Abstract
Colloidal perovskite CsPbX3 (X = Cl, Br, I) nanocrystals (CPNCs)/polymers composites have attracted extensive attention due to their potential to be developed as flexible phosphor films for lighting applications. However, to maintain high quantum efficiency and photo stability of CPNCs in such composites remains a daunting challenge. Here, we have demonstrated a layered composite structure consisting of CPNCs and polydimethylsiloxane (PDMS) with multi-color emission and long-term stability. By tuning the molar ratio between CsPbCl1.58Br1.42 and CsPbBr1.35I1.65, flexible fluorescent films as down-converter layers with a high luminescent efficiency and a controllable color temperature spanning from 3194 K to 5901 K have been demonstrated. Furthermore, due to embedding inside such composites, the quantum efficiency of CPNCs exhibited negligible changes during seven months in ambient conditions. The carrier dynamics based on time-resolved photoluminescence (PL) and transient absorption spectroscopy reveal that the hot electron tunneling and trapping process are significant in the composite film. This work provides a good understanding of CPNC materials in complex composite for the development of flexible, robust, color controllable fluorescent films for lighting applications.
Export citation and abstract BibTeX RIS
1. Introduction
Colloidal perovskite CsPbX3 (X = Cl, Br, I) nanocrystals (CPNCs) have shown extraordinary performance in light-emitting diodes (LEDs), due to their excellent photophysical properties, such as high photoluminescence quantum yield (PLQY) and narrow emission line width [1–9]. More importantly, the energy bandgap of CPNCs can be tuned by a simple mixing-induced anion exchange to cover the entire visible spectral region of 400–700 nm, further improving their favorability for lighting applications [10–13]. Unfortunately, CPNCs still suffer from stability issues since the material structure is sensitive to external stimuli (e.g. temperature, oxygen and moisture environment) [14–18]. Enhancing the stability of CPNCs in LEDs is crucial to bringing the materials towards practical applications. To achieve this goal, utilization of polymers (e.g. PMMA [19], PVP [20] and EVA [1]) to encapsulate CPNCs has been demonstrated as an efficient strategy to stabilize the emission properties of CPNCs. Specially, the PMMA to encapsulate perovskite nanowires are stable for over 30 d [19], the PVA to encapsulate to perovskite nanocrystals show high reproducibility [20], and the EVA to encapsulate to CsPbBr3 perovskite quantum dots are repeatedly bent for 1000 cycles at a constant photoluminescence intensity [1]. However, the majority of the work focuses on the performance characterization, while the mechanism responsible for the stabilization remains elusive.
Herein, we have proposed and demonstrated highly flexible fluorescent films for down-converting layers based on CPNC/PDMS composite films. The proposed flexible films exhibit a wide range of tunable correlated color temperatures (CCT) with negligible performance degradation after more than seven months of storage in ambient conditions. Through analyzing the carrier dynamics of CPNCs in polymers and in solutions, we have revealed that the PDMS can significantly maintain the quantum efficiency of CPNCs. This work offers a deeper insight into the quenching mechanism of CPNCs and proposes enlightenment for the design of perovskite-based light emitting devices.
2. Experimental section
The CsPbCl3, CsPbBr3 and CsPbI3 nanocrystals were initially synthesized by a hot-injection method, which is described in [9]. In order to get a blue emission solution, we mixed the CsPbCl3 with CsPbBr3 nanocrystal solutions in the ratio of 1:1 by volume. Similarly, mixing CsPbBr3 with CsPbI3 (at 1:1 volume ratio) generates an orange emission solution. Then we diluted the concentrations of blue and orange emission solution to 10 mg ml−1 using hexane. Finally, we extracted 5 ml from each of these CPNC solutions, then injected this into a separate PDMS curing agent resin solution in a weight ratio of 10:1. The concentration of CPNCs in PDMS is 10 mg ml−1 as well. After stirring under nitrogen (N2) gas for 5 h, the liquid mixture was deposited onto glass substrates by spin-coating. The flexible CPNC/PDMS films were ultimately stripped off from glass substrates after annealing at 80 °C. In order to demonstrate the flexible and tunable warm and cold emission film, we adjusted the molar ratio of the CPNCs and placed the different emission thin films on UV-light emitting chip.
3. Results and discussion
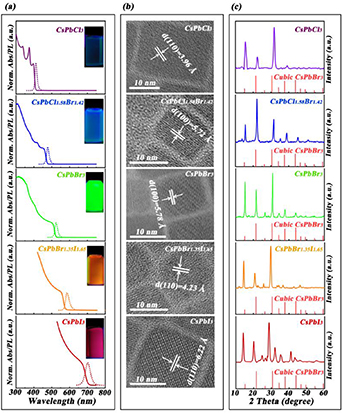
The edge of these CPNCs absorption spectra and the peak wavelength of PL emission was shifted from 400 to 700 nm by tuning the halogen element ratio in our synthesis. Figure 1(a) shows five different emission spectra of CPNCs corresponding to CsPbCl3 (411 nm), CsPbCl1.58Br1.42 (465 nm), CsPbBr3 (524 nm), CsPbBr1.35I1.65 (583 nm), and CsPbI3 (694 nm), respectively. The photos of these CPNCs emission solutions are inserted as insets into figure 1(a). These CPNCs exhibited cubic perovskite phase, consistent with previous reports [21–26]. Their average sizes were 10 to 20 nm. The different distinct lattice fringe spacing distances are 3.96 Å, 5.72 Å, 5.78 Å, 4.23 Å, and 6.22 Å corresponding to the (110), (100), (100), (110), and (100) crystal planes of cubic CsPbBr3 crystal (JCPDS No. 54–0752 of XRD standard card), respectively (figure 1(b)). Certainly, the slight deviation from the cubic CsPbBr3 crystal is normal in fringe spacing distances due to the existence of halogen substitution. The XRD patterns show these CPNCs have cubic phase similar to the normal CsPbBr3 nanocrystal. Only the diffraction peaks are shifted gradually to a lower diffraction angle (2θ) range with the anion changing from chloride to bromide and iodide, owing to the enlarging lattice induced by the substitution smaller-radius halogens with larger radius ones in figure 1(c). In particular, the crystalline phases of CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 nanocrystal are unchanged despite their partial halogen substitution.
Figure 1. (a) Absorption and photoluminescence spectra of these CPNCs dispersed in hexane under 365 nm lamp excitation. (b) High-resolution transmission electron microscopy (HRTEM) images of these CPNCs. (c) XRD patterns of these CPNCs, respectively.
Download figure:
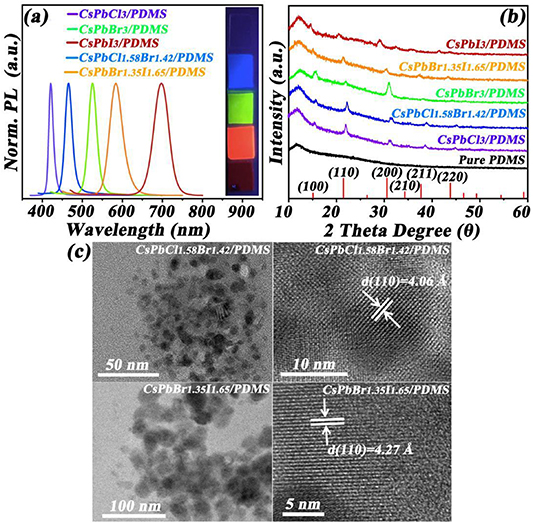
Standard image High-resolution imageFollowing the previous description, we injected the CPNCs into the PDMS polymer respectively. The colorful flexible polymer films contained CsPbCl3, CsPbCl1.58Br1.42, CsPbBr3, CsPbBr1.35I1.65, and CsPbI3 nanocrystals are presented as insets in figure 2(a). The PL peaks of CPNCs/PDMS composite film were centered at 416 nm, 466 nm, 526 nm, 586 nm, and 698 nm, with a narrow full width half maximum (FWHM) value of about 35 nm, which were similar to that of the CPNC solution. The result shows the PL peak of CPNCs in PDMS polymer is negligibly changed under the 365 nm excited after seven months storage in ambient conditions (figure 2(a)). The XRD patterns of the above CPNC/PDMS flexible films in figure 2(b) are typical of when the combination of crystalline phase and non-crystalline phase patterns coexist. Note that no sharp crystalline peaks are observed in pure PDMS flexible films, indicating a typical non-crystalline phase.
Figure 2. The optical properties and nano-structure of CPNCs in PDMS flexible films. (a) PL emission spectrum of CPNC/PDMS composites, and their photographs under the excitation of 365 nm. (b) XRD patterns of these flexible films. (c) HRTEM images CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 CPNC/PDMS flexible films.
Download figure:
Standard image High-resolution imageWith the CPNC mixed PDMS flexible films, some sharp crystalline peaks matched well with the main peaks in corresponding CPNCs cubic structure. This indicates that the crystalline phases of the CPNCs are partially maintained after having been embedded into PDMS flexible films. However, the HRTEM images in figure 2(c) show that the morphologies of the CPNCs in PDMS flexible films were different from those in hexane solution as presented in figure 1(b). It can be seen from figure 2(c) that some black nanoparticles with irregular shapes and disorder distribution are observed in the CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 CPNC/PDMS flexible films. The previous study also observed the similar irregular situation in the organic MAPbX3 QDs/PVA flexible films [26].
The main reasons could be summed up as follows: after the CPNCs were injected into PDMS resin, these original CPNCs were naturally damaged under polymer solution slowly and continuously. With further curing process, the mixed CPNCs appear obvious random characteristics in PDMS flexible films. In order to accomplish TEM tests, the CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 CPNC/PDMS flexible films need to be cut and thinned by ultrathin cryo-section technology. Nevertheless, the lattice fringes of the CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 CPNCs in PDMS flexible films are still observed in figure 2(c), where the fringe spacing distances of the CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 CPNCs are determined to be 4.06 Å and 4.27 Å, locating between the CPNCs in (110) crystal plane of CsPbCl3 (d= 3.98 Å), CsPbBr3 (d= 4.12 Å), and CsPbI3 (d= 4.43 Å) [26, 27]. It is an obvious monotonic increase trend of d from 3.98 Å to 4.43 Å, suggesting a monotonic decreasing trend of 2θ for the same crystal plane according to Bragg's law [28]. This, indeed, is reflected in experimentally measured XRD patterns as in figure 2(b). More importantly, the crystalline structure of the CPNCs in PDMS flexible films is still cubic, similar to CsPbBr3 crystals. The HRTEM results show clear lattice fringes for CsPbCl1.58Br1.42 and CsPbBr1.35I1.65 CPNCs which are unchanged due to embedding into PDMS flexible films.
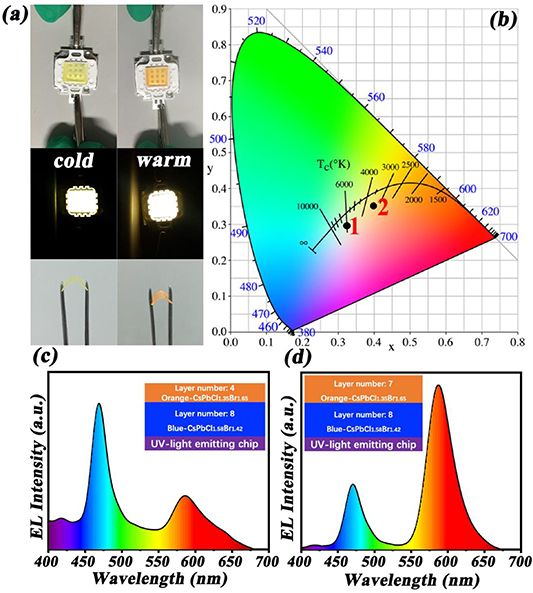
To demonstrate the color-tunable flexible fluorescent film for lighting purposes from cold to warm white light, the fluorescent film is designed with multi-layer film structures using the above CsPbCl1.58Br1.42 (blue emission) and CsPbBr1.35I1.65 (orange emission) CPNC/PDMS flexible films. The layered flexible films of the tunable white LEDs can be made by coating CsPbCl1.58Br1.42 (blue emission) CPNC/PDMS flexible films on the UV-light emitting chip, then followed by CsPbBr1.35I1.65 (orange emission) CPNC/PDMS flexible films. The thickness of each single layer in the multi-layer film structures is about 30 nm at a spin speed of 2000 rpm for 30 s. Through adjusting the layer thickness of CPNC/PDMS flexible films, the emission can be adjusted from cold through to warm light. We changed the layer thickness of CsPbCl1.58Br1.42 (blue emission) and CsPbBr1.35I1.65 (orange emission) CPNC/PDMS flexible films on the UV-light emitting chip by spin coating. Namely, 8:4 and 8:7 are the ratio of the blue to red layer number for the cold and warm white light in figure 3(a), respectively. Also, the designed multi-layer film structures exhibit highly flexible characteristics. Figure 3(b) shows the Commission Internationale de l'Eclairage (CIE) chromaticity coordinates of the two flexible white LEDs at the layer number ratio of 8:4 and 8:7. The two CIE chromaticity points are located at (0.3325, 0.2964) and (0.3996, 0.3503), corresponding to the CCT of 5901 K and 3194 K. It is obvious that the two CIE chromaticity points are all within white region, and their corresponding CCT values are associated with the cold and warm white light. The spectra of the white LEDs in figures 3(c)–(d) reveal the spectral components of cold and warm white light in the designed flexible fluorescent films (the film structures see illustrations). The intensity ratio of two emission peaks at 466 nm (blue) and 586 nm (orange) are controlled arbitrarily with the layer ratios of two materials, allowing a large range of tuning from cold to warm white light. Thus, the designed flexible fluorescent films with multi-layer film structures are simple and convenient for realizing the tunable fluorescent film for solid state lighting.
Figure 3. Device performance of the designed flexible fluorescent films for lighting applications with two layers ratios of blue-emitting CsPbCl1.58Br1.42 to orange-emitting CsPbBr1.35I1.65 CPNC/PDMS flexible films at 8:4 (cold) and 8:7 (warm). (a) Photographs of the devices and the flexible films. (b) CIE chromaticity coordinates images. (c), (d) Luminescence spectra of the devices in the visible spectral range (The film structures see in illustrations).
Download figure:
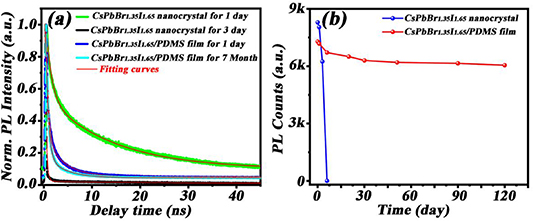
Standard image High-resolution imageIn order to investigate the optical stability of these composite CPNC flexible films, we explored the dynamic optical properties of the CsPbBr1.35I1.65 CPNC/PDMS flexible films. The characteristics of the CsPbBr1.35I1.65 CPNCs in PDMS flexible films and hexane solution are discussed and compared. Figure 4(a) shows the time-resolved PL of the CsPbBr1.35I1.65 in PDMS flexible films and hexane solution with different storage times, where the PL intensity shows double-exponential decay behavior with two time constants τ1 (fast) and τ2 (slow) as presented in equation (1):
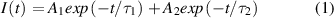
Figure 4. (a) PL decay of CsPbBr1.35I1.65 CPNCs in hexane solution and in PDMS flexible films after different storage time in ambient conditions. The red solid lines are double-exponential fits to these kinetics. (b) Intensity of PL for the CsPbBr1.35I1.65 CPNCs in the hexane and PDMS as a function of storage time.
Download figure:
Standard image High-resolution imagewhere I(t) is the PL intensity at time t; A1 and A2 are constants. And on this basis, the average decay lifetime (τave) is determined by the following equation (2) [29]:
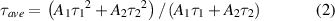
The calculated results show that the τave values in hexane solution decrease rapidly from 14.88 to 0.24 ns within 3 d, whereas the τave values in PDMS flexible films showed a negligible change from 2.68 to 2.18 ns during the 7 months of storage in ambient conditions. Obviously, the protection of PDMS flexible films makes the CsPbBr1.35I1.65 CPNCs more stable in long term. However, it is also noted that the τave value of 2.68 ns in PDMS flexible films for 1 d is far less than 14.88 ns in hexane solution for 1 d. The reason is most likely related to with the ligand dissociation of the CsPbBr1.35I1.65 CPNCs in PDMS flexible films, making the quick drop of luminescence efficiency at the beginning of process. Compared with these CPNCs in the hexane solution, their morphologies in PDMS flexible films, as shown in figure 2(d), are irregular and stacking to each other and form a larger cluster; thus causing the defect state and trapping, induced the short decay lifetime. However, in the long term, PDMS flexible films prevent these CPNCs from contacting with external environment such as oxygen and moisture etc Of course, hexane solution does not provide enough protection for the CPNCs due to diffusion of air or moisture, resulting in damage to these CPNCs in a very short time. Finally, we compare the PL emission intensity with CPNCs in the hexane and PDMS over a long period of time. The results show in the figure 4(b), signifying the stability of the CsPbBr1.35I1.65 CPNCs/PDMS over a long time.
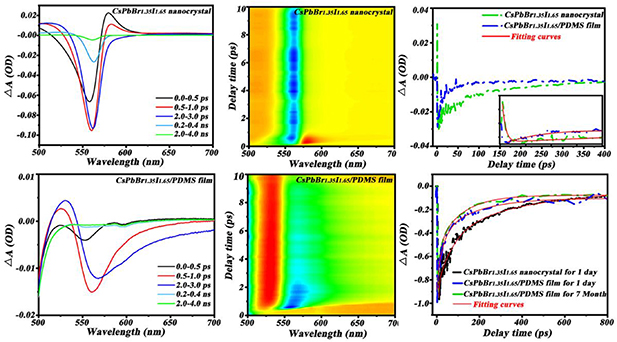
In order to further understand the carries dynamics in the flexible composite films, we utilized the ultrafast transient absorption spectroscopy to investigate the dynamic process of the CsPbBr1.35I1.65 CPNCs in hexane solution and PDMS flexible films. Briefly, a pump pulse at 450 nm with an average power intensity of 30 mW cm−2 was used to excite the CsPbBr1.35I1.65 QDs, and the induced absorption changes, as functions of both wavelength and time, were recorded by a delayed supercontinuum white light probe pulse with respect to the excited pulse. As shown in figures 5(a) and (d), two bleaching signals were located at 553 nm and 557 nm corresponding to the CsPbBr1.35I1.65 CPNCs in hexane solution and PDMS flexible films, respectively. And the peak positions of ground state absorption basically match very well with those of steady-state absorption spectra in figure 1(a). However, it is noted from figure 5(a), that the negative bleaching signal intensities of ground state absorption in PDMS flexible films are much weaker than those in hexane solution. Compared to CPNCs in hexane solution of figure 1(d), the forming larger clusters of the CPNCs in PDMS flexible films, as shown in figure 2(d), are indications of ligand dissociation [30, 31], causing a lot of surface defects surrounding these CPNCs. At an early delay time (0–2 ps) of CsPbBr1.35I1.65 CPNC solution showed an exciton bleach signal at ∼533 nm and exciton absorption signal at ∼570 nm (figure 5(a)). This can be attributed to state-filling-induced bleach and hot-exciton-induced red-shift. We fitted the hot-exciton-induced lifetime about 2.59 ps shown in figure 5(c) black curve, which is similar to a previous study [32]. After hot-exciton relaxations, the exciton-induced shift was much smaller at about 33 nm, and the spectroscopy signal was dominated by the exciton bleach feature. The two-dimensional transient absorption spectroscopy also clearly showed a broad photo-induced absorption feature throughout 570–600 nm (figure 5(b)). This is different to CsPbBr1.35I1.65 CPNCs in PDMS film where in the early delay time is dominated by electron relaxation and not existing hot-exciton-induced red-shift (figure 5(d)). Compared to the two-dimensional transient absorption spectroscopy of CsPbBr1.35I1.65 CPNC solution, it was also clearly showed electron relaxation features and no hot-exciton-induced red shift figure 5(e).
Figure 5. Transient absorption spectrum of CsPbBr1.35I1.65 CPNCs in PDMS flexible films and hexane solution. (a) and (d) TA spectra of CsPbBr1.35I1.65 nanocrystal probed at indicated time delays following the excitation by 450 nm pulse. (b) and (e) 3D transient absorption (TA) spectra of CsPbBr1.35I1.65 nanocrystal and CsPbBr1.35I1.65/PDMS film. (c) and (f) TA kinetics probed band bleaches following the excitation by a 450 nm pulse.
Download figure:
Standard image High-resolution imageKinetics of transient features are shown in the figure 5(f). By fitting the kinetic curves of the conducting electrons, we obtained the decay lifetimes of the CsPbBr1.35I1.65 nanocrystals in hexane and PDMS flexible films for different storage times.
It is seen that when stored for 1 d, the decay lifetime of conducting electrons of CPNCs in PDMS flexible films is 119 ps, which is far less than that of 287 ps in hexane solution. The fast decay lifetime of CPNCs in PDMS flexible films derives from the rapid conducting electron recombination, which is due to a lot of surface defects surrounding the CsPbBr1.35I1.65 CPNCs, caused by the ligand problems as described in figure 2(d). When stored for more than 1 d, there is already no signs of the kinetic curves which can be detected in a hexane solution, indicating a rapid decay process of the conducting electrons. This further confirms the unstable properties of CsPbBr1.35I1.65 CPNCs in hexane. By comparison, the kinetic curves in PDMS flexible films are substantially stable over a period of up to 7 months. It means that the PDMS flexible films effectively protect the CPNCs from oxygen and moisture. The trend of the kinetic curves of the conducting electrons in the transient absorption spectra is in good agreement with the PL decay curves in the steady-state fluorescence spectra as in figure 4. Therefore, from the carrier dynamics point of view, the transient absorption spectra provide another direct evidence to explain why the CsPbBr1.35I1.65 CPNCs in PDMS flexible films have a long-term stability compared to those in hexane solution.
4. Conclusion
In summary, we successfully demonstrated the white LEDs based on UV LED and CPNC/PDMS flexible films can be easily tuned from cold to warm white light merely by adjusting the layered ratios of the blue-emitting CsPbCl1.58Br1.42 to orange-emitting CsPbBr1.35I1.65 CPNC/PDMS flexible films. Interestingly, the crystalline structures of the CPNCs in PDMS flexible films are in good agreement with the cubic CsPbBr3 crystals although their morphologies show some irregular shapes and clusters compared to those in a hexane solution. Most significantly, PDMS flexible films provide a long-term and stable environment for the CPNCs. Note that the PL decay lifetime of the CPNCs in PDMS flexible films only changes from 2.68 ns to 2.18 ns during seven-month storage, whereas extremely short PL decay signals are detected in hexane solution after only one-day of storage. The time-resolved transient absorption spectra provide the carrier dynamics to describe the stability of the CPNCs in PDMS flexible films. The carrier dynamics curves show that in initial stage, the decay lifetime of the CPNCs in PDMS flexible films is obviously less than those in hexane solution, which is ascribed to the fast electron recombination due to a lot of surface defects in the CPNCs in PDMS flexible films. However, as time goes on, the protection of PDMS flexible films prevents further damage to the CPNCs, and conversely, the CPNCs in hexane solution degrades badly due to exposure to external conditions, causing the obvious luminescent quenching effects. Our designed CPNC/PDMS flexible films provide a good candidate for the stable flexible fluorescent film for solid state lighting.