Abstract
Hydrogen has been considered as one of the most promising alternative energy source to solve the future energy demands due to its high energy capacity and emission-free character. The generation of hydrogen from non-fossil sources is necessary for the sustainable development of human life on this planet. The hydrolysis of sodium borohydride can quickly produce a large amount of hydrogen in situ and on-demand in the presence of the catalyst, which can be used as an alternative energy source. So, it is crucial to fabricate the highly efficient, robust, and economical catalyst for the production of hydrogen via hydrolysis of sodium borohydride. Herein, a facile and efficient approach for the synthesis of metal-functionalized reduced graphene oxide for the production of hydrogen at room temperature was used. Moreover, the synthesized catalyst has also been tested in the field of environmental catalysis for the reduction of toxic 4-nitrophenol to valuable 4-aminophenol in the presence of sodium borohydride. The enhanced activity of prepared metal-functionalized reduced graphene oxide is ascribed to a strong affinity between Fe–NX and reduced graphene oxide which facilitates electron transfer as well as synergistic effect. Overall, this work presents a crucial procedure for green chemistry reactions when a carbonaceous material is selected as a catalyst.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Currently, the global energy demand and burden on the environment are increasing at a very fast rate due to the rapid growth of population and industrialization. Therefore, it is highly desirable to design alternative clean, and sustainable efficient energy sources, which can fulfill the energy demand of the fast-growing population. Among all renewable energy resources, undoubtedly hydrogen is considered the most promising alternative energy source to fossil fuels due to its cleanness, high energy capacity, and environmentally friendly nature [1]. Moreover, hydrogen is emission-free fuel, can be utilized in rocket fuel, aircraft, internal combustion engines, and proton exchange membrane fuel cells. Hydrogen is also regarded as 'green' and sustainable energy as the only product of the combustion process is water, thereby eliminating air pollution without discharging greenhouse gases [2, 3]. Currently, the majority of hydrogen (∼95%) is generated from fossil fuels using high-temperature steam reforming of natural gas, methane, and coal gasification [4–6]. Various other methods also reported for the production of hydrogens such as water splitting or oxidation (electrolysis, photocatalytic, and thermolysis), bio-hydrogen production, and hydrolysis of hydrogen-holding materials (hydrides, amines, etc) [7, 8]. Among all given approaches, catalytic hydrolysis of metal hydride is considered as the most potential, suitable, economic, and powerful method for the production of hydrogen [9, 10]. Especially, sodium borohydride is the most studied metal hydride and promising candidate for hydrogen production on-demand via hydrolysis in the presence of suitable catalyst due to its high gravimetric capacity (∼10.8 wt%), non-toxic, and non-flammable nature, as well as high stability in air [11, 12]. Moreover, the only by-product from hydrolysis of sodium borohydride is sodium metaborate (NaBO2, which can be recycled), which is water-soluble and environmentally benign [11]. Thus, different catalysts such as Pd [13], Pt [14], Ru [15], Rh [16], and Ru–Pd–Pt [17] on various support have been explored for getting excellent activity for hydrogen production via hydrolysis of sodium borohydride [1, 13, 18]. Although, the high cost and limited supply of noble metals restrain their use for industrial applications.
Undoubtedly, 4-nitrophenol (4-NP) is considered one of the most prevalent organic pollutants in wastewater generated from agricultural and industrial sources [19]. Moreover, 4-NP is a very toxic and refractory pollutant and difficult to remove due to its good chemical and biological stability against degradation as reported by the US Environmental protection Agency [20]. It has been reported that the presence of 4-NP in the environment may cause health issues due to its carcinogenic and mutagenic nature [21]. Therefore, the removal of such hazardous and toxic compounds is of great importance for a healthy environment. Various methods have been applied for the removal of 4-NP pollutants from the environment. Recently, the conversion of 4-NP to reusable 4-aminophenol (4-AP) without using organic solvents has drawn much attention [22]. As the conversion of 4-NP to 4-AP is not only a model reaction to assess the catalytic activity of certain catalysts, but also their reduced product (4-AP) has industrial application as well as an important intermediate for the manufacturing of fungicides, cosmetic, dyes, and pharmaceuticals, etc [23]. Accordingly, the reduction of 4-NP to 4-AP is an essential and critical issue for scientists. The reduction of 4-NP to 4-AP in the presence of sodium borohydride on the surface of catalysts has emerged as a key reaction due to its low-cost and simplicity [24]. The mainly reported catalysts for the reduction of 4-NP to 4-AP are expensive noble metals such as Au, Ag, Pt, Pd, etc [25–29]. However, limited availability, high cost, and serious trend in the aggregation of noble metal are the major problem for practical applications [27]. For this reason, an alternative catalyst such as metal-functionalized reduced graphene oxide for the catalyzed reaction is needed.
Graphene oxide is a novel two-dimensional atomic sheet of sp2 hybridized carbon atoms packed in a hexagonal lattice [30, 31]. The properties of graphene oxide such as fast charge carrier, high thermal conductivity, large specific surface area, high young's modulus, remarkable chemical and mechanical stability, good optical and electrical properties may be due to its extended honeycomb network and long-range π conjugation [32–39]. Presently graphene oxide has been extensively studied in various admissible industrial applications such as bio and chemical-sensing, energy conversion/storage, adsorption, photo-catalysis, electro-catalysis, aerospace, touch screen panel devices, catalysis and so on [32, 40–49]. Thus, the scientific community is devoted for the development of various top-down and bottom-up manufacturing approaches for graphene oxide and its derivatives [50]. However, pristine graphene oxide is needed to be activated for catalytic application via doping. Heteroatom doping is a promising approach for tailoring the electronic structure, introducing local change, and manipulating the surface chemistry of graphene oxide which can be used for various applications [51–53]. It has been reported that noble metals such as Pd, Pt, Au, Ag, etc doped carbon nanotubes and graphene are being extensively used in various applications [54–58]. Then, it is desirable to explore alternative non-precious metal-free materials due to scarcity and the high cost of noble metals [54, 59]. Hence, a lot of research attention has been focused on noble metal-free catalytic systems for improved catalytic activity. Doping of nitrogen has been considered as a good alternative to noble metals due to its low-cost, comparable atomic size, and capability to make a strong bond with a carbon of graphene oxide. Moreover, nitrogen doping can change the electronic properties of graphene oxide via forming three different bonds with the sp2 carbon lattice of graphene oxide. Depending on the nature of nitrogen doping it can be p-type in the case of pyridine-N or may be n-type in the case of pyrrolic N, and graphitic/quaternary-N [60–62]. Nitrogen-doped graphene oxide has been widely used as an excellent catalyst that exhibits outstanding catalytic properties. Despite such properties of nitrogen-doped graphene oxide, the catalytic activity still require improvement for industrial application. Therefore, greater attention is being devoted to further enhancement of the catalytic activity of nitrogen-doped graphene oxide and building up a better understanding of its underlying catalytic mechanism. Therefore, doping with heteroatoms having electron donor property is critical to increases the structural defects and electronic polarization, which intern improve the catalytic activity. Transition metals such as Fe, Co, and Ni are considered the best dopants capable of enhancing the graphitization of graphene oxide for improved catalytic activity. Moreover, transition metal-nitrogen-containing complexes supported on carbon material enriched with M–Nx (M = Fe, CO) are treated as the most promising non-precious metal group-based catalysts (NPMGCs) [63]. Interestingly, co-doping will further improve the catalytic activity by synergistic effects. Experimental reports and quantum chemical calculations have declared that nitrogen and/or M–Nx moieties play a crucial role in catalytic activity [64, 65]. Therefore, noble NPMGCs exhibited comparable durability and catalytic activity to precious metal-based catalysts [66]. However, the activity and durability of transition metal-based NPMGCs depend highly upon the choice of precursors and method. Transition metal−nitrogen coordinations supported on carbon materials are treated as the most auspicious NPMGCs for replacing precious-metal-based catalysts due to easy coordination of pyridine and pyrrolic nitrogen to Fe and CO, which boost the construction of M–Nx /C active centers for excellent catalysis [63, 66, 67]. Among numerous accessible catalysts Fe–Nx /C enriched carbon-based materials bear the potential to take over novel metal-based catalysts as the binding energy of Fe–Nx /C (4.45 eV) is approximately equal to precious metal Pt (4.46 eV) [68].
Herein, a facile approach was used to synthesis of metal-functionalized (Fe–Nx /C) reduced graphene oxide for the production of hydrogen and 4-NP in the presence of sodium borohydride at room temperature (R.T). The improved catalytic activity of the as-prepared catalyst may be attributed to the strong affinity between Fe–Nx /C and the synergistic effect. This work presents an efficient and facile method for the engineering of pristine graphene oxide with Fe and N co-dopants for making valuable catalysts. Moreover, the great potential of doping for tailoring the applications of graphene oxide and may pave a new way for architecture and designing a stable non-precious metal-free graphene oxide-based catalyst. Overall, the present work provides a promising method in the field of green chemistry for hydrogen production and 4-NP reduction.
2. Experimental section
2.1. Reagents and chemicals
Graphitic flakes, 4-NP, sodium borohydride (NaBH4), and urea were obtained from Sigma Aldrich. Sodium nitrate, ethylene glycol, sulfuric acid, and hydrogen peroxide were taken from SDFCL. Potassium permanganate (KMnO4), N, N dimethylformamide, and iron (III) nitrate monohydrate were supplied by fisher scientific. The double-distilled water was used in all experiments.
2.2. Synthesis of graphene oxide
The graphene oxide was synthesized using graphite flake via a modified Hummers' method [69]. In a typical experiment, graphite flakes and sodium nitrate were exploited in previously cooled sulfuric acid with continuous stirring in an ice bath. Then a desirable amount of potassium permanganate was added slowly to the above suspension with continuous stirring. After that, the suspension was heated at 40 °C with continuous stirring followed by slow addition of double-distilled water. The obtained thick paste was heated again at 90 °C for 2 h. Finally, the gradual addition of hydrogen peroxide to stop the reaction resulted in the color change. The obtained suspension was filtered and washed with HCl and double-distilled water to remove the sulfate. The obtained product was dried in the oven at 60 °C.
2.3. Synthesis of iron and iron/nitrogen co-doped reduced graphene oxide
The iron and nitrogen co-doped reduced graphene oxide was synthesized by the solvothermal method. In a typical approach, prepared graphene oxide (100 mg) was dispersed in N, N dimethylformamide (80 ml) to form a homogenous solution ultrasonically. After that, a desired amount of Iron (III) nitrate (1 wt%) and urea (5 wt%) was added to the above solution, which was then transferred to the hydrothermal bomb and heated at 160 °C for 15 h. The obtained product was filtered and washed several times with ethanol and water after being cooled naturally. Then the product was dried at 60 °C in an oven. Nitrogen-doped graphene oxide was synthesized using the same procedure without using iron (III) nitrate. The products are named G-1, G-2, and G-3 for graphene oxide, nitrogen-doped reduced graphene oxide, and iron/nitrogen co-doped reduced graphene oxide respectively.
2.4. Characterization
The phase structure of the prepared materials was investigated using an x-ray diffractometer (Rigaku MiniFlex-300) with Cu Kα radiation as the x-ray source (λ = 0.154 nm). The scanning electron microscopy (SEM Evo18) was employed to see the surface morphology. Transmission electron microscopy (TEM), high-resolution electron microscopy (HRTEM), and selected area electron diffraction (SAED) were carried out (JEOL JEM-1400) at an accelerating voltage of 150 kV. The elemental analysis of the G-3 sample was performed using energy-dispersive x-ray spectroscopy (EDX) by Hitachi S5500 field emission SEM. x-ray photoelectron spectroscopy (XPS) was employed to detect the bonding, oxidation, and elemental analysis using PHI VersaProbe II with an AES photoelectron spectrometer. Raman spectroscopy of the prepared samples was carried out using Renishaw Micro Raman Spectrometer operating at 514 nm.
2.5. Hydrogen production measurement
The catalytic activity of the as-synthesized samples was investigated by calculating the hydrogen production by hydrolysis of NaBH4 using the water displacement method as shown in figure S1 (available online at stacks.iop.org/NANO/32/495404/mmedia). Typically, 50 mg catalyst and 100 mg NaBH4 were kept at the bottom of a conical flask on the magnetic stirrer. The reaction flask was closed tightly with a septum and connected with the burette to control the water flow into it. Moreover, the reaction flask was also connected with other filled (water) flask with the help of a glass tube. The water-filled (second) flask was connected with a measuring cylinder. The generation of hydrogen gas was calculated by measuring the displaced water in the measuring cylinder. The activation energy (Ea) was investigated by repeating the experiments at different temperatures.
2.6. 4-NP reduction
The reduction of 4-NP was carried out in the presence of NaBH4 at R.T to further investigate the catalytic activity of the as-synthesized materials for environmental application. In a typical experiment, a desired amount of NaBH4 (100 mg) was added to the aqueous solution of 4-NP (4 ml, 30 ppm) and the color was abruptly changed from light yellow to deep yellow due to the formation of phenolate ions. After that desired amount of catalyst (150 μl) was added to the aqueous solution of 4-nitro-phenol and time-dependent UV–vis spectra were recorded. The degradation and conversion of 4-NP to 4-AP were investigated by a change in absorbance. The progress of the experiment was investigated using UV–vis Perkin Elmer lambda 900 spectrophotometer.
3. Results and discussion
3.1. XRD analysis
The XRD analysis was carried out to examine the phase purity and crystallinity of the prepared materials and presented in figure 1(A). The XRD pattern of G-1 exhibits a sharp peak at around 2θ = 11.0° corresponding to (001) diffraction peak of graphene oxide indicating the lamellar structure [70]. After hydrothermal treatment and doping of nitrogen and iron the peak of G-1 shifted towards a higher 2θ value (25.4°), which is assigned to the (002) plane of reduced graphene oxide [70]. The characteristic peak of graphene oxide 11.0° moved to 25.4° indicating the reduction of graphene oxide. The d value for G-1 and G-3 are found to be 0.8 nm and 0.3 nm indicating the reduction in interlayer spacing, which confirms the removal of oxygen and water, captured between G-1 sheets, further confirm and support the reduction of G-1 [70–72].
Figure 1. (A) XRD and (B) Raman spectra of G-1, G-2, and G-3.
Download figure:
Standard image High-resolution image3.2. Raman analysis
Raman spectroscopy is a non-destructive and powerful analysis to characterize the notable structural changes/defects and to observe the presence of dopants in carbon-based materials.
It could be seen from figure 1(B) that all three catalysts are exhibiting a peak at 1346 cm−1 which is corresponding to the D band due to structural defects of the A1g mode of the sp3 carbon atom [73]. Moreover, a peak around 1590 cm−1 is characteristic of the G band which is related to the in-plane vibration of the E2g mode of sp2 carbon atom in the hexagonal plan [73]. Generally, the intensity ratio of D and G bands (ID/IG) is used to study the structural defects/disorders and reduction in the average size of sp2 domains in graphitic materials [71]. The calculated ID/IG values for G-1, G-2, and G-3 are found to be 0.77, 1.05, and 1.10 respectively. The increase in the ID/IG value for G-3 indicates the reduction of G-1 and large structural defects in reduced graphene oxide, which provides new graphitic domains resulting in an increase in D band due to the introduction of iron and nitrogen [74, 75].
3.3. SEM and elemental mapping
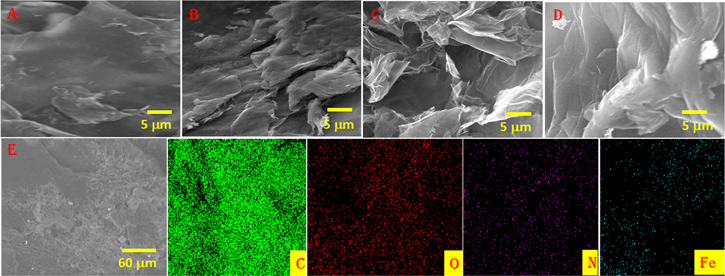
The surface morphology of the G-1, G-2, and G-3 was investigated using SEM analysis and exhibited in figure 2. It could be seen from figures 2(A) and (B) that sheets of G-1 are stacked and curved to form a flat-like structure. Moreover, sheets of G-1 are linked with each other to form a honeycomb structure as shown in figures 2(A) and (B). Moreover, figures 2(C) and (D) displays the SEM images for G-2 and G-3 indicating an exfoliated lamellar structure with plenty of wrinkles. Furthermore, the SEM image of G-3 emerges as a curved, crumpled, and scrolling structure. To confirm the consistency of the spatial distribution of nitrogen and iron, elemental mapping was also carried out. The elemental mapping indicates the presence of carbon, oxygen, nitrogen, and iron as shown in figure 2(E).
Figure 2. SEM image of (A) and (B) G-1, (C) G-2, (D) and (E) G-3 and corresponding elemental mapping.
Download figure:
Standard image High-resolution image3.4. TEM, HRTEM, SAED and EDX analysis
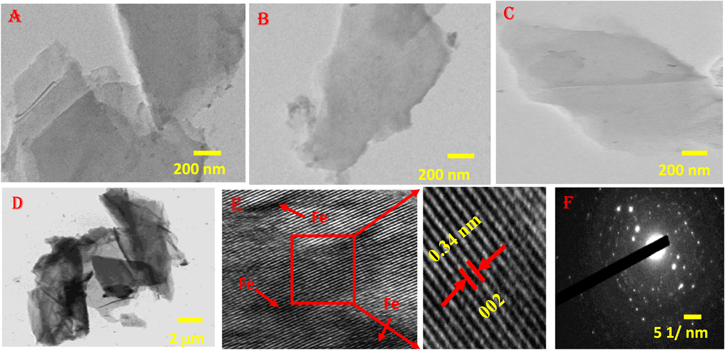
TEM, HRTEM, SAED, and EDX analyses were further carried out to study the microstructure and crystalline nature as well as the distribution of dopants on the reduced graphene oxide sheets. Figures 3(A) and (B) displays the TEM image of G-1 indicating the folded sheets and layered structure with wrinkles. Figures 3(C) and (D) exhibits the TEM image of reduced graphene oxide (G-2 and G-3), which appears transparent, wrinkled, and a few layers entangled with each other. The TEM image of G-3 exhibits exfoliated layers with sheet-like morphology and uniform distribution of dopants on the reduced graphene oxide sheets as shown in figure 3(D). The HRTEM image of G-3 displays a lattice fringe spacing of 0.34 nm corresponding to the (002) plane of sp2 graphitic carbon as shown in figure 3(E). Figure 3(F) indicates the SAED pattern of G-3 with defined spots showing its polycrystalline structure. The EDX analysis indicates the presence of carbon, oxygen, nitrogen, and iron elements as shown in figure 2S (supplementary information).
Figure 3. TEM image of (A) and (B) G-1, (C) G-2, (D)–(F) and (H) TEM, HRTEM and SAED pattern of G-3.
Download figure:
Standard image High-resolution image3.5. XPS analysis
XPS is a powerful tool to measure and elucidate the surface chemical composition and valance state of prepared materials. Figure 4(A) exhibits the XPS wide scan spectra of G-3 indicating the presence of C, O, N, and Fe elements. Wide survey spectra show the presence of nitrogen and iron species on the carbon base which is helpful for the construction of strong Fe–N interactions as well as enlightening the co-doping of carbon with nitrogen and iron. The high-resolution XPS (HR-XPS) spectra of C 1s shows the peak at 284.6 eV, which can be deconvoluted into four peaks with binding energies at 284.6, 286.3, 287.5 and 289.1 eV, that is assigned to C=C, C–C, C–N and C=O, C–O species respectively as shown in figure 4(B) [76–78]. The peak at 284.6 eV is analogous to sp2 carbon atom bonded with other carbon atoms indicating that most carbon is organized in a honeycomb lattice. The peak at around 286.3 eV is attributed to the sp3 carbon atom bonded with other carbon atoms, whereas the peak at around 287.5 eV is assigned to sp2 carbon bonded with a nitrogen atom. However, the peak at 289.1 eV is corresponding to the bonding of carbon with oxygen-rich functional groups [76–78]. The HR-XPS spectra of O 1s show the peak at 532.2 eV, which is deconvoluted into three different peaks at 531.0, 532.8, and 534.1 eV corresponding to C=O, C–O, and O=C–O respectively as shown in figure 4(C) [75]. The HR-XPS spectra of N 1s exposed five different types of nitrogen moieties, namely pyridinic N (399.4 eV), pyrrolic N (400.7 eV), graphitic N (403.1), and N-oxide (404.1 eV) as shown in figure 4(D). Moreover, an additional peak at around 401.5 eV is corresponding to the bonding of nitrogen with iron i.e Fe–Nx moieties as shown in figure 4(D) [79]. It has been reported that among all available nitrogen moieties pyridinic and pyrrolic nitrogen can be easily coordinated with transition metals like Fe to form Fe–Nx complexes [80]. These Fe–Nx complexes are remarkably helpful for catalytic activity and act as active centers [80]. Likewise HR-XPS spectra of Fe 2p shows that both Fe2+ and Fe3+ present on the G-3 as shown in figure 4(E) [81, 82].
Figure 4. (A) XPS survey scan spectra of G-3 and high-resolution XPS spectra of (B) C 1s, (C) O 1s, (D) N 1s, and (E) Fe 2p.
Download figure:
Standard image High-resolution image3.6. Catalytic hydrogen generation in the presence of NaBH4
To investigate the catalytic activity of the synthesized materials, experiments for the production of hydrogen using the water displacement method were employed in the presence of NaBH4. It is well-established that the generation of hydrogen via hydrolysis of NaBH4 is an automatic, spontaneous, and exothermic reaction, but is very slow without a catalyst. The spontaneous hydrolysis is so slow that it can take more than a day for the generation of a very small amount of hydrogen even in the presence of a large amount of water if carried out without any catalyst. NaBH4 is an excellent material for the storage of hydrogen fuel as it is having a high percentage of hydrogen ions (10.8%), an environmentally friendly nature, good stability, and easy availability. Production of hydrogen can take place by the hydrolysis of NaBH4 in the presence of water and a suitable catalyst. Herein, we explore the activity of G-3 material which has been demonstrated as an efficient catalyst for hydrogen generation via hydrolysis of NaBH4. It could be seen from figure 5(A) production of hydrogen in the presence of G-1, G-2, and G-3 via hydrolysis of NaBH4. The production of hydrogen may take place by hydrolysis of NaBH4 using prepared materials according to the given equation (1), which can give nearly 4 moles of hydrogen per NaBH4 molecule
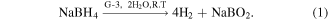
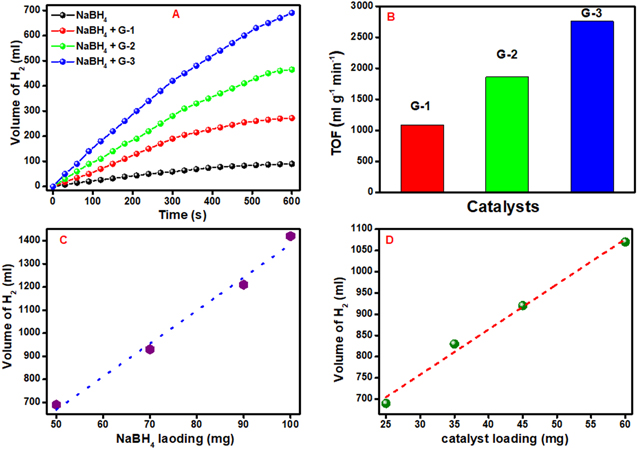
Figure 5. (A) Time-dependent plot for the hydrolysis of NaBH4 catalyzed by G-1, G-2, G-3 and absence of catalyst, (B) turn over frequency in the presence of G-1, G-2, and G-3, (C) effect of NaBH4 loading and (D) effect of catalyst loading on hydrogen production in the presence of G-3.
Download figure:
Standard image High-resolution imageIn our experiment, the catalytic reaction was started after the addition of water into the mixture of NaBH4 and catalyst. The amount of generated hydrogen was calculated by the water displacement method in terms of conversion of sodium borohydride as a function of time at ambient conditions. It could be seen from figure 5(A) that G-3 exhibited the highest hydrogen generation as compared to all other catalysts. We also did a blank experiment via hydrolysis of NaBH4 in the absence of catalyst exhibiting very low production of hydrogen as shown in figure 5(A).
To determine the catalytic efficiency significantly of the as-prepared catalysts, the turnover frequency (TOF) was predicted using equation (2)

Figure 5(B) shows the TOF of the as-prepared catalysts, where G-3 exhibits the highest TOF towards hydrogen production as compared to the other catalysts. It may be due to the synergistic effect of nitrogen and iron present in G-3. Moreover, the TOF of G-3 was found to be comparable to the various previously reported catalysts for the production of hydrogen [83–85].
To investigate the effect of NaBH4 loading on the production of hydrogen, several experiments were employed without changing other experimental conditions. It could be seen from figure 5(C) that the production of hydrogen increases almost linearly with NaBH4 loading. Therefore, the controlled loading of NaBH4 is an effective way to regulate the hydrogen yield.
To explore the effect of catalyst more accurately on hydrogen generation, a series of experiments were carried out with different loadings of the most active catalyst (G-3) without changing any other conditions. Figure 5(D) displays the improvement in the rate of hydrogen production with catalyst loading, which in turn indicates that the concentration of catalyst also plays a key role in controlling the hydrogen production rate.
To investigate the effect of a basic solution (NaOH) on the production rate of hydrogen, a series of experiments were also conducted without changing other experimental conditions. It has been reported that the hydrolysis of NaBH4 starts with the strike of hydronium ion on the borohydride anion in acidic conditions. However, the self-hydrolysis of NaBH4 can be prevented by the addition of alkali (NaOH) in the experimental solution. In alkaline solution (NaOH), the rate of hydrogen production has been found to decrease due to a decline in the hydrolysis of NaBH4 with a subsequent decline in the proton concentration. Four different experiments were done to investigate the effect of NaOH concentration (0, 10−2, 10−4, and 10–6 M) on the hydrolysis of NaBH4 at R.T in the presence of G-3. It could be seen from figure 6(A) that the rate of hydrogen production decreased gently with the NaOH concentration. Therefore, the concentration of acidic and basic solutions has a great effect on the production of hydrogen.
Figure 6. (A) Effect of NaOH concentration on hydrogen production in the presence of G-3, (B) on requirement hydrogen production in the presence of HCl and NaOH, (C) Arrhenius plot for hydrolysis of NaBH4 from the kinetic data and (D) reusability of the G-3 catalyst for hydrolysis of NaBH4.
Download figure:
Standard image High-resolution imageTo explore the industrial applications for on-demand hydrogen production, several experiments were carried out. It could be seen from figure 6(B) that the controlled production of hydrogen was studied by the addition of the appropriate amount of HCl and NaOH. The figure shows the on–off controlled production of hydrogen, where NaOH (10–1 M) plays a key role to completely terminate the production of hydrogen. However, hydrolysis of NaBH4 was found to be increased in the presence of HCl, where H+ plays an effective positive role for hydrogen production.
It is well known that activation energy is an important parameter for assessing the kinetic rate of hydrogen production. However, activation energy is temperature dependant phenomenon. Therefore, to obtain Arrhenius activation energy, the hydrolysis of NaBH4 was employed at different temperatures. The activation energy was calculated from the Arrhenius plot as shown in figure 6(C) and found to be = 15.98 kJ mol−1.
In order to demonstrate the industrial value of the synthesized G-3, a stability and conversion efficiency test was also carried out by reusing the G-3 catalyst. Therefore, four successive hydrolysis experiments were employed by adding the same amount of NaBH4 without changing other parameters at R.T. It could be seen from figure 6(D) that the conversion efficiency of NaBH4 hydrolysis was found to be 100%, however, a reduction in the catalytic activity of G-3 was observed at each cycle. This may be due to the formation of by-products during hydrolysis of NaBH4 and its dilution in water. Therefore, G-3 may prove to be of high industrial value.
3.7. The mechanism for the hydrolysis of sodium borohydride for hydrogen production
Santos and Sequeira reported the procedure for the determination of hydrogen evolution via hydrolysis of sodium borohydride (hydride hydrolysis) [86]. This method is based on gasometric measurement of hydrogen evolution upon hydrolysis of BH4 −. Here the used apparatus is very simple and less complicated than the conventional hydrogen evolution approaches (figure S1) and importantly does not require any control on temperature and pressure during the whole measurement process. The production of hydrogen takes place via hydrolysis reaction of sodium borohydride over catalyst as shown in equations [87]. Firstly, the formation of MH takes place via reversible adsorption and desorption surface reaction of borohydride (equation (3)). In the next step formation of borane (BH3) occur with the release of electron (equation (4)). In the next step, borane can react with OH− anion leads to the formation of a stable intermediate ion (BH3OH−) as shown in equation (6). The stable intermediate can go through similar steps (equations (3)–(5)) to form B(OH)4 −. Finally, the hydrogen can be evolved from the hydrolysis of BH4 − in the presence of a catalyst (equation (7)).
Where M is active catalyst surface (G-3)
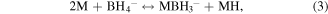
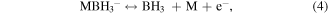
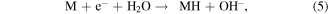
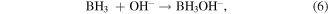
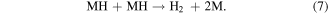
The higher hydrolysis activity of G-3 for the production of more hydrogen mainly due to synergistic effect as well as improvement in the active surface center due to co-doping of nitrogen and iron.
3.8. Catalytic activity towards the reduction of 4-NP
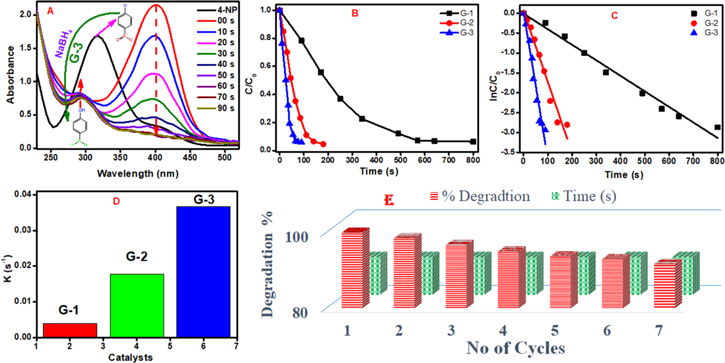
The pollution of water by phenol and phenolic compounds is attracting great scientific attention due to environmental concerns. Among all, nitro-phenols are the highest refractory pollutants as they are by-products of insecticides, herbicides, synthetic dyes, and pesticides, etc. This study is important academically as well as technologically due to the great demand for aromatic amino compounds in organic synthesis and industries. To investigate the catalytic activity of the synthesized materials quantitatively, a well-established model catalytic reaction under ambient conditions was applied for the reduction of 4-NP to 4-AP in the presence of NaBH4, and the progress of the reaction was observed spectrophotometrically. It could be seen from figure 7(A) that the absorption peak of 4-NP is shifted from 317 to 400 nm due to the formation of phenolate ions after the addition of NaBH4. Figure 7(A) exhibits the decrease in the absorption peak of 4-NP (400 nm) after the addition of catalyst (G-3). The spectral changes were quantitatively noticed with the time that completely vanished after 90 s in the presence of G-3 indicating the reduction of 4-NP. A new peak at λmax = 295 nm appeared, which is corresponding to the production of 4-AP, indicating conversion of 4-NP to 4-AP as shown in figure 7(A) [88]. Figure 7(B) shows the change in concentration versus time in the presence of G-1, G-2, and G-3 and exhibiting a complete reduction of 4-NP. G-3 displays the highest reduction of 4-NP among all catalysts due to the presence of nitrogen and iron dopants and their synergistic effect. Interestingly, the plot between ln C/C0 versus time gives a straight line indicating pseudo-first-order reaction kinetics with respect to the concentration of 4-NP only as the NaBH4 was present in excess as shown in figure 7(C). It could be seen from figure 7(C) that the reduction of 4-NP is well fitted with linear correction for all catalysts. The kinetic reaction rate constants k were calculated from the slopes of linear fitting and shown in figure 7(D). The above results precisely express that G-3 shows the highest reduction of 4-NP compared to other catalysts due to the presence of a combination of nitrogen and iron dopants that play an effective role in catalysis due to the synergistic effect. Industrial application is an important aspect that determines the practical utility of a catalyst. Therefore reusability and stability of the prepared catalyst (G-3) were also examined by reusing it for 7 successive periodic cycles as shown in figure 7(E). Herein, 4-NP was periodically added into the stock solution of converted 4-AP containing NaBH4 and the catalyst. G-3 catalyst exhibiting strong stability (92%) after being reused for seven cycles indicates its great potential for the reduction and conversion of 4-NP to 4-AP.
Figure 7. (A) Time-dependent UV–visible spectra for the reduction of 4-nitrophenol in the presence of G-3, (B) conversion of 4-nitrophenol to 4-aminophenol as a function of time, (C) reaction kinetic as a function of time, and (D) variation of the rate constant in the presence of G-1, G-2 and G-3 and (E) stability for the degradation of 4-NP during seven repeated cycles in the presence of G-3.
Download figure:
Standard image High-resolution image3.9. Proposed mechanism for the reduction of 4-NP to 4-AP in the presence of G-3
Based on the above results and the literature, a plausible mechanism for the reduction of 4-NP to 4-AP in the presence of NaBH4 over G-3 catalyst is given in figure 8. It has been reported that this model reaction is a thermodynamically feasible process as this reaction involves standard reduction potential for 4-NP/4-AP (0.76 V) and H3BO3/BH4− (−1.33 V) versus normal hydrogen electrode, but this reaction is kinetically blocked in the absence of the catalyst [89]. Generally, 4-NP shows an absorption peak at 317 nm, but after the addition of NaBH4 peak immediately undergoes a redshift to 400 nm due to the generation of a 4-nitrophenolate ion at the alkaline condition as shown in figure 7(A). However, the peak at 400 nm remains unchanged for a long period of time in the absence of catalyst, indicating 4-NP is stable in the presence of NaBH4. Whereas after the addition of catalyst (G-3), peak at 400 nm (4-nitrophenolate ion) start decreasing gradually indicating the reduction of 4-NP. A new absorption peak at 295 nm (4-AP) appears suggesting the successful conversion of 4-NP to 4-AP as shown in figure 7(A). The catalytic reduction is carried out on the surface of the catalyst (G-3), where adsorption of BH4 − and 4-NP take place. In this process, π–π stacking interaction plays an important role in the binding of 4-NP and reduced graphene sheets leading to promoting the reduction reaction [90, 91]. However, BH4 − and 4-nitrophenolate ions show a very high energy/kinetic barrier due to mutual repulsion between two negative ions, which is possible down by the presence of a catalyst (G-3) [92]. Therefore, the presence of a catalyst (G-3) is compulsory, which stimulates the catalytic reduction by transferring the electron from BH4 − to 4-nitrophenolate ion and also generate active hydrogen atoms via the cleavage of the B–H bond [93]. The generated active hydrogen atoms are thermodynamically unstable and therefore readily react with 4-nitrophenolate ions and as a result, nitroso compound is produced. After that hydroxylamine compound is produced by the reductive addition of two hydrogen atoms and then finally aniline compound is produced in the slow reaction step as shown in figure 8.
Figure 8. Schematic illustration for the reduction of 4-NP to 4-AP in the presence of NaBH4 over G-3 catalyst.
Download figure:
Standard image High-resolution image4. Conclusion
Herein, a low-cost, active, and stable non-precious metal-free catalyst for the first time was developed. A simple solvothermal strategy was employed for the synthesis of robust and attractive nitrogen and iron co-doped reduced graphene oxide. The reduction of G-1 was done in situ by hydrothermal treatment in a single step which increased the active sites on reduced graphene oxide. A highly potent G-3 catalyst was prepared with active nitrogen ions and homogenously installed iron species on reduced graphene oxide. Modified reduced graphene oxide provides a large surface area, structural defects, high conductivity, and outstanding catalytic performance. The improved catalytic activity of the as-prepared G-3 catalyst towards hydrogen production and 4-NP reduction may be due to the modulation of the electronic structure of graphene oxide to reduced graphene oxide. This may have happened as a result of co-doping with nitrogen and iron and their strong positive synergistic effect as well as enhancement in the active sites. Therefore, it is assumed that this new Fe and N co-doped reduced graphene oxide can be used as a low-cost, decisive, and booming catalyst to attain dynamic conversion of hazardous waste 4-NP to 4-AP and hydrogen production. The present work introduces a facile approach for the engineering of innovative and lightweight catalysts. This also exposes the great potential of doping for tailoring the applications of graphene oxide and may pave a new way for architecture and designing a stable non-precious metal-free catalyst.
Acknowledgments
This study was supported by the Regional Industrial Technology Development Program (P0017744) funded by the Ministry of Trade, Industry & Energy (MI) of Korea, and the National Research Foundation of Korea (NRF) [grant number:NRF-2018R1A1A3A04076752] funded by the Korean government (MSIT). Waseem Raza acknowledges the financial support from the Department of Science and Technology (DST), Government of India through the National Post-Doctoral Fellowship (NPDF No. PDF/2016/001471). Department of Chemical Enginerring, Indian Institute of Technology are greatly acknowledged.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).