Abstract
Magnetic iron oxide nanoparticles are among metal nanoparticles that attract huge attention in many biotechnological fields especially in the biomedical area. Their extensive capabilities and easy separation methodology drive them to be an interesting point to many researchers. Biosynthesis is of a major importance among different methods of nanoparticles production. Microbial synthesis of these nanoparticles by bacteria and yeasts have been reported on a wide scale. However, biosynthesis using halophilic archaea is still in an early stage. This study reveals the first contribution of the haloarchaeon Halobiforma sp. N1 to the nanobiotechnology field. It reports a rapid and economical one-step method of fabricating functionalized superparamagnetic iron oxide nanoparticles and their feasibility for hyperthermia treatment for cancer therapy. Herein, we have focused on optimizing the quantity of these fascinating nanoparticles, obtaining a very high yield of 15 g l−1 with high dispersion in water solution. Their unique characteristics enable them to participate in medical applications. They are nearly spherical in shape with a high degree of homogenity and uniformity with average diameter of 25 ± 9 nm. Also, the magnetic properties and elemental structure of the formed nanoparticles tend to be superparamagnetic like behavior with saturation magnetization of 62 emu g−1 and purity of 98.38% of iron oxide, respectively. The specific absorption rate (SAR) was measured and the particles induced significant heating power at lower frequencies which is a promising result to be applied for in vitro/in vivo hyperthermia studies in the near future.
Export citation and abstract BibTeX RIS
Introduction
Magnetic nanoparticles (MNPs) attain a great attention not only for the fundamental scientific interest but also for biomedical applications such as targeted drug delivery systems, magnetic resonance imaging, hyperthermia cancer therapy, cellular labeling and separation, or bio-sensing. Most of MNPs show superparamagnetic behavior, which allows them to gain magnetism in an applied alternating magnetic field (AC) and lose it upon the removal of the field. This property was fully realized when they were used as drug delivery agents, whereby chemotherapeutic drugs can target the desired locations in the body when external magnetic field is applied [1]. Iron oxide MNPs exist in many types; magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3) are the most common forms [2]. The chemical stability and biocompatibility of these MNPs have been providing immense benefits to medicine [3]. The application of small magnetic iron oxide nanoparticles (MIONPs), has been practiced and intensively developed for nearly 40 years [4].
One of the most promising applications of these fascinating nanoparticles is localized hyperthermia which is a clinical approach aimed at raising the temperature of cancerous regions of the body to 40 °C–45 °C [5] and able to induce cancer cell death by enhancement of the cytotoxicity of chemotherapy and radiotherapy. However, the hyperthermia clinical approach is still under research due to the agglomeration of MNPs leading to insufficient heat dissipation known as specific absorption rate (SAR) under therapeutic limit of applied AC magnetic field [5]. Therefore, researchers still need to optimize the synthesis of such MNPs before their application for in vitro/in vivo and clinical stages. The synthesis of MNPs through chemical and physical routes are popular and used extensively, but they have many drawbacks such as the participation of toxic chemicals and the production requirements of high-energy [6, 7], limiting their implementation in biomedical applications [7]. Thus, biological synthesis has become a promising, environmental/eco-friendly, nontoxic alternative pathway for the synthesis of MNPs [7].
Briefly, biosynthesis of nanoparticles is among the bottom-up methods for producing MNPs and more advantageous than physical and chemical methods [8–10]. Microorganisms possess an incredible capability of fabricating extremely specialized inorganic nanostructures. These magnificent skills of living organisms have attracted the attention of many material scientists towards these biological systems to gain knowledge and recover the skills for the exact formulation of nanostructures. Recently, the biomass or cellular extracts of many strains of bacteria have been used for synthesis of various types of metal oxide nanoparticles with a large scale of morphologies. For example, magnetotactic bacteria and S-layer bacteria whose cell walls are wrapped by a proteinaceous substance, are some of the widely used bacteria for the synthesis of these nanoparticles [11]. Bacterial synthesis of MNPs is a well-known approach as it is cost-effective, environmentally friendly, and nontoxic. It also possesses fine control over the magnetic and thermal properties of the MNPs, control of particle size, and its higher saturation magnetization (Ms) compared to typical chemically synthesized materials [12]. Biosynthesis is carried either intracellularly through cellular extract or extracellularly. However, not much attention has been directed towards the usage of cellular extract [7], because the extraction of NPs from the cell is difficult.
Extremophiles in particular are advantageous for potential applications due to their more pronounced ability to withstand harsh conditions that may occur within the process [13]. Archaea, a group of extremophilic bacteria has not been well investigated in the field of nanoparticle formation. Haloarchaea can adapt to a wide range of osmolality's. Some of them live near NaCl saturation, and they are the predominant population of thalassohaline and athalassohaline environments where salinity reaches up to 300 g l−1 and contribute to the red coloration of solar salt crystallizer. Haloarchaea are also known to encounter metals in their environment, but their metal tolerance has not been well documented [14].
Biosynthesis of MIONPs using halophilic archaea has not been demonstrated yet. They are advantageous in many respects. They have simple growth requirements and the presence of high salt concentrations in their growth media prevents any kind of contamination from other organisms. Therefore, the requirements for sterile conditions can be reduced [15] and the ability of cells to lyse easily will lead to the release of intracellular and membrane components. Although biosynthesis approaches possess many advantages such as nontoxicity of biosynthesized NPs, the major disadvantages are the low yield-production and nonhomogeneous particles size.
Therefore, this work presents for the first time, the one-step large-scale intracellular production of superparamagnetic MIONPs by using the haloarchaeon Halobiforma sp. N1 isolated from Wadi-Al-Natrun, Egypt.
Materials and methods
Bacterial strain and growth condition
The Haloarchaeon Halobiforma sp. N1 isolated from Wadi Al-Natrun, Egypt was used throughout this study. It was cultivated on Horikoshi-I medium [16] composed of (g l−1): Glucose 10; peptone 5; di-potassium hydrogen phosphate 1; magnesium sulfate 0.2; sodium carbonate 10, casein hydrolysate 1, and sodium chloride 175, pH was adjusted to 9. No sterilization was required.
Biosynthesis of functionalized MIONPs
Halobiforma sp. N1 was grown in liquid Horikoshi medium for 7 days under shacked condition at 37 °C and 150 rpm. Culture was centrifuged for 10 min at 6000 rpm to separate cellular biomass from the culture filtrate. Both cell mass (CM) and filtrate were examined for the biosynthesis of MIONPs by challenging 10 ml bacterial supernatant or 0.13 g cellular biomass with 10 ml ferrous sulfate (FeSO4) of 1 mM concentration and incubated at shaker incubator for 48 h at 37 °C, using a modified method of Fatemi et al [17]. Liquid Cultures of Haloarchaeon Halobiforma sp.N1 were prepared using Horikoshi-I medium at pH = 9 and the bacterial inoculum was always set to be 10% (V/V) of the experimental volume. The halo-archaeal morphological structure was observed by using phase contrast microscopy (figure S1(A), available online at stacks.iop.org/NANO/32/09LT01/mmedia), scanning electron microscopy (SEM) (figure S1(B)) and Gram staining technique (figure S1(C)). The cells appeared to be Gram-negative cocci of 0.25 microns in liquid medium. We observed positive results by the formation of particles that show high dispersion in water solution and a magnetic attractive force towards a permanent magnet.
Screening for optimum physical conditions using 2n factorial design
A complete factorial design that is useful with a small number of factors was applied. It reveals the interaction between physical and chemical parameters while the effect it implies on the biosynthesized MIONPs is recognized. The factors studied were the concentration of precursor (FeSO4), FeSO4 volume, bacterial biomass, and pH. Table 1 illustrates the abbreviation and concentration of each factor under study at basal (0), low (−), and high levels (+). Accordingly, the experiment included sixteen possible treatment combinations corresponding to 24.
Table 1. Abbreviation and concentration of each factor at −, 0, + levels.
| Factor | Symbol | — | Level | + |
|---|---|---|---|---|
| 0 | ||||
| Cell mass (mg) | CM | 0.5 | 1 | 2 |
| FeSO4 conc. (mM) | FC | 50 | 100 | 200 |
| FeSO4 volume (ml) | FV | 10 | 50 | 100 |
| pH | pH | 5 | 7 | 9 |
Characterization of functionalized MIONPs
Several characterization techniques have been carried out for a better understanding of the comprehensive properties of the produced MNPs. These include x-ray diffraction (XRD) using Bruker XRD-D2 Phaser, high resolution transmission electron microscopy (TEM) using JEM-2100UHR, and energy dispersive x-ray (EDX) analysis using a SEM Jeol JSM- 5300 equipped with an at EDX Unit. Magnetic properties was measured using Lakeshore 7410 vibrating sample magnetometer (VSM) and functionalization groups were confirmed using Fourier transform infrared spectrometry (FTIR) by Bruker Tensor 37. These techniques allow us to study crystal phase structure, surface morphology, size distribution, chemical composition, the spatial distribution of the functional group, and magnetic properties of the synthesized MIONPs, respectively. Feasibility for hyperthermia of 5 mg MIONPs dispersed in 1 ml of water was measured by using a G2-D5 Series Multi-mode 1500 W Driver from Nanoscale Biomagnetics. The sample solution was placed into a G2-D5 coil, where the alternating magnetic field and frequencies were applied. The induced temperature of the solution was measured and recorded using a fiber-optic sensor. The feasibility of MIONPs for magnetic hyperthermia treatment depends considerably on the SAR of these particles dispersed in water solution under applied AC magnetic field. The SAR expresses the heating capabilities of MIONPs in water solution. The applied AC magnetic field was in the range of 100–500 Oe and at frequencies of 144, 164, and 304 kHz.
Results and discussion
Biosynthesis of functionalized MIONPs and screening for optimum physical conditions using 2n factorial design
The intracellular biosynthesis of the MIONPs from the haloarchaeon Halobiforma sp. N1 is unconventional. No reports are available on MIONPs formation from haloarchaea, while some literatures reported the use of some archaeal species in silver nanoparticles formation such as Halococcus salifodinae BK3, BK18 [14, 18]. We successfully biosynthesized MIONPs by the formation of nanoparticles when bacterial biomass was challenged against a 1 mM FeSO4 substrate solution. S-layer (surface layer) proteins/glycoproteins commonly found in the cell envelope of archaea are capable of self-assembling into monomolecular layer on suitable surfaces or suspension [19]. Results were recorded visually by observing the magnetic attractive force of MIONPs towards amagnet. First experiments showed formation of only a small amount of particles, but by the aid of 24 factorial experimental design, a full conversion occurred. Conditions employed in trial #15 appeared to provide successful biosynthesis of MIONPs (table 2). The conditions applied in trial #15 are CM 2 mg/100 ml, FeSO4 concentration (FC) 50 mM, FeSO4 volume (FV) 100 ml, and pH 9. The solution turned to a dark brown color with a high attraction of particles towards the permanent magnet leaving a clear solution (figure 1). Moreover, under this condition the highest yield (∼15 g l−1) was obtained showing a high attraction force towards the magnet.
Table 2. Design matrix and results of the 24 factorial experimental design.
| Trial | Factors under study | Variables | ||||
|---|---|---|---|---|---|---|
| CM | FC | FV | pH | Attraction to magnet | ||
| 1 | Base | − | − | − | − | − |
| 2 | CM | + | − | − | − | − |
| 3 | FC | − | + | − | − | − |
| 4 | FV | − | − | + | − | − |
| 5 | pH | − | − | − | + | − |
| 6 | CM, FC | + | + | − | − | − |
| 7 | CM, FV | + | − | + | − | − |
| 8 | CM, pH | + | − | − | + | − |
| 9 | FC, FV | − | + | + | − | − |
| 10 | FC, pH | − | + | − | + |

|
| 11 | FV, pH | − | − | + | + | − |
| 12 | CM, FC, FV | + | + | + | − | − |
| 13 | CM, FC, pH | + | + | − | + | − |
| 14 | FC, FV, pH | − | + | + | + | − |
| 15 | CM, FV, pH | + | − | + | + |

|
| 16 | CM, FC, FV, pH | + | + | + | + | − |
Figure 1. Full color change and formation of MIONPs with trial #15 of the 24 factorial design observed using permanent magnets.
Download figure:
Standard image High-resolution imageThis experiment aimed to reach higher yield than that of the primary experiments, but the results were fascinating and surprisingly turned to a large-scale production of functionalized MIONPs. It is clear too from figure 1 that the formed MIONPs are highly dispersive in solution, which is an important property for our future in vitro/in vivo studies of hyperthermia. However, to confirm the optimization of these biosynthesized MIONPs, the physical properties such as crystallography, size, homogeneity, magnetic, and hyperthermia must be characterized.
Characterization of functionalized MIONPs
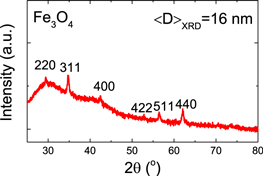
The crystal phase structure of the optimized MIONPs was characterized using XRD [20, 21] as shown in figure 2. Figure 2 shows XRD patterns of the synthesized MIONPs. Typically, the 2θ peaks found at 30.1, 35.5, 42.6, 53.6, 57.0, and 62.8° are assigned to diffraction of the (220), (311), (400), (422), (511), and (440) planes, respectively. Those peaks are characteristic for inverse spinel, with O2− ions forming a face-centered cubic magnetite (Fe3O4) lattice and iron cations occupying interstitial sites as referenced by Shukla et al [22] and Rasouli et al [23]. In addition, from the full width at half maximum of the peaks, we determined the average crystalline size using Scherer equation [24] to be 16 nm.
Figure 2. X-ray diffraction (XRD) pattern of Fe3O4 MIONPs.
Download figure:
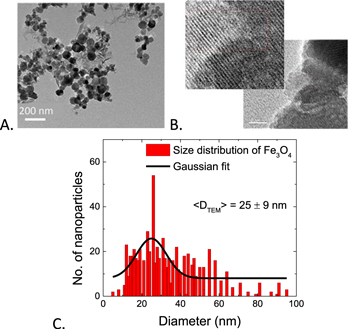
Standard image High-resolution imageThe morphology including the particle size of the formed Fe3O4 MIONPs was determined using TEM as shown in figure 3. The TEM image (figure 3(A)) illustrates that the particles have spherical like shape. High-resolution TEM images showed polycrystalline features and d spacing (lattice fringes) of 0.25 nm, which is consistent with the XRD data of 311 planes (figure 3(B)) of magnetite (Fe3O4) nanoparticles. In addition, the particle size distribution shown in figure 3(C) was determined using more than 250 particles and was fitted using Gaussian distribution to reveal average size of 25 ± 9 nm. The result from TEM was in reasonable agreement with XRD where the calculated crystalline size using Scherer equation lies in the range of size distribution determined from TEM.
Figure 3. TEM of biosynthesized iron oxide nanoparticles (scale is 200 nm) (A), high-resolution TEM showing polycrystalline features and d spacing (scale is 10 nm) (B), and size distribution of MIONPs (C).
Download figure:
Standard image High-resolution imageThe obtained results from TEM analysis are comparable with other biologically synthesized MIONPs by Fatemi et al [17] who reported spherical IONPs from Bacillus cereus with size ranges from 18.8 to 28.3 nm, while Sundaram et al [12] reported the spherical biosynthesized IONPS by Bacillus subtilis with size range between 60 and 80 nm. Spherical iron oxide nanoparticles of 20 nm were observed by high resolution of TEM in green synthesized iron oxide by Amutha and Sridhar [24]. VG and Prem [25] reported the green synthesis of nearly square iron oxide nanoparticles using Phyllanthus niruri extract with size of 10 nm. Polycrystalline character of iron oxide nanoparticles synthesized by Pyrodictium delaneyi and Pyrobaculum islandicum was reported by Kayshap et al [26]. The biosynthesized Fe3O4 MIONPs in our study were of higher uniformity and smaller size compared to other microbial synthesized MIONPs; however, our study can be extended to present particle size control in the future.
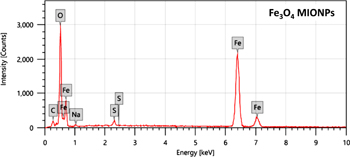
The purity of our MIONPs was determined by the EDX analysis as shown in figure 4. EDX spectrum confirms the formation of pure iron oxide nanoparticles. It displays 94.71% of iron oxide purity. The EDX confirmed the presence of both iron and oxygen in NPs. The composition was 70.45% and 24.26% of oxygen. Since our nanoparticles were biosynthesized from biological source, it confers some impurities that explains the low intensities in XRD. The impurities were displayed in the EDX as peaks for C, Na and S elements as seen in figure 4. By comparing our results to the published literatures, we found that Jagathesan and Rajiv [27] produced iron oxide nanoparticles biosynthesized from Eichhornia composed of 77.08% of iron and 22.97% of oxygen. Rasouli et al [23] reported that the EDX spectra of the Fe3O4-NPs synthesized by co-precipitation in presence of natural honey confirm the presence of elemental Fe without any impurity peaks. Therefore, the MIONPs biologically synthesized using natural honey show a very high purity as well as the NPs biosynthesized in this study. However, the production of large quantities of NPs from natural honey will be of a high cost, in contradiction with our method where the NPs were biosynthesized in an eco-friendly and cost-effective manner in large quantities (15 g l−1).
Figure 4. EDX spectrum of biosynthesized iron oxide nanoparticles.
Download figure:
Standard image High-resolution imageIn order to investigate the magnetic properties of our biosynthesized MIONPs, we measured the magnetization dependence on magnetic field up to 2 T using VSM. Figure 5 represents the closed hysteresis loops revealing superparamagnetic-like behavior of our biosynthesized Fe3O4 MIONPs with high saturation magnetization (MS) of 62 emu g−1, small coercivity (HC) and small spontaneous magnetization. Based on these results, the magnetization studies can provide another alternative to obtain more information on the magnetic domain size (Dmag) from the initial slope of the closed hysteresis loop [28, 29]. Therefore, one can estimate an upper boundary for the magnetic domain size to be 8 nm which confirms the superparamagnetic behavior of our Fe3O4 MIONPs.
Figure 5. Magnetization dependence on magnetic field at room temperature for biosynthesized Fe3O4 MIONPS.
Download figure:
Standard image High-resolution imageIn comparison to the published work by others, the magnetic properties of the iron oxide nanoparticles of Krishnan et al [3] showed a magnetization of 50 emu g−1, and that of Walujkar et al [30] showed magnetization value of 28 emu g−1. Fatemi et al [17] showed IONPs with MS of 59 emu g−1. Darroudi, et al [31] showed IONPs with MS ranging 17.5–42.1 emu g−1. Kandpal, et al [32] reported Fe3O4 with MS of 66 emu g−1. In comparison with the previously mentioned literatures, the MIONPs produced throughout this study are of very high magnetization compared to that of NPs produced by biological methods, and comparable to those NPs produced by chemical methods such as co-precipitation with less cost-effective microbial method using Halobiforma sp.N1.
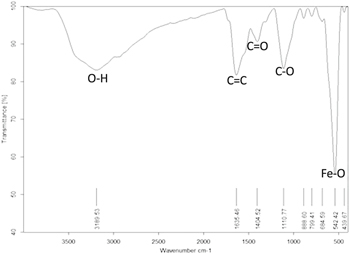
In order to investigate the functionalization groups that are responsible for the high dispersion in water solution and formed on the surface of the MIONPs, FTIR have been used. In the IR spectrum of biosynthesized nanoparticle (figure 6), absorption bands around 3189.53 cm−1 are characteristic stretching vibration of the hydroxyl functional group (O–H) on the surface of nanoparticles. The stretching vibration of the carboxylate group (C=O) is observed around 1404.52 cm−1. The stretching vibration of the C=C group was also localized at 1635.46 cm−1. The absorption band around 1110.77 cm−1 is assigned to the stretching vibration of the C–O group. Multiple peaks appearing at 439.67 and 542.42 cm−1 are due to Fe–O in conformity with the literature Kumar et al [33]; Srivastava et al [14], and Mirza et al [34]. The formation of Fe3O4 NPs was confirmed by characteristic peaks laying in the region between 400 and 600 cm−1 corresponding to Fe3O4 as mentioned by VG and Prem [25] in which the IR spectrum also showed a peak at 583.02 cm−1 corresponding to the Fe-O stretching band of magnetite (Fe3O4). Also the FTIR analysis and presence of organic groups (C=O) and C=C group observed around 1404.52 cm−1 and 1635.46 cm−1 respectively may act as a natural coating for the particles which explain the reduced agglomeration and the high dispersion of the highly magnetic Fe3O4 nanoparticles. As previously reported, in FTIR spectrum the protein functional groups may confer the effect of a capping agent for nanoparticles due to sorption of proteins on surface of nanoparticles from biological routes [17]. Comparison of the different iron oxide nanoparticles from different routes of synthesis are illustrated in table 3.
Figure 6. FTIR analysis of biosynthesized Fe3O4 MIONPs.
Download figure:
Standard image High-resolution imageTable 3. Comparison between routes of synthesis, particle shape, and particle size.
| Literature | Route of synthesis | Particle shape | Particle size | Magnetization | Dispersion in Solution |
|---|---|---|---|---|---|
| (nm) | (Ms) | ||||
| Our work | Biological by Halobiforma sp.N1 | Spherical | 26 nm | 62.7 emu g−1 | High dispersion |
| Fatemi et al 2018 [16] | Biological by Bacillus cereus strain HMH1 | Spherical | From 18.8 to 28.3 nm | 58.96 emu g−1 | High dispersion due to presence of a protein cap |
| Rasouli et al 2018 [22] | Co-precipitation method using NaOH and honey | Spherical | Between 2.22 nm and 3.21 | 28.98 emu g−1 | Well dispersed |
| Walujkar et al 2019 [29] | Biological by Bacillus sp. with visible light active catalytic activity | Mostly cubic, few spherical in shape | 81.3 nm | 28.1 emu g−1 | NA |
| Tadic et al 2019 [35] | Colloidal chemistry and magnetic field-induced self-assembly of nanoparticle clusters. | Chain like morphology and core-shell structure | 8–12 nm | 3.0 emu g−1 | NA |
| Aivazoglou et al 2018 [36] | Microwave-assisted synthesis of iron oxide nanoparticles | uniform faceted particles | 8.5 nm ± 0,5 nm | 70 emu g−1 | Partial agglomeration |
| Krishnan et al 2017 [3] | β-cyclodextrinEDTA-Fe3O4 nanoparticleconjugated nanocarriers | Spherical shape | 168 nm | 50 emu g−1 | Homogenous dispersion |
We have found that our nanoparticles biosynthesized by Halobiforma sp.N1 have a significant MS; also, the obtained size is very good in such a one-step approach, which is cost-effective, easy, and not time consuming. The biological synthesis offered our MIONPs a protein cap from cellular material leading to a very high dispersion, solving the agglomeration problem of highly magnetic IONPs.
SAR of Halobiforma sp. N1 MIONPs
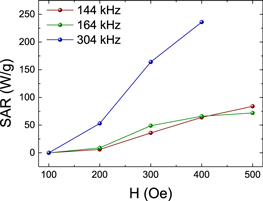
The SAR expresses the heating ability of a magnetic material and, therefore, the feasibility of a material for application in magnetic hyperthermia [37]. The value of SAR increases as the frequency of AC magnetic field increases [38]. The SAR value is calculated from the initial slope of the T versus t curves (figure S2, supplementary information) using the following equation:
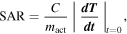
Here, mact is the mass of the magnetically active material and C the heat capacity of water (C = 4.18 J g−1 K−1). As shown in (figure 7), at f = 304 kHz SAR values are 50, 155, and 230 W g−1 corresponding to the magnetic field applied (H) of 200, 300, and 400 Oe, respectively. At f = 164 kHz, SAR values are 25, 50, 75, and 77 W g−1 corresponding to applied magnetic field (H) of 200, 300, 400, and 500 Oe, respectively. At f = 144 kHz, SAR values are 25, 45, 75, and 80 W g−1 corresponding to applied magnetic field (H) of 200, 300, 400, and 500 Oe, respectively. It is clear that SAR increases when applied field and frequencies are increased.
Figure 7. SAR dependence on AC magnetic field at different frequencies of 144, 164 and 304 kHz for Fe3O4 MIONPs.
Download figure:
Standard image High-resolution imageIn addition, intrinsic loss power known as normalized SAR, was calculated as shown in FIGURE S3, confirming that SAR of Halobiforma sp. N1 MIONPs induced a very good heat at the low tested frequencies in comparison to all the published values for commercial materials prepared by physical and chemical methods [39]. Therefore, the biosynthesized MIONPs in this study have very promising properties to be used efficiently for the magnetic hyperthermia in vitro/in vivo and clinical approach in the future.
Conclusions
We have successfully produced superparamagnetic Fe3O4 MIONPs using a one-step biologically safe, environmentally friendly and cost-effective method. To be specific, the present work reported a novel technique for the intracellular biosynthesis of MIONPs from the haloarchaeon Halobiforma sp.N1. We have succeeded to overcome the low yield and the agglomeration problem, which are major drawbacks of biosynthesis techniques. Biosynthesized magnetic NPs exhibited a very high dispersion which is necessary for biomedical applications. Under our experimental conditions, a significant high yield of the nanoparticles (∼15 g l−1) was obtained, which is high compared to other methods. Finally, economically, this method is very promising in the biosynthesis of MIONPs at low cost and of unique properties that allow their use in the medical field. The MINOPS bio-fabricated by our strain showed very high purity (98%). Particles are uniform and almost all have the same size (25 ± 9 nm), which is a unique character compared to other synthesized MIONPs. The MIONPs produced throughout this study showed a high magnetization similar to those produced through chemical methods. Our biosynthesized MIONPs showed feasibility to hyperthermia treatment of cancer, which is a promising approach in cancer therapy. The nanoparticles have a good heat absorption rate at low frequency, which is safer for clinical treatment application. Based on our results, in vitro/in vivo studies will be performed in the near future.
Acknowledgments
AAE acknowledges the start-up and rising stars funds by UTEP and UT-system respectively. In addition, the research reported in this paper was partially supported by the US-National Science Foundation under award number 2009358, and National Institute of General Medical Sciences of the National Institutes of Health under linked award numbers RL5GM118969, TL4GM118971, and UL1GM118970.
Conflicts of interest
There are no conflicts to declare.
Authors contribution
NFAS synthesized, characterized the samples and drafted the manuscript under supervision and guidance of SS, HG, and AAE S.S.A helped NFAS for the synthesis, microbiological data analysis and drafting the manuscript. AMY helped NFAS in doing the experimental lab' work. BPM did measure and analyze the hyperthermia part with guidance and supervision of AAE AAE designed the idea, analyzed and discussed the results with the co-authors, revised and edited the manuscript.