Abstract
As a key component in many industrial heterogeneous catalysts, the surface structure and reactivity of ceria, CeO2, has attracted a lot of attention. In this topical review we discuss some of the approaches taken to form a deeper understanding of the surface physics and chemistry of this important and interesting material. In particular, we focus on the preparation of ultrathin ceria films, nanostructures and supported metal nanoparticles. Cutting-edge microscopic and spectroscopic experimental techniques are highlighted which can probe the behaviour of oxygen species and atomic defects on these model surfaces.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Ceria (cerium dioxide) is a key component of many heterogeneous catalyst systems with wide-ranging applications in critical industrial processes including water-gas-shift, methanol synthesis, methane reforming, and CO oxidation [1–6]. In addition to its role in catalysis, ceria also has important applications in solid oxide fuel cells and in optical and electronic devices [7–9].
A major reason for the catalytic activity of ceria is its ability to switch easily between the Ce4+ and Ce3+ oxidation states, which enables it to store and release oxygen during the course of reactions [10, 11]. This variability in oxygen stoichiometry is reflected in the structure of the ceria surface. This will be illustrated and discussed in the course of this topical review, which summarises our work in this area in the context of the field in general.
In heterogeneous catalysts, ceria is usually employed as a support material for various active metal particles, stabilising them under often quite extreme temperatures and pressures. The local structure of the ceria surface is vital to this and will also be examined in this work. Bulk CeO2 is insulating and as such often suffers from unwanted charging problems when interrogated using electron-based experimental techniques. As a result, it has become common to study ceria surfaces via the formation of ultrathin films, generally a few nm thick, supported on a metal single crystal surface. If the dimensionality of the ceria is further constrained by preparing islands of oxide supported on a catalytically active metal, a so-called 'inverse model catalyst' is formed. This allows access to the metal–ceria interface, which has uncovered novel behaviour in some instances, in particular involving spillover processes [12–14].
The most stable termination of CeO2 is the oxygen terminated (111) plane. This has been prepared on a wide variety of supports as films and nanoscale 'islands' including Ru(0001) [15], Pt(111) [16], Rh(111) [17], Ni(111) [18], Au [19], Cu(111) [1], Re(0001) [20] and Pd [21]. Figure 1 shows micrographs and associated low energy electron diffraction (LEED) patterns of ceria islands prepared on a wide range of substrates and shows some of the morphological variations due to the influence of the support. Key attributes of the support that influence the ceria include the propensity for alloying with Ce (Pt, Rh), the tendency of oxide formation (Ni, Cu) and the strain between the metal and CeO2(111) due to lattice mismatch, as indicated in figure 1.
Figure 1. Micrographs (STM and low energy electron microscopy (LEEM) images, 200 × 200 nm2) and accompanying LEED patterns of CeO2(111) surfaces prepared on different supports. The ratio of the metal to CeO2 lattice constants are indicated. Reprinted with permission from [12]. Copyright (2016) American Chemical Society.
Download figure:
Standard image High-resolution imageUltrathin ceria layers of up to tens of nanometres thickness can be formed with good crystallinity on many metal supports. This is usually achieved via physical vapour deposition of cerium metal, followed by oxidation and annealing steps to order and control the stoichiometry of the resultant structures. Fine control of the deposition rate, temperature, and oxygen partial pressure is critical to the final film structure and has been extensively reviewed in the literature [22].
2. Imaging and structure of ceria surfaces
A wide range of experimental techniques have been used to characterise ceria surfaces during and after their preparation. X-ray photoelectron spectroscopy (XPS) is the standard technique for quantifying the oxidation state of the cerium, typically probing the 3d and 4d core levels [22–26]. Resonant photoemission (RESPES) has been used to great effect to study minor changes to the Ce4+/Ce3+ ratio with very high surface sensitivity [27–29]. Synchrotron soft x-ray absorption spectroscopy has also been used to probe the Ce M4,5 absorption edge, which is also sensitive to oxidation state changes of the cerium and provides a more bulk-sensitive measurement [30, 31].
A popular approach to study the larger scale structure of ceria films and nanostructures has been LEEM and the complementary technique of x-ray photoemission electron microscopy (XPEEM). LEEM/XPEEM are full-field microscopy techniques with an ultimate resolution <10 nm and can be applied in situ in a range of experimental conditions, including during the initial cerium deposition stage and subsequent thermal treatments [32, 33]. As it is a diffractive imaging technique, LEEM instruments can also provide localised LEED measurements of small sample regions (few hundred nm) and more detailed structural information through I-V LEEM measurements [34]. XPEEM combines the high resolution imaging capability of LEEM with elemental and chemical sensitivity via photoemission and absorption processes, as will be discussed later in this topical review.
A key strength of LEEM when applied to the preparation of ceria and other oxide films is the ability to image an area of tens of nanometre in diameter with high spatial resolution (<5 nm) even at elevated temperatures [33, 35, 36]. In the case of the formation of ceria nano-islands on Rh(111), bright-field LEEM imaging was used during the post-deposition oxidation process. Figure 2 shows a series of snapshots (6 μm field of view) taken at different stages of the temperature ramp of ∼1 MLE cerium/Rh(111) in 1 × 10−7 mbar O2 [17]. In the first image, figure 2(a) the contrast is just from the Rh(111) surface where the step edges have clear contrast and the deposited cerium is not resolved. As the temperature is increased, figures 2(b) and (c), dark ceria islands are observed to form and ripen, with their morphology clearly influenced by the support structure. The full movie of this process is available in the supporting information (https://stacks.iop.org/JPCM/34/253001/mmedia).
Figure 2. LEEM images during the preparation of CeO2(111) islands on Rh(111). See supporting information for the full movie of the growth process. Reprinted with permission from [17]. Copyright (2014) American Chemical Society.
Download figure:
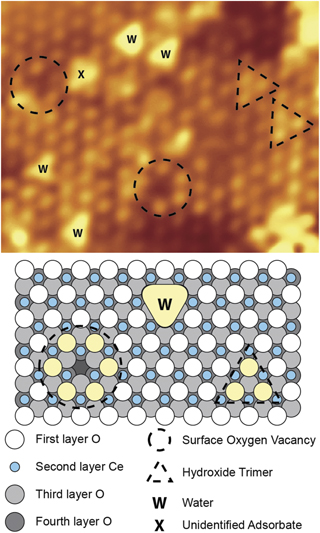
Standard image High-resolution imageThe ultimate experimental tool to probe the atomic scale structure and interactions with adsorbates is scanning probe microscopy. Both scanning tunnelling microscopy (STM) and non-contact atomic force microscopy (NC-AFM) have been extensively applied to ceria single crystals and ultrathin film surfaces for over two decades [37–41]. Despite some high resolution imaging of single crystal CeO2, investigations on thin films have proved more popular due to their relative ease of study, particularly at low temperatures. As an extremely sensitive real-space imaging technique, STM has proved particularly well-suited to probing the defect structure of CeO2(111) surfaces [16, 42] as illustrated in figure 3. Figure 3 is an atomically-resolved filled states (bright lattice points are located at the top layer oxygen atoms) image of ultrathin film CeO2(111)/Pt(111) with a number of point defects visible including surface oxygen vacancies and water molecules adsorbed from the background vacuum [16]. Comparison of these images with similar NC-AFM studies on single crystal CeO2(111) demonstrated the validity of the thin film as a model for the (1 × 1) surface [16, 43]. Such imaging is now relatively routine at both room temperature and cryogenic temperatures, where scanning tunnelling spectroscopy has also been employed to study the local electronic structure of the stoichiometric surface and around defect sites [42, 44, 45]. The behaviour of such defects, especially oxygen vacancies, is clearly crucial to the oxygen chemistry of ceria surfaces and as such has been a major focus of both experimental and theoretical studies. Similar studies with NC-AFM, which is less affected by charging than STM, but is experimentally significantly more challenging, has also shown the strength of real-space imaging to identify and study different ceria surface reconstructions [46, 47].
Figure 3. Atomically-resolved STM image of CeO2(111)/Pt(111). Reprinted with permission from [16]. Copyright (2010) American Chemical Society.
Download figure:
Standard image High-resolution imageDue to the facile formation of oxygen vacancies in the top and subsurface regions, there have been many experimental observations of stable arrangements of such vacancies resulting in various surface reconstructions with stoichiometries accommodating varying degrees of reduction from CeO2 (fully Ce4+) to Ce2O3 (fully Ce3+) [48–50]. Typically these intermediate reconstructions are prepared by tuning the growth conditions of the ceria layers, the key parameters being oxygen partial pressure (chemical potential) and the temperature [48, 51]. The metal single crystal support and its interface with the ceria is also critical to the formation of these metastable reconstructions [23, 52, 53]. In some cases, post-growth treatments have been used to induce such reconstructions, such as vacuum annealing [54], deposition of metallic cerium [49], or exposure to other reducing agents e.g. hydrogen [55]. Figure 4 shows LEED patterns depicting the transformation of CeO2(111) to CeO2−x (111) via irradiation with soft x-rays (∼120 eV, highly-focussed undulator beamline source) [56]. After just a few seconds these x-rays, tuned to the Ce 4d–4f absorption leading to a very high absorption cross-section, formed ordered vacancies. The associated reconstruction had a stoichiometry and symmetry of Ce7O12(111)(7 × 7)R19.1 relative to the CeO2(111)(1.4 × 1.4) and the underlying Pt(111)(1 × 1) support. Such synchrotron beam-induced changes have been widely reported for oxide surfaces [57, 58] and illustrate some of the experimental challenges which exist when trying to study redox processes on these materials.
Figure 4. LEED patterns of CeO2(111) films before and after irradiation with high-flux soft x-rays (∼120 eV). Reprinted with permission from [56]. Copyright (2016) American Chemical Society.
Download figure:
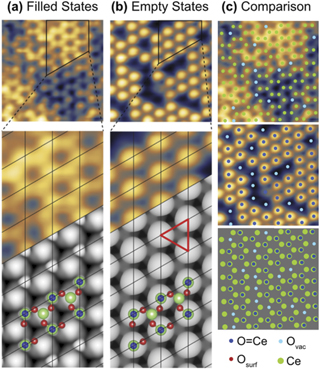
Standard image High-resolution imageThe oxygen chemistry of ceria surfaces is clearly of great importance considering its role in oxygen storage and release in heterogeneous catalysis, and novel surface reconstructions often display interesting bonding structures. Figure 5 shows atomically-resolved STM images (top panels) of a reconstructed CeO2(111)-( ×
×  )R30° film prepared on Pt(111) under oxygen-lean conditions. Empty (a) and filled (b) states STM images were obtained simultaneously and showed an excellent match to DFT simulations. The registry of the empty and filled states images suggested a novel termination with O atop Ce, that is a rare Ce=O species. This was confirmed by comparison with DFT calculations and high resolution electron energy loss spectroscopy measurements which detected a characteristic Ce=O stretch [51].
)R30° film prepared on Pt(111) under oxygen-lean conditions. Empty (a) and filled (b) states STM images were obtained simultaneously and showed an excellent match to DFT simulations. The registry of the empty and filled states images suggested a novel termination with O atop Ce, that is a rare Ce=O species. This was confirmed by comparison with DFT calculations and high resolution electron energy loss spectroscopy measurements which detected a characteristic Ce=O stretch [51].
Figure 5. (a) Filled (+1.7 V) and (b) empty (−4 V) states STM images (colour, upper panels) and simulations (monochrome, lower panels) of a Ce=O terminated CeO2(111)-( ×
×  )R30° film. The origins of the bright protrusions in the STM images are indicated and compared in (c), highlighting some surface oxygen vacancies (light blue) and the Ce=O species (dark blue). The structural model used for the DFT-simulation is shown in (a) and (b) with green representing Ce, red the surface O, and blue the Ce=O. Reproduced from [51]. CC BY 4.0.
)R30° film. The origins of the bright protrusions in the STM images are indicated and compared in (c), highlighting some surface oxygen vacancies (light blue) and the Ce=O species (dark blue). The structural model used for the DFT-simulation is shown in (a) and (b) with green representing Ce, red the surface O, and blue the Ce=O. Reproduced from [51]. CC BY 4.0.
Download figure:
Standard image High-resolution image3. Supported metals and reactivity
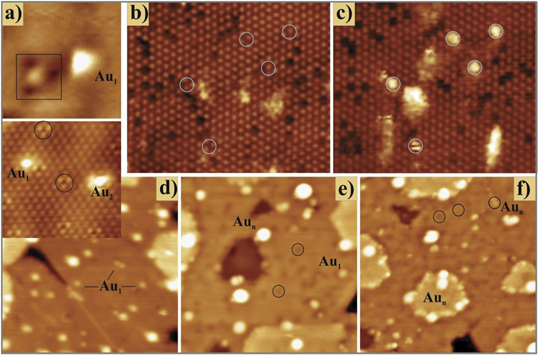
The interaction of metal adatoms and nanoparticles with oxide supports such as ceria has been under intense study for many years, probing key issues such as nucleation, stabilisation under reaction conditions, charge states, metal–support interactions, and deactivation mechanisms [59–62]. Ceria-supported noble metal particles, in particular gold, have attracted much attention due to their catalytic activity in applications such as water-gas-shift and CO oxidation [63–65]. For many metals, including Au, Ag, Pt and Pd, charge transfer from the ceria to the metal has been observed using photoelectron spectroscopy, leading to partial reduction of Ce4+ to Ce3+ at the surface in line with DFT calculations [27, 66–69]. The combination of STM and DFT is a powerful method to directly probe the influence of surface defects on the adsorption behaviour of Au on slightly reduced ceria surfaces, as shown in the work of Lustemberg et al [15]. Figure 6 shows STM images of two types of partially-reduced ceria surfaces: (b) and (c) from a reconstructed CeO2(111)-( ×
×  )R30° surface, and (a), (d)–(f) from CeO2−x
(111)-(1 × 1). For the CeO2(111)-(
)R30° surface, and (a), (d)–(f) from CeO2−x
(111)-(1 × 1). For the CeO2(111)-( ×
×  )R30° surface, figure 6(b) is the as-prepared surface with a number of dark depressions indicative of surface oxygen vacancies. Figure 6(c) shows the same region after deposition of Au at 300 K (1–3 Au atoms per cluster marked with white circles)—by comparison with the image in (b) there is no clear preference for nucleation at the surface defect sites.
)R30° surface, figure 6(b) is the as-prepared surface with a number of dark depressions indicative of surface oxygen vacancies. Figure 6(c) shows the same region after deposition of Au at 300 K (1–3 Au atoms per cluster marked with white circles)—by comparison with the image in (b) there is no clear preference for nucleation at the surface defect sites.
Figure 6. STM images of gold atoms and clusters on partially-reduced ceria surfaces. (a) A regular and defect-bound (square box) Au atom on CeO2−x
/Ru(0001); (b) and (c) Areas of CeO2(111)-( ×
×  )R30° before and after Au deposition (white circles show adsorption sites); (d) Au deposited on CeO2−x
/Ru(0001) at 15 K and then annealed to 200 K (e) and 400 K (f). (Black circles highlight defect sites.) Reprinted figure with permission from [15], Copyright (2016) by the American Physical Society.
)R30° before and after Au deposition (white circles show adsorption sites); (d) Au deposited on CeO2−x
/Ru(0001) at 15 K and then annealed to 200 K (e) and 400 K (f). (Black circles highlight defect sites.) Reprinted figure with permission from [15], Copyright (2016) by the American Physical Society.
Download figure:
Standard image High-resolution imageFigure 6(a) shows images of defect bound (black square) and regular Au1 atoms on CeO2−x (111)-(1 × 1). Figures 6(d)–(f) show the same surface at 15 K, 200 K and 400 K respectively, with surface vacancies highlighted with black circles. Again, there is no clear preference for adsorption at the surface defect sites even as the temperature is increased. Instead, the step edges of the ceria act as trapping sites. This leads to their decoration with larger Au clusters than on the as-deposited surface. The latter, which is shown in figure 6(d), consists mainly of single Au atoms evenly distributed across the film. To understand this somewhat surprising behaviour, DFT was used to calculate the adsorption energies of the various surface sites of these ceria surfaces and also the kinetics of surface diffusion. Although the defect sites are thermodynamically favoured, their population is kinetically hindered by the presence of a significant barrier resulting from the strong diabatic character of the diffusion process. On the stoichiometric surface, Au single atoms were shown to keep a constant 1+ charge, and the barriers towards surface diffusion are relatively small, however since further electron exchange with nearby Ce3+ ions is required to populate an oxygen vacancy, this process is kinetically blocked [15]. This result shows the complex nature of the interactions between reducible oxide surfaces and metallic adsorbates, even under carefully controlled ultrahigh vacuum conditions.
There has been a drive towards in situ spectroscopic studies of metal nanoparticles supported on ceria surfaces to investigate changes to both metal and ceria during exposure to elevated temperatures and pressures closer to those experienced in industrial catalysts. One key technique here is ambient pressure x-ray photoelectron spectroscopy (AP-XPS), which permits XPS measurements up to tens of mbar, and is now readily available at synchrotron light sources and home laboratories around the world [70, 71]. One system that has been extensively studied using AP-XPS is that of low coverage Ni nanoparticles on CeO2(111)/Ru(0001). Well-ordered ceria films, completely covering the Ru surface form the oxide support upon which ∼0.1 ML Ni was evaporated and annealed in vacuum to prepare the catalyst, with nanoparticles of ∼2 nm diameter. One key application of this catalyst is methane reforming with CO2 to form syngas (CH4 + CO2 → 2CO + 2H2), where it aids the low temperature activation of the C–O and C–H bonds in the reactants [2, 4, 24, 70, 72]. Figure 7 shows the behaviour of the Ni-CeO2(111) catalyst under 100 mTorr CH4 + 100 mTorr CO2 as the temperature of the system is increased to the operating conditions (700 K) [24]. The C 1s region is dominated by gas phase contributions from the reagents as well as an intermediate activated COx species at 300 K which is a result of room temperature activation of the methane. As the temperature is increased, this desorbs from the surface and under reaction conditions some CO gas phase product can even be observed. The Ni nanoparticles remain as 2+ until 700 K, where they are reduced to metallic Ni. The oxidation state of the Ce remains mainly 4+ until 700 K, where significant Ce3+ formation is observed.
Figure 7. Reactivity of Ni-CeO2(111) from AP-XPS. C 1s and Ce 4d core levels were measured under UHV (pristine) and when exposed to 100 mTorr CO2 + 100 mTorr CH4 at 300, 500 and 700 K. [24] John Wiley & Sons. © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Download figure:
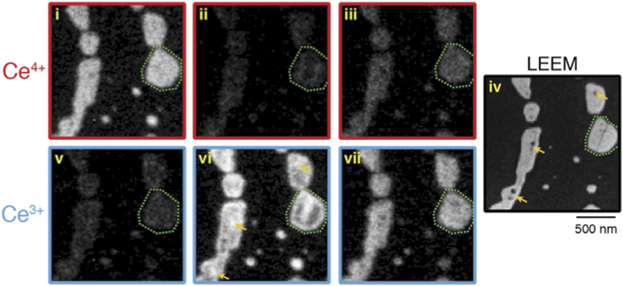
Standard image High-resolution imageCorrelating structure and reactivity of ceria surfaces is another major aim of the surface science approach to studying these materials. The chemical sensitivity of XPEEM, allowing element-and oxidation state specific imaging up to a few Hz acquisition rates has given us new insights into various redox processes on ceria inverse model catalysts [17, 20, 31, 56]. Figure 8 illustrates how the local reduction of ceria islands can be tracked in real time while the soft x-ray beam reduces the top surface of the ceria [56]. Using resonant energy-selected imaging it is possible to make maps of Ce4+ (red, panels (i)–(iii)) and Ce3+ (blue, panels (v)–(vii)) and compare it to the structure observed in LEEM (panel (iv)). As the reduction proceeds ((i)–(iii), and (v)–(vii)) over a couple of minutes, there is a clear decrease in intensity of Ce4+ and concomitant increase in Ce3+, with the reaction happening most readily at the edges of the islands and at defect sites. (In this case step edges and holes within the islands.) The combination of LEEM, LEED and XPEEM in a single instrument also permitted the study of the reverse of this process; during the formation of the ceria, an activated (2 × 2) oxygen overlayer was formed on the Rh(111) support in between the islands. By gently heating (∼400 K) the beam-altered surface, this oxygen was able to reverse spillover onto the ceria, reoxidising it, and showing the complex interplay between the oxide and the metal (Rh).
Figure 8. In situ resonant micro-XPS following the soft x-ray induced reduction of CeO2(111) islands supported on Rh(111). Reprinted with permission from [56]. Copyright (2016) American Chemical Society.
Download figure:
Standard image High-resolution image4. Future outlook
While characterisation of model CeO2 systems in UHV conditions has now achieved a good degree of maturity, studies at ambient pressure using a number of techniques have much to offer of relevance to catalysis. In particular, AP-PES could be further exploited to investigate surfaces under conditions closer to those employed in catalysis, including recent instrument developments permitting XPEEM measurements in the mbar regime [73]. Methods are also being developed to run PES measurements at significantly higher pressures, such as above 1 bar. These use reaction cells sealed by, for instance, graphene membranes which are photoelectron transparent [74]. Ambient pressure scanning probe microscopy offers a means to follow the morphology of metal nanoparticles as a reaction to gas phase species. It has previously found application in the study of inverse model ceria catalysts supported on Cu(111) [75].
Refection absorption infrared spectroscopy has previously been used under UHV conditions to monitor adsorption sites of CO on supported Pd on CeO2 ultrathin films [76]. Because RAIRS is a photon in-photon out technique it can also operate at elevated pressures, which has been exploited in studies of ethanol steam reforming over Ni-CeO2(111) [77]. Measurements of the dynamics of catalytic reactions are now becoming possible through the advent of fs pulsed x-ray sources in the form of x-ray free electron lasers [78]. Such studies present an unprecedented opportunity to evaluate reaction mechanisms in exquisite detail sufficient to inform future catalyst design.
Acknowledgments
This work was supported by the European Research Council Advanced Grant ENERGYSURF (GT), European Cooperation in Science and Technology Action CM1104, the EPSRC through Grant EP/L015277/1, the Royal Society through a Wolfson Merit Award to GT, and the Alexander von Humboldt Stiftung.
Data availability statement
No new data were created or analysed in this study.