%/240 min | 5 | [208] |
| g-C3N4/NiO/ZnO/Fe3O4 | Hydrothermal | 36.76 | ·O2 −, ·OH | 38.78 | 3.101/ | Esomeprazole | 23 W white LED | 95.05%/70 min | 5 | [209] |
| Fe3O4/g-C3N4/C/MnO2 | In-situ and low temperature assembling | — | — | 120.25 | 6.35/0.31 | MO | 400 W metal halide lamp | 94.11%/140 min | 4 | [210] |
| Fe3O4@ns-TiO2/Ag/g-C3N4 yolk-shell | Hydrothermal and chemical reduction | 15.14 | ·O2 −, ·OH | 130 | 6/ | Mo | 500 W Xenon lamp | ∼70%–80%/360 min | 4 | [211] |
2.3.2. g-C3N4/Fe3O4-based quaternary NCs
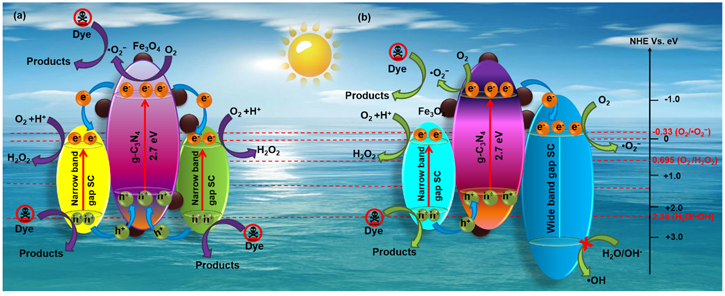
As discussed, the ternary NCs are composed of narrow bandgap semiconductors that exhibit broad absorption in the whole visible region. In contrast, the NCs composed of wide bandgap semiconductors effectively reduce the direct recombination of photo-generated EHP. Meanwhile, to achieve both the goals, several quaternary NCs (as shown in figure 15) are synthesized and extensively explored in the degradation of dyes. For example, Choi et al [199] have prepared quaternary NCs of g-C3N4/Fe3O4/Ag3VO4/Ag2CrO4 using two narrow bandgap semiconductors. In contrast to ternary composites, the quaternary NCs promise substantial improvement in the photodegradation process due to the generation of EHPs in their individual counterparts upon visible-light illumination. Accordingly, the complementing interfaces amongst g-C3N4, Ag3VO4, and Ag2CrO4 in the NCs significantly improve the separation of photo-induced EHPs. Further, the combination of narrow and wide bandgap semiconductors, for example, g-C3N4/Fe3O4/AgBr/Ag2WO4 [203], the electrons and holes are generated only in g-C3N4 and AgBr, not in Ag2WO4 due to the large bandgap. The CB and VB energies of g-C3N4 are more negative than AgBr and Ag2WO4, which assists the electrons in moving from CB of g-C3N4 to CB of AgBr/Ag2WO4 effortlessly. Therefore, the effective separation of photo-generated EHPs leads to enhanced photocatalytic activity. The literature demonstrates that the g-C3N4/Fe3O4/AgBr/Ag2WO4 (30%) NCs exhibits 94.7, 17.4, and 26.7-fold enhanced activity relative to g-C3N4, g-C3N4/Fe3O/Ag2WO4 (30%), and g-C3N4/Fe3O/AgBr (30%), respectively for the degradation of MO [203]. The possible mechanism of EHPs separation and improvement in photoactivity of the quaternary NCs is presented in figure 16. In another study, Kumar et al have prepared Fe3O4/BiOCl/g-C3N4/Cu2O heterojunction via the facile co-precipitation method [204]. The composites show superior photocatalytic activity for Sulfamethoxazole (SME) degradation with 99.5% (60 min) under visible light. In general, the CB edge of g-C3N4 (−1.09 eV) lies above Cu2O (−0.295 eV) and BiOCl (0.62 eV). It leads to effortless movement of electrons from g-C3N4 to Cu2O and BiOCl, and the holes flow in the reverse direction. During the formation of p–n–p (BiOCl-g-C3N4-Cu2O) heterojunction, the energy bands of p-type and n-type semiconductors move upward and downward direction, respectively. The electrons transport is favorable from BiOCl and Cu2O to g-C3N4, which strengthens an inbuilt electric field. Upon light illumination, this inbuilt electric field propels the charge carriers to flow and minimizes carrier recombination. The photocatalytic performance of various ternary and quaternary NCs is summarized in table 3 [174–198, 200, 201, 202, 205–213]. So, for quaternary NCs, the leading active species are ·O2 − and ·OH, which promotes simultaneous photo-reduction and photo-oxidation at the reduction and oxidation sites, respectively. Therefore, the quaternary NCs show relatively higher photocatalytic performance compared to ternary NCs. This superior performance is also attributed to the efficient separation of EHPs which consequently suppresses the recombination rate.
Figure 15. Various g-C3N4/Fe3O4-based quaternary NCs for the application in dye degradation. Reproduced with permission from [14] Copyright 2018, Springer Nature.
Download figure:
Standard image High-resolution imageFigure 16. Schematic illustration of proposed degradation mechanism of organic dye over the (a) g-C3N4/Fe3O4/narrow band gap/narrow band gap semiconductor and (b) g-C3N4/Fe3O4/narrow band gap/wide band gap semiconductor quaternary NCs [199, 203, 204]. Reproduced with permission from [199] Copyright 2016; [203] Copyright 2018, Elsevier.
Download figure:
Standard image High-resolution image2.4. Ferrites/ g-C3N4-based photocatalyst
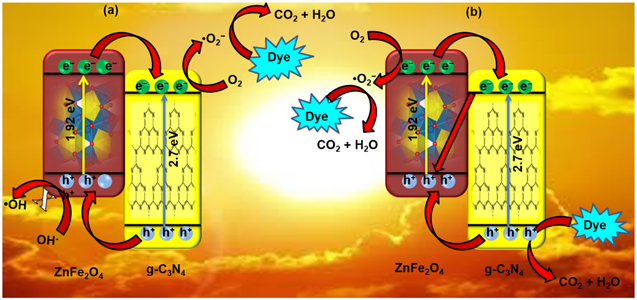
The most popular magnetic photocatalysts including transition metal oxides are termed as spinel ferrites. These spinel ferrites are stable both thermally and chemically and formulated by way of MFe2O4, where M is a transition metal with a +2-oxidation number. In the context of anchoring sites of M(II) and Fe(III), three types of spinel arrangements are possible, labeled as normal ferrite, inverse ferrite, and mixed ferrite structures [214]. Recently, various spinel ferrites are thoroughly studied in different domains due to their unique internal magnetic assets, availability of numerous photo-active sites [215], narrow bandgap, and upright response to visible light. Among all ferrites, spinel Zinc ferrite (ZnFe2O4) nanoparticles (with reasonably 1.9 eV narrow bandgap) put forth an ever-increasing consideration for its potential application in solar photocatalysis, photo-chemical H2 production, and CO2 reduction [29, 216]. Besides, these materials are very convenient to synthesize following cost-effective techniques and possess substantial magnetic property and sensitivity. For example, ZnFe2O4 has a face-centered cubic (FCC) crystal structure with divalent Zn2+ ions in the tetrahedral site and Fe3+ ions in the octahedral site [217]. Below Neel temperature (∼10.5 K), ZnFe2O4 shows Anti-ferromagnetic behavior and becomes paramagnetic at room temperature [216]. The magnetic properties of ZnFe2O4 nanoparticles allow effective separation and reusability of the catalyst after the treatment or purification process, providing a favorable and cost-effective route for practical application [92, 218]. However, the shorter lifetime of the photoexcited carriers is the major limitation, responsible for the poor photocatalytic activity of spinel ZnFe2O4 [92, 215, 217]. Therefore, a heterojunction structure comprising ZnFe2O4 and g-C3N4 is materialized to boost the photocatalytic performance [215, 217, 218].
The heterojunction structures create a built-in electric field at the interface, diminishing the recombination rate by driving the photo-generated carriers immediately in the opposite direction to improve the redox process [219]. Moreover, the photoreaction mechanisms are studied through possible charge transfer route analysis as shown in figure 17.The traditional type II double charge transfer route structure is expressed in figure 17(a), where the diffusion of electrons takes place from the CB of ZnFe2O4 to CB of g-C3N4 and the diffusion of holes takes place from the VB of g-C3N4 to the VB of ZnFe2O4. In such case, the photooxidation reaction (·OH generation) is not possible as the oxidation potential of ZnFe2O4 is more negative than the standard redox potential of H2O/·OH. The figure 17(b) describes the Z-Scheme charge transfer route [220–223], where the CB electrons of ZnFe2O4 is moved to combine with VB holes of g-C3N4 on the contact interface. Therefore, the reduction potential of ZnFe2O4 is more negative than that of O2/·O2 − redox potential and the oxidation potential of g-C3N4 is more positive than ZnFe2O4. Hence, the enhanced photocatalytic activity via efficient charge separation is expected via Z-scheme charge transfer mechanism [220]. Zhang et al have prepared ZnFe2O4/g-C3N4 nanocomposite through a one-step solvothermal route [223]. The structural characterization of the NCs reveals uniform dispersion of magnetic ZnFe2O4 lying on the g-C3N4 sheets. The synergistic interfacial effect, high solubility in the aqueous medium, and large specific area of the g-C3N4/ZnFe2O4 composites augment the catalytic degradation of MO. More interestingly, the catalytic performances of the ZnFe2O4/g-C3N4 composite strongly depend on the content of g-C3N4. Moreover, the high saturation magnetization (63 emu g−1) of the g-C3N4/ZnFe2O4 (80%) composite facilitates an efficient and fast separation of catalyst from the treated suspension through an external magnet. In another study, Borthakur et al [216] have fabricated g-C3N4/ZnFe2O4 using a template-free chemical co-precipitation method. The g-C3N4@ZnFe2O4/H2O2 composites exhibit notable photocatalytic activity under the visible light towards discoloration of rhodamine B. The as-prepared (10%) ZnFe2O4@g-C3N4 NCs degrades 93.7% of RhB within 30 min, nine folds higher than that of ZnFe2O4. Interestingly, the ZnFe2O4/g-C3N4 (10%) NCs do not show any significant loss in photocatalytic activity even after five successive runs. Several studies show that the performance of heterojunction-based NCs still lacks many aspects and needs further amendments. Das et al have developed n–n heterojunction-based on polypyrrole sensitized ZnFe2O4/g-C3N4 following an in-situ polymerization method. Polypyrrole (PPY), a conducting organic polymer, possesses a large no. of π- conjugated backbones, facilitating better charge transfer in a photocatalytic process. It exhibits high electrical conductivity, exciting redox properties, fantastic stability, and is environmentally compatible [215]. The polypyrrole sensitized n–n heterojunctions display fantastic photocatalytic activity and are used as a potential candidate in removing the non-biodegradable waterborne contaminants.
Figure 17. Schematic illustration of proposed charge transfer mechanism in ZnFe2O4/g-C3N4 n–n composite (a) Type-II hetero-junction (b) Z-Scheme [220–223]. Reprinted from [220], © 2020 Elsevier Ltd. All rights reserved.
Download figure:
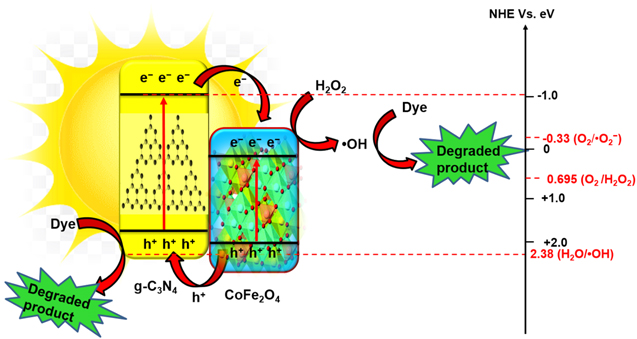
Standard image High-resolution imageInverse spinel ferrite such as CoFe2O4 shows significant recognition in the process of photocatalytic discoloration of pollutants due to their fascinating magnetic properties, low bandgap energy, high coercivity, stable crystalline structure, and low solubility. Subsequently, the high saturation magnetization eases the isolation of NPs from the solution effortlessly [224, 225]. The heterojunction interface of CoFe2O4 NPs anchored g-C3N4 enhances the photocatalytic efficiency due to favorable energy band positions and complementing material properties [226–230], as shown in figure 18. In an investigation, the CoFe2O4/g-C3N4 hybrid has been synthesized by Huang et al [231], employing a calcination method. The synthesized hybrid composites display superior photocatalytic activity in the presence of H2O2 oxidant upon visible-light illumination. The traits of CoFe2O4/g-C3N4 at the heterojunction interfaces are harnessed to boost EHPs generation and separation. In that framework, Yao et al [93] have synthesized CoFe2O4/g-C3N4 NCs via a facile self-assembly method with mass ratio 2:1, which completely degrades RhB within 210 min under artificial light irradiation. The enhanced photocatalytic performance is attributed to the Z-scheme carrier transportation amongst the interfaces of g-C3N4 and CoFe2O4 [226]. The strong magnetic properties of the NCs help isolate the catalysts from the reaction medium using an external magnet. A plausible photocatalytic mechanism is proposed to figure out the generation process of EHPs in the NCs under visible light. The CB/VB energies of g-C3N4 and CoFe2O4 are −1.09/1.56 eV and +0.42/1.75 eV, respectively, with respect to NHE. The photo-induced electrons/holes in the CB/VB of g-C3N4 are transported towards the respective bands of CoFe2O4, facilitating the charge separation and diminishing the recombination rate. In the same framework, honey mediated sol-gel auto combustion synthesis method is reported by Inbaraj et al [232] The as-prepared NCs reveal broad absorption in the visible region and display excellent degradation efficacy towards MB of about 77% within 30 min Besides, the NCs also remove a significant amount of heavy metal such as lead from water in a short time. In another attempt to narrow down the bandgap energy (1.85 eV), Kamal et al [233] have incorporated CoFe2O4 onto sulfur-doped g-C3N4 via the ultrasonication method. The aggregated CoFe2O4 NPs on a sulfur-doped g-C3N4 sheet effectively improve the catalytic activity. In the presence of H2O2, the NCs demonstrate an excellent degradation efficiency of 96% within a reaction time of 20 min [233].
Figure 18. Schematic illustration of proposed degradation mechanism of organic dye over CoFe2O4/g-C3N4 NCs [224, 226–231]. Reproduced with permission from [231] Copyright 2015, Elsevier.
Download figure:
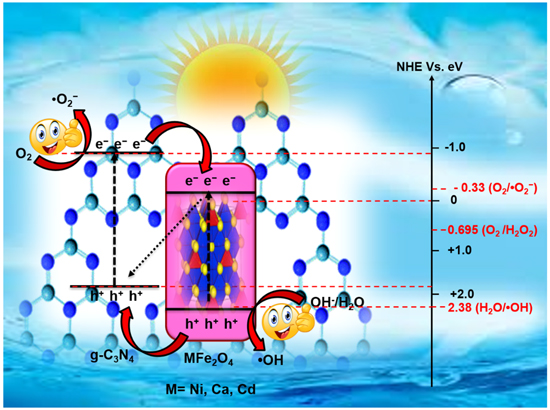
Standard image High-resolution imageVery recently, Cu2V2O7/CoFe2O4/g-C3N4 ternary NCs have been synthesized via a cost-effective hydrothermal approach. The nanocomposite displays better catalytic activity towards the eradication of 4-NP to 4-AP using NaBH4 upon visible-light illumination, and the process completes in just 60 s at room temperature. Moreover, the catalyst shows outstanding stability and reusability up to ten successive runs without any substantial loss in the activity [225]. Another magnetic semiconductor is the inverse spinel Nickel ferrite (NiFe2O4) [234, 235], Calcium ferrite (CaFe2O4), and Cadmium ferrite (CdFe2O4) [101] with an optical bandgap of ∼2 eV and appropriate positioning of CB and VB energies corresponding to redox potential. Few notable features of these ferrites (M = Ni, Ca, Cd, etc) include broad visible-light absorption, high electrical resistivity, and chemical stability, making them perfect choices. For inverse spinel structure, the M2+ and Fe3+ ions reside equally in numbers at the octahedral sites and balance through Fe3+ ions located at the tetrahedral sites, bearing antiparallel spins lead to ferrimagnetism. The Fe2+ ions are responsible for the system's n-type behavior, and the conductivity arises due to the jumping of electrons from Fe2+ to Fe3+. The presence of Fe3+ ions and transferring of holes from the M3+ site to the Fe3+ site is responsible for the p-type behavior of the system [236, 237]. However, the quick recombination of photo-generated EHPs makes MFe2O4 a poor photocatalyst. Therefore, the researchers have followed different compelling methodologies, including combining with inorganic metal oxide semiconductors. The MFe2O4/g-C3N4 composites have well-matched energy levels, which helps to abet the EHP recombination. Gebreslassie et al [238, 239] have fabricated NiFe2O4/g-C3N4 nanocomposite with a modification of direct Z-scheme charge transport mechanism via a facile one‐pot hydrothermal process. The same has been evaluated for the eradication of MO upon visible-light illumination and found to be 4.4 and 3 times higher than individual NiFe2O4 and g-C3N4, respectively. The proposed degradation mechanism [237, 240–243] of MFe2O4 (M = Ni, Ca, Cd)/g-C3N4 NCs is shown in figure 19. Researchers have reported excellent photocatalytic activity of NiFe2O4/Boron [244] and NiFe2O4/Phosphorus [76] doped g-C3N4 prepared via the sol-gel, and Fe3O4@NiFe2O4/Phosphorus doped g-C3N4 via co-precipitation method. The results reveal that all these are attributable to the increased SSA, improved charge transfer rate, and bandgap narrowing via non-metal doping.
Figure 19. Schematic illustration of proposed degradation mechanism over MFe2O4 (M = Ni, Ca, Cd)/g-C3N4 NCs [235, 237–243]. Reproduced with permission from [238] Copyright 2019, John Wiley and Sons.
Download figure:
Standard image High-resolution imageCopper ferrite (CuFe2O4), a p-type magnetic semiconductor, has a moderate bandgap of 1.4 eV and offers good chemical or thermal stability. Considering this, CuFe2O4 is employed in various catalytic applications. The matching electronic band structures of CuFe2O4 and g-C3N4 conveniently enhance the process of photo-generated charge separation, and the magnetic property helps the segregation of photocatalysts from the suspension. For example, Yao et al have reported CuFe2O4/g-C3N4 core–shell photocatalyst, which exhibits an enhanced photocatalytic activity compared to pristine CuFe2O4 or g-C3N4. The combination of g-C3N4 and CuFe2O4 under visible-light illumination displays an excellent response towards the degradation of Orange II dye in the presence of an H2O2 oxidizing agent [19, 245].
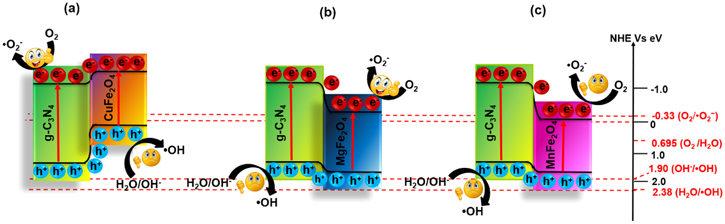
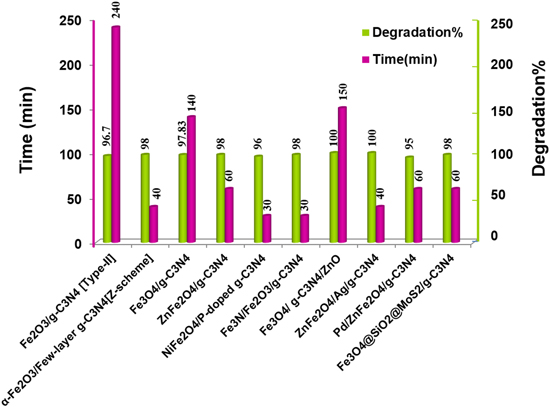
Compared to other ferrites (NiFe2O4, CoFe2O4, and CuFe2O4) [246], the manganese ferrite (MnFe2O4) displays moderately high adsorption, high mechanical hardness, biocompatibility, high magnetic susceptibility, and photochemical stability. But, pure MnFe2O4 is photo catalytically inactive because of the lower VB edge potentials and unsatisfactory performance towards photoelectric conversion [247]. Hence, the magnetic NCs of MnFe2O4/g-C3N4/TiO2 is successfully synthesized using the chemical impregnation approach by Vignesh et al and effectively utilizes it as a solar light-driven photocatalyst for the degradation of MO. In another attempt, the magnetic g-C3N4@MnFe2O4-Graphene photocatalyst is synthesized through a facile combination of solvothermal and impregnation synthesis approach [248]. The composite undergoes a photo-Fenton-like reaction and exhibits superlative catalytic performance to eradicate many antibiotics upon visible-light illumination. The synergistic interfacial effects, high visible-light response, and large SSA appear to be responsible for the fast and effortless separation and transportation of photo-excited EHPs. From this perspective, we can conclude that the degree of magnetization plays a vital role in the isolation of catalysts without any significant loss even after successive runs. Table 4 provides information about various combinations of ferrites, and g-C3N4 NCs, which were designed and executed for the purification of aqueous solutions consisting of numerous organic–inorganic contaminants [96, 249–266]. These magnetic heterojunctions display exciting properties, such as high SSA, enough magnetism, convenient particle size, effortless recovery and outstanding photocatalytic performance in various AOPs. Therefore, from the energy band diagram and above discussion, it can be inferred that most of the g-C3N4/ferrites (MFe2O4) composites (type-II or Z-scheme) such as (M = Zn, Cu, Mn, and Mg) shows stronger reduction ability rather than oxidation. The type II and Z-scheme mechanism of the g-C3N4/ferrites are schematically shown in figures 20 and 21, respectively [96, 267]. On the other hand, ferrites with M = Ni, Co, Ca, and Cd offers strong oxidation ability to the g-C3N4/ferrite composite for simultaneous photoreduction and oxidation reactions on the active sites. Besides, the self-redox properties of M and Fe atoms in MFe2O4 induced H2O2 to be facile for the generation of ·OH. The redox reactions are as shown in figure 22. The features mentioned above inspire the designing of g-C3N4-based ferrite NCs. Based on the literature review, a comparison of photocatalytic performances among the different magnetic NCs is made towards the degradation of RhB dye under 300 W Xenon lamp irradiation (figure 23). Among the binary composites, NiFe2O4/P-doped g-C3N4 and α-Fe2O3/few-layer g-C3N4 (Z-Scheme) show excellent photocatalytic performances. Hence, it is suggested that the combining magnetic materials with exfoliated g-C3N4 or doped g-C3N4 is preferable to constructing new materials with superior performances.
Table 4. Photocatalytic performances of MFe2O4/g-C3N4-based magnetic photocatalyst.
| Photocatalyst | Method of preparation | Saturation magnetization (emu g−1) | Reactive species | Specific surface area (m2 g−1) | Pore diameter (nm) and pore volume (cm3 g−1) | Targeted pollutant | Light source | Degradation rate/time (min) | Runs | References |
|---|---|---|---|---|---|---|---|---|---|---|
| ZnFe2O4@g-C3N4(80%) | Solvothermal | 63.8 | ·OH | 49.8 | — | MO | 500 W Xenon lamp | 98%/180 min | 5 | [223] |
| ZnFe2O4/g-C3N4 + H2 O2 | Refluxing | — | ·O2 −, ·OH, h+ | — | 19.1/— | Orange II | 500 W Xenon lamp | 97%/240 min | 5 | [92] |
| ZnFe2O4/g-C3N4 | Hydrothermal | 52.1 | ·O2 −, ·OH | 61.285 | — | Spiramycin | 300 W Xenon lamp | ∼95%/240 min | 5 | [221] |
| ZnFe2O4/g-C3N4 | Solvothermal | 10.9 | ·O2 −, ·OH | — | — | MB TC | 300 W Xenon lamp | 98%/120 min 95%/210 min | 10 | [218] |
| ZnFe2O4/g-C3N4/Fe2O3 | Hydrothermal | — | — | 47.62 | 16.49/— | RhB | Visible light | 85%/180 min | 5 | [29] |
| ZnFe2O4/g-C3N4@polypyrrole (PPY) | Polymerization | — | ·O2 − · OH | 29.52 | 1.86 and 0.05 | Ciprofloxacin (CIP) | Solar light irradiation | 92%/120 min | 4 | [215] |
| (10%) ZnFe2O4/g-C3N4 + H2 O2 | Template free chemical co-precipitation | — | ·OH | — | — | RhB | 9 W LED lamp | 93.4%/30 min | 5 | [216] |
| ZnFe2O4/g-C3N4 + H2 O2 | Sol-gel followed calcinations | — | ·OH | — | — | MB | LED Sunlight | 90%/60 min 99.5%/60 min | 5 | [217] |
| Pd/ZnFe2O4/g-C3N4 | ultrasonic-assisted self-assemble and photoreduction route | — | ·O2 −, h+, e− | — | 200/- | RhB Cr(VI) | 300 W Xenon lamp | 95%/60 min | 4 | [249] |
| ZnFe2O4/g-C3N4 | Microwave irradiation | — | ·O2 − | 97.5 | 35–45/- | MO RhB | 500 W Xenon lamp | 86%/60 min 98%/60 min | 5 | [250] |
| ZnFe2O4/Ag/g-C3N4 | Thermal pollymerizatio, Photoreduction, Solvothermal, Calcination | — | ·O2 −, h+ | 65.8 | 2–50/- | RhB | 300 W Xenon lamp | ∼100%/40 min | 5 | [251] |
| ZnFe2O4/g-C3N4 | Hydrothermal | — | ·O2 − | 121 | 30–35/- | Nitrobenzene(NB) and Cr(VI) | Visible light | 89%/100 min 97%/100 min | 5 | [252] |
| ZnFe2O4/g-C3N4 | Acid sonication | — | ·OH, h+ | 202 | — | Acridine orange dye | 500 W Xenon lamp | 90%/60 min | 5 | [222] |
| (41.4%) CoFe2O4/g-C3N4 + H2 O2 | Calcinations | 76.6 | ·OH, h+ | — | — | Methylene blue (MB) | 300 W Xenon lamp | 97.3%/180 min | 3 | [231] |
| Co0.5Zn0.5Fe2O4/g-C3N4 | Hydrothermal | — | ·OH | — | — | Chloromycetin | 300 W Xenon lamp | 96%/240 min | — | [23] |
| CoFe2O4/g-C3N4 + H2 O2 | Self-assembly | 1.8 | ·OH | — | — | RhB, MG, MB and Acid Fuchsin Orange II, Methyl Violet, MO and Fuchsin basic | 500 W Xenon lamp | 100%/210 91.1%/210 91.9%/210 85.4%/210 95%/210 | 5 | [93] |
| CoFe2O4/mp g-C3N4 | Thermal decomposition and self- assembly | ·OH | 92.33 | 14.74/0.68 | MB | 400 W ultrasonic bath apparatus | 92.81%/45 min | 5 | [253] | |
| CoFe2O4/mp g-C3N4 | Thermal decomposition and sonication | 6.1 | ·O2 −, ·OH | 163 | 9/0.6 | MG | 16 W UV light | 93.4%/120 min | 5 | [254] |
| CoFe2O4@g-C3N4 + PMS | Calcinations | — | ·O2 −, ·OH, ·SO4 − | — | — | RhB | 500 W Halogen Tungsten lamp | 96%/30 min | — | [224] |
| CoFe2O4/g-C3N4 | Honey mediated sol-gel auto combustion and hydrothermal | 20 | — | — | — | MB | — | 77%/30 min | 3 | [232] |
| CoFe2O4/Cu2V2O7/g-C3N4 + NaBH | Hydrothermal | 19.21 | — | — | — | 4-nitrophenol | 40 W LED light | 100%/60sec | 10 | [225] |
| CoFe2O4/g-C3N4 | Microwave irradiation | — | — | 92.1 | 26.7/- | MO | 500 W Xenon lamp | 93.5%/60 min | 5 | [227] |
| CoFe2O4/g-C3N4 | Hydrothermal | — | ··O2 − | 49.79 | — | MB | 300 W Xenon lamp | 93.96%/120 min | 3 | [228] |
| CoFe2O4/S-doped g-C3N4 + H2 O2 | Ultrasonication | — | — | — | — | MB | — | 96%/20 min | 5 | [233] |
| (4%)CoFe2O4/g-C3N4 | Soft and hard tamplates | 11.07 | — | 151 | 5–10/- | Hg(II) | 300 W Xenon lamp | 100%/60 min | — | [255] |
| rGO/Co Fe2O4/g-C3N4 + H2 O2 | Hydrothermal | — | ·OH | — | — | 4-NP | 40 W LED light | 97%/40 min | 4 | [256] |
| NiFe2O4/g-C3N4(7.5%) +H2 O2 | Chemisorption | 40 | ·OH | — | — | MB | 300 W Xenon lamp | 87%/240 | 5 | [257] |
| (2.0wt%) NiFe2O4/g-C3N4 | Co-precipitation | — | ·O2 −, ·OH, h+ | — | — | MO | 300 W Xenon lamp | — | 5 | [258] |
| NiFe2O4/g-C3N4 | Thermal polycondensation | 20.54 | ·OH | 15.5 | — | Oxytetracycline antibiotic (OTC) | Solar light irradiation | 97%/— | 10 | [234] |
| NiFe2O4/g-C3N4 + H2 O2 | Sol-gel followed calcinations | 4.36 | ·O2 −, ·OH | 46.502 | — | MB RhB | 40 W LED light | 99%/225 min 90%/225 min | 3 | [17] |
| (20wt%) NiFe2O4/P-doped g-C3N4 | Sol-gel and calcination | — | ·OH | — | — | Phenol, RhB | 150 W Xenon arc lamp, solar light | 96%/30 min, 98%/60 min | 3 | [237] |
| NiFe2O4/g-C3N4 | Hydrothermal | 28.8 | ·O2 −, ·OH, h+ | 85.2 | 16.5/0.128 | MO | 10 W LED light | 100%/210 min | 3 | [238] |
| NiFe2O4/graphene/g-C3N4 | Hydrothermal | 39.4 | ·OH, h+ | 77.6 | 16.8/0.29 | MO | 10 W LED light | 100%/120 min | 6 | [239] |
| NiFe2O4/Boron doped g-C3N4 | Sol-gel | — | ·O2 −, ·OH | — | — | MB | 350 W mercury-xenon lamp | 98%/80 min | 3 | [244] |
| Fe3O4@NiFe2O4/P-doped g-C3N4 | Co-precipitation | 28 | ·OH | 147.143 | — | CIP | Solar light and 150 W Xenon arc lamp | 90%/60 min | 4 | [236] |
| NiFe2O4/g-C3N | ionic liquid self-combustion | — | ·OH, h+ | — | — | MB | 300 W Xenon lamp | 100%/75 min | 3 | [259] |
| NiFe2O4/Ag/g-C3N | Self-assembly | 18 | ·O2 −, ·OH | 34 | 40/- | TC | 150 W Xenon lamp | 92.1%/ | — | [235] |
| NiFe2O4/g-C3N | Microwave irradiation | — | ·OH | 67.5 | 25–35/- | MO RhB | 500 W Xenon lamp | 97%/100 min 89%/100 min | 5 | [260] |
| NiFe2O4/BiVO4/g-C3N | Hydrothermal | 2.72 | ·O2 −, h+ | — | — | TC Chlorotetracycline(CTC) MB Ofloxacin(OFl) | 300 W Xenon lamp | 93%/60 min 87/60 min 96/60 min 93.8%/20 min | 5 | [261] |
| BiFeO3/ g-C3N4/Cu2O | Hydrothermally wet precipitation and ultrasonic dispersion | 0.062 | — | 121.4 | 22.1/0.24 | RhB | 500 W Halogen lamp | 98.5%/140 min | 5 | [77] |
| CuFe2O4@g-C3N4 +H2 O2 | Self-assembly | — | ·O2 −, ·OH | 21.6 | — | Orange II | 500 W Xenon lamp | 90%/180 min | 5 | [245] |
| CuFe2O4/g-C3N4 | Calcinations | — | ·O2 −, ·OH, ·SO4 −, h+ | — | — | Propranolol (PRO) | 350 W Xenon lamp | 82.2%/120 min | — | [19] |
| MnFe2O4/g-C3N4/TiO2 | Chemical impregnation | 0.653 | ·O2 −, ·OH, h+ | 42.77 | 53.68/0.5741 | MO | 150 W Xenon arc lamp | 99.27%/180 min | 3 | [247] |
| MnFe2O4/g-C3N4/Graphene | Solvothermal | — | ·O2 −, ·OH, ·SO4 −, h+ | — | — | Metronidazole | 300 W Xenon lamp | 94.5%/— | — | [248] |
| MnFe2O4/g-C3N4 + PMS | auto-combustion sol-gel | ·O2 − | 98.93 | 15.83/0.448 | triclosan (TCS) | — | 97%/60 min | 7 | [96] | |
| (30%)MnFe2O4/g-C3N4 + PMS | Self-assembly | 39.03 | ·SO4 − | 46.82 | 2.448/0.115 | sulfamethoxazole (SMX) | — | 100%/35 min | 4 | [262] |
| (30%) CaFe2O4/g-C3N4 | Two steps | — | ·O2 −, h+ | — | — | MB | 150 W Tungsten halogen lamp | 94%/120 min | 3 | [263] |
| CaFe2O4/g-C3N4/CNT | Hydrothermal | — | ·O2 −, ·OH | — | 2.37/— | Cr(VI) TC | 300 W Xenon lamp | 98%/120 min | [264] | |
| SnFe2O4/g-C3N4 | Solvothermal | 21.73 | ·O2 −, h+ | — | 50 nm/— | Chlorotetracycline | 300 W Iodine tungsten lamp | 67%/300 min | 3 | [265] |
| (30%)SnFe2O4/g-C3N4 | In situ precipitation | — | ·O2 − | 95.9 | 38.3/0.268 | RhB | 500 W Halogen lamp | 99%/30 min | 5 | [266] |
Figure 20. Schematic illustration of proposed Type-II charge transfer mechanism in (a) CuFe2O4/g-C3N4 (b) MgFe2O4/g-C3N4 (c) MnFe2O4/g-C3N4 NCs [95, 96, 267].
Download figure:
Standard image High-resolution imageFigure 21. Schematic illustration of proposed Z-Scheme charge transfer mechanism in (a) CuFe2O4/g-C3N4 (b) MgFe2O4/g-C3N4 (c) MnFe2O4/g-C3N4 NCs [19, 95, 96, 245, 267].
Download figure:
Standard image High-resolution imageFigure 22. Schematic diagram of MFe2O4 photocatalyst (where M = Zn, Co, Ni, Cu, Mn, Ca, Mg, Ba, Sn, Cd etc) upon visible light illumination [87, 88, 245]. Reprinted from [245], Copyright © 2015 Elsevier B.V. All rights reserved.
Download figure:
Standard image High-resolution imageFigure 23. Photocatalytic performance of g-C3N4-based binary, ternary and quaternary magnetic nanocomposites towards the degradation of RhB dye upon 300 W xenon lamp irradiation.
Download figure:
Standard image High-resolution image2.5. Computational (Density Functional theory) study of g-C3N4-based magnetic photocatalysts
The First‐principles calculation using density functional theory (DFT) is a fundamental and powerful technique to determine the material's intrinsic properties by optimizing the geometric structure and calculating the system energy [268]. Further, the geometric optimization is performed using the ultra-soft projector augmented wave (PAW) pseudo-potential method, and the GGA (generalized gradient approximation) depicts the functional exchange-correlation interaction [269, 270]. The computational study demonstrates that heteroatoms [271] doping modifies the chemical and electronic properties of the g-C3N4 sheets and improves photocatalytic activity significantly. In the theoretical models [272], the nitrogen linker is primarily replaced by various heteroatoms or aromatic groups to obtain the 2D or 3D multilayered structure [273]. Many transition metal ions, including noble metals, are incorporated into the caves of the g-C3N4 [274] to improve carrier mobility by narrowing the energy band gap and increasing the visible light absorption ability. Similar types of cave doping using the alkali-metal ions [272, 275] cause nonuniform charge distribution on the sheets favorable for free carrier generation, transportation and separation. Hence, understanding the effects of element doping and interaction between different components or layers, including the photocatalytic reaction process on catalysts' surfaces, are crucial for designing new materials. To date, the major theoretical studies are concentrated on the electronic and magnetic behavior of the g-C3N4 catalysts.
The precise architecture of g-C3N4 has two accepted crystal structures: tri‐s‐triazine (C6N7) and s‐triazine (C3N3) rings [276], as shown in figure 24. Tri-s-triazine- based g-C3N4 is found to be a more stable photocatalyst at ambient conditions [277, 278]. For non-metal doped tri‐s‐triazine‐based g-C3N4 systems, Stolbov et al and Ma et al have revealed that B and S atoms preferred to substitute the C and two‐coordinated N atoms, respectively (shown in figure 25) [279, 280]. In another study by Yousefi et al, a similar verdict is observed for the s-triazine-based system [281]. The monolayer of g-C3N4 exhibits a larger bandgap than bulk g-C3N4 because of the quantum confinement effect. The bandgap of bulk g-C3N4 is indirect, whereas monolayer g-C3N4 is direct. The work function (Φ) of g-C3N4 is around 4.50 eV, which weakly correlates with the number of layers [276]. The energy bandgap (Eg) of g-C3N4 is experimentally estimated to be 2.7 eV; however, the DFT calculation estimates higher values depending on the constructed model. A model for g-C3N4 is proposed with a supercell consisting of 27 Carbon atoms and 36 nitrogen atoms. A vacuum spacing of 15 Å is considered to avoid the interaction of the complex system with periodic counterparts [282]. The DFT study demonstrates that the oxidation and reduction reactions of different species take place at the sites, overlapping of atomic orbitals (Pz) of nitrogen and Carbon [283], creating the VB (+1.6 eV) and CB (−1.1 eV) of g-C3N4. Thus, the C and N atoms act as sites where reduction and oxidation of O2 and H2O occur, respectively [278, 284]. Till now, comprehensive knowledge of DFT calculation methods and results of g-C3N4 atomic arrangements has expounded, such as pristine g-C3N4, nonmetal doped g-C3N4, metal decorated g-C3N4, and g-C3N4 based composites [276]. Recently, many alkali metals decorated g-C3N4 and transition metals decorated g-C3N4 systems have been investigated by DFT calculation. For example, Zhang et al have studied Ti or Sc decorated monolayer g-C3N4 system and introduced it as a promising candidate for hydrogen storage due to its excellent hydrogen adsorption capacity [285, 286]. Zhang et al have found that the decoration of transition metal atoms (Sc, Ti, V, Cr, Mn, Fe, Co, and Ni) [285, 286] resulted in various band edge potentials of bulk g-C3N4. It can be inferred that the photogenerated electrons of Sc‐, Ti‐, and V‐decorated g-C3N4 systems had strong reduction ability. The band edge potentials of Cr‐, Mn‐, and Fe‐decorated g-C3N4 systems met the requirement of overall water splitting [275, 287]. Figure 26 shows different symmetric and asymmetric cave doping of transition metals. Recently, computational investigation of g-C3N4-based metal oxides gained enormous attention towards understanding the interaction at the interface by calculating the charge density difference in the composite or comparing the work functions of different components.
Download figure:
Standard image High-resolution imageFigure 25. Schematic diagram of non-metal (a) sulfur, (b) phosphorus and (c) boron doping on g-C3N4 framework [89, 273]. Reprinted from [273], © 2019 Elsevier Ltd. All rights reserved.
Download figure:
Standard image High-resolution imageFigure 26. Schematic diagram of transition metals (a) symmetrical and (b) asymmetrical cave doping on g-C3N4 framework [85].
Download figure:
Standard image High-resolution image3. Conclusions and future perspectives
This review presents an overview of recent developments in the fabrication of g-C3N4-based NCs, including their potential utilization as visible-light active photocatalyst for water purification and environmental remediation. Despite many appealing features and competencies as metal-free catalysts, the applications of bulk g-C3N4 are limited and suffer many challenges, especially in the degradation of dyes and antibiotics. Therefore, various magnetic NPs, namely hematite (α-Fe2O3), magnetite (Fe3O4), ferrites (MFe2O4), including metals and semi-metals, are combined judiciously with a few-layered g-C3N4 to make binary, ternary, and quaternary NCs to improve reusability and recovery of catalysts. The integration of magnetic NPs improves the visible-light response, facilitates the photogenerated EHP, expedites the charge separation, and enhances the photocatalytic activity. Besides, the high Ms value of the magnetic NCs eases the process of catalyst extraction and recovery. The magnetic NCs display decent constancy to use in consecutive cycles without any loss of activity. The computational study demonstrates that the Tri-s-triazine-based g-C3N4 framework is more stable than s-triazine- based g-C3N4 which allows the selection of suitable structures for the development of magnetic composites. The doping of metal/or non-metal elements at the cave site or substitution of C/or N significantly alters the electronic properties of g-C3N4. At the same time, heterojunction-based magnetic composites are preferred over doping due to inducing high magnetization and interfacial electric fields. Further to augment the efficacy of the catalyst's performances, binary, ternary and quaternary semiconductor grafted g-C3N4 are developed where the synergistic interfacial effect at the heterojunction is exploited to enhance the light absorption, EHP generation, carrier separation, and transportation. The separation efficacy of the magnetic NCs depends on the value of high saturation magnetization (binary composite > ternary composite > quaternary composites) with increasing the contents of Fe3O4. The g-C3N4/ferrites (MFe2O4; M = Zn, Cu, Mn, and Mg) composites (type-II/ or Z-scheme) show strong reduction ability rather than oxidation. However, the g-C3N4/ferrites composites (M = Ni, Co, Ca, and Cd) displays strong oxidation and reduction due to the suitable positioning of VB and CB in the Z-scheme charge transfer mechanism. Among the different charge transfer mechanisms, the recombination rate of the photogenerated EHPs is suppressed in the following order: Indirect Z-scheme > Z-scheme > Type-II. The coupling of noble metal NPs with magnetic g-C3N4 composites is another way to boost the photocatalytic activity by exploiting both the surface plasmon resonance effect and the magnetic property. However, the performances of magnetic photocatalysts are not adequate for commercialization at a larger scale. Therefore, further research is required to design and develop magnetic NCs with superior visible-light absorption capacity by following a cost-effective preparation method. This comprehensive review summarizes the experimental and theoretical aspects of the g-C3N4 based magnetic photocatalyst and discusses the charge transfer mechanisms, recovery, and reusability property of the complex catalytic system. However, making a suitable catalyst based on g-C3N4 with all desirable traits is challenging and needs further investigation. The charge transfer mechanisms in the g-C3N4-based ternary and quaternary NCs, including Z-scheme heterojunction interfaces, are very complicated, and, hence, advanced spectroscopic techniques are required for further study. Additionally, the current studies using theoretical DFT calculation concentrate primarily on the single element doped g-C3N4; however, more studies on the complex system need to be performed to design new materials and optimize the catalytic efficiency. Relatively new processes, such as Sonocatalysis and Sonophotocatalysis, can be employed using ultrasonic waves to enhance free radicals and quickly remove the organic pollutants from the wastewater.
Data availability statement
No new data were created or analysed in this study.