Abstract
Bacterial biofilms are surface adhered communities of bacteria encased within a protective extracellular polymeric matrix. These heterogeneous microbial communities are characterized by elevated tolerance to antimicrobial agents, host immune clearance mechanisms and physical disinfection approaches. Atmospheric pressure non-thermal plasmas have proven to be highly effective in the eradication of bacteria and fungi in both planktonic and biofilm modes of growth at low temperatures, making it a promising approach for surface decontamination of both biotic and abiotic surfaces alike. In addition, non-thermal plasmas as a putative non-antibiotic approach to controlling infectious microorganisms, holds significant promise as an antibiotic alternative infection control strategy, with demonstrated efficacy against antibiotic resistant microorganisms. This topical review introduces the reader to key concepts in biofilm tolerance mechanisms relevant to treatment and control of these surface adhered bacterial communities with cold plasmas. In addition, the ability of plasma-derived active species to interact with both biofilm extracellular matrix components and bacterial cellular targets will be discussed in order to elucidate the mechanisms of antimicrobial and antibiofilm action. By understanding these fundamental interactions, plasma sources may be precisely tailored for antimicrobial applications, specifically for biofilm control where bacterial and fungal physiology (and sensitivity to physical and chemical decontamination) is markedly different from that of their planktonic, or free swimming, counterparts. Recently, novel roles for reactive oxygen and nitrogen species in the activity of conventional antibiotics have been proposed. This extends the possibility that plasmas may enhance the activity of conventional antibiotics and biocides in controlling these highly tolerant microbial populations. Lessons from classical biofilm microbiology can be usefully translated and applied to the design of plasma-based approaches aimed at biofilm control, while potential for tolerance and persistence to plasma in bacterial communities will be reviewed.
Export citation and abstract BibTeX RIS
Introduction
In his pioneering observations of microorganisms, made possible by his expertly fashioned microscopes, van Leeuwenhoek also described for the first time, the growth of microorganisms within dental plaque (a biofilm) which exhibited variable sensitivity to antimicrobial insult: 'from whence I conclude that the vinegar with which I washt my teeth, kill'd only those Animals which were on the outside of the scurf, but did not pass thro the whole substance of it' [1]. This discovery was arguably ahead of its time, as the relevance and impact of this new microscopic world was not realized until the 19th century with the work of Pasteur and Koch. Pasteur introduced the concept of sterilization and related food spoilage with the presence of microorganisms; along with Koch's postulates, credence was provided for the germ theory of disease. The understanding of microbial life and its impact on infectious diseases and food spoilage provided a platform for scientists and engineers to innovate and investigate methods for the complete or partial removal of all microorganisms on biotic (biological) and abiotic (non-biological) surfaces.
In 1857, the dielectric barrier discharge (DBD) device commonly cited as the earliest example of an engineered plasma source, was designed by von Siemens to generate ozone for water decontamination [2]. The commercialization of similar technologies for disinfection occurred in the early 1900s with the development of ozone generators [3] and mercury-vapor lamps as UV sources for water disinfection [4]. It was not until more than a century later however that a patent was approved for a device for plasma sterilization in 1968. The patent was for sterilization of surfaces without appreciable damage of the surface from high temperatures using short exposure of a high voltage argon/nitrogen plasma [5]. Similar techniques were developed over the next 20 years using various gas compositions at low pressures with an emphasis on sterilization of items at low temperatures without damage to the product or packaging [6]. These devices were designed for closed system sterilization, requiring vacuums something not associated with contemporary atmospheric pressure non-thermal devices. Similar low pressure systems are still commercially available such as the Sterrad® systems. During this period of new plasma sterilization devices, within the field of microbiology a theory was to evolve that has environmental and healthcare consequences that are still very much relevant and pertinent today.
In 1978, the 'biofilm theory' was first proposed by Costerton and colleagues, in a publication in Scientific American entitled 'How bacteria stick' [7]. In addition to coining the term biofilm, this work, which built on earlier observations of bacteria preferentially growing as sessile consortia on submerged or partially submerged substrates, as opposed to moving freely as single cells (planktonically) in the bulk medium [8, 9], laid down the basic conceptual framework and fundamental principles of biofilm microbiology [7]. In the following four decades, the role of biofilms in, for example, chronic disease, industrial biofouling, environmental microbiology, biocatalysis, bioremediation, and geochemistry have been increasingly recognized. Table 1 provides a summary of positive and negative consequences of biofilms providing the interested reader an insight into their socio-economic significance.
Table 1. A summary of the positive and negative consequences of biofilms.
| Positive | Negative |
|---|---|
| Bioremediation of polluted sites and wastewater [10, 11] | Medical device infections, pathogenic interactions with host cells and reservoirs of infection [12–14] |
| White biotechnology [15] | Food safety [16] |
| Agriculture—biofilm formation in root nodules aids nutrient transfer and provides defense against disease [17] | Biofouling [18, 19] |
Although undergoing constant evolution the term biofilm is generally defined as surface-associated microbial consortia encased within a self-produced, protective matrix of extracellular biopolymers exhibiting distinct phenotypic variation from their planktonic counterparts and are characterized by enhanced persistence and elevated tolerance to antimicrobials and normal immune clearance [20, 21]. The enhanced persistence and elevated tolerance is the hallmark of biofilms that has stretched antimicrobials and disinfectants to the boundaries of permissible use.
As with the requirement for sterility and decontamination of products that Pasteur identified and Siemens attempted to remedy, there is a need for the removal and destruction of microbial biofilms that exist in dynamic environments such as medical devices, body cavities, chronic wounds and foodstuffs [16, 22]. Engineering advances during the 1980s and 1990s led to the creation of non-thermal plasma devices that did not require vacuums and which could operate successfully at atmospheric pressure. This presented an opportunity for more practical applications of atmospheric pressure non-thermal plasmas including skin disinfection and infected wound healing applications, as well as sterilization where in-packaging sterilization was not a requisite, with the first reports in 1996 of its ability to inactivate the bacterium Pseudomonas fluorescence in yeast extract polypeptone glucose media [23]. Treatment of prokaryotic cells using atmospheric pressure non-thermal plasmas continued [24, 25] alongside studies investigating plasmas effects on eukaryotic organisms [26, 27] coming together to form a 'new field at the intersection of plasma science and technology with biology and medicine' called plasma medicine [28]. This field has progressed rapidly from initial experiments on single cells of bacteria to treatment of patients with head and neck cancer [29]. Alongside these advancements there has also been greater understanding of non-thermal plasma's interaction with microbes from a biological perspective with an appreciation of differences between planktonic bacteria and biofilms.
There are already several well placed reviews on plasma's antimicrobial activity and its applications [30, 31]. This review however, will introduce the reader to the biofilm paradigm that contributes significantly to the current understanding of the mechanisms by which communities of bacteria tolerate and resist traditional antibiotics and host immune challenges, with a focus on how this relates to atmospheric pressure non-thermal plasmas interaction with microbial biofilms and its application within a next generation antimicrobial strategy.
Fundamental principles governing plasma's biological effects
Atmospheric pressure non-thermal plasmas, sometimes referred to as partially ionized gases can be produced using a variety of gases, configurations and power supplies to generate an array of reactive oxygen and nitrogen species (RONS) (such as the superoxide anion radical ( ), hydroxyl radical (
), hydroxyl radical ( ), hydrogen peroxide (H2O2), nitric oxide (NO), nitrite (
), hydrogen peroxide (H2O2), nitric oxide (NO), nitrite ( ), nitrates (
), nitrates ( ) and peroxynitrite anion (
) and peroxynitrite anion ( )), charged particles, UV photons and electrical energy. The antimicrobial effect of non-thermal plasma has been extensively characterized using a variety of bespoke experimental sources and set ups. Further discussion on the experimental and commercial plasma sources has recently been provided by Laroussi et al [32] and Weltmann and von Woedtke [33]. In addition, the 2017 plasma roadmap explores the opportunities and challenges of translating non-thermal plasma technology within agri-food and medical fields [34]. Currently, it is understood and accepted that two fundamental principles underpin the observed effects and applications of non-thermal plasma on biological entities.
)), charged particles, UV photons and electrical energy. The antimicrobial effect of non-thermal plasma has been extensively characterized using a variety of bespoke experimental sources and set ups. Further discussion on the experimental and commercial plasma sources has recently been provided by Laroussi et al [32] and Weltmann and von Woedtke [33]. In addition, the 2017 plasma roadmap explores the opportunities and challenges of translating non-thermal plasma technology within agri-food and medical fields [34]. Currently, it is understood and accepted that two fundamental principles underpin the observed effects and applications of non-thermal plasma on biological entities.
- Biological effects are induced predominately by plasma generated RONS.
- These RONS can either directly interact with biological molecules, tissue and organisms or may act indirectly via plasma-mediated modification of the biological milieu and intracellular compartment.
These principles broadly encompass the observed effects in eukaryotic cells and tissue as well as plasma's antimicrobial action. Plasma-liquid interactions have received attention due to its role in mediating biological effects and the long lived species produced from plasma treatment. Indeed plasma activated water (PAW) has demonstrated antimicrobial action [35] with pH considered a key parameter in driving and maintaining its antimicrobial activity [36, 37].
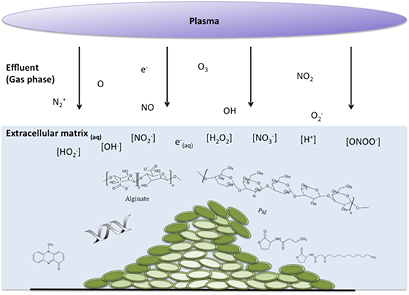
Within a biofilm, cells account for less than 10% of the dry weight, with the extracellular mass accounting for over 90% and water being the largest component [38]. This provides a highly hydrated microenvironment of extracellular components creating pH, oxygen and nutrient gradients resulting in bacterial cells at various metabolic stages, a key factor in determining biofilms antimicrobial tolerance [39]. Figure 1 coalesces the fundamental principles of atmospheric pressure non-thermal plasmas biological action and the complex microenvironment of the exemplar Pseudomonas aeruginosa biofilm model. This can be interpreted in two ways, firstly that the extracellular components (carbohydrate polymers, proteins, DNA, signaling molecules and virulence factors) and biomass of a biofilm presents a barrier to plasma generated RONS interacting with cells, but also that considering plasmas ability to alter the physiochemical nature of water it is logical to assume that in the hydrated biofilm microenvironment short exposures which do not result in desiccation or biofilm inactivation may alter pH and oxygen gradients and consequently cellular metabolism.
Figure 1. Non-thermal plasma treatment of a model P. aeruginosa biofilm: plasma derived reactive species encounters physical barriers to diffusion of species to a heterogeneous population of cells (intensity of green color reflecting the bacteria metabolic rate), which exist in their own microenvironment in comparison to the external environment.
Download figure:
Standard image High-resolution imageThe private life of biofilms
The biofilm phenotype is the predominant mode of growth of bacteria in the natural environment and is recognized as a principle virulence factor in infections such as chronic infected wounds arising from diabetic ulcers, pressure sores and burns where it is estimated that over 90% of wounds contain bacterial biofilms [40]. Biofilms are associated with the failure of medical devices [12] and the persistent recurrence of the same infection after treatments [22]. Not restricted to the field of medicine, the adaptability and environmental recalcitrance of microbial biofilms such as Listeria monocytogenes results in their emergence within and on food produce as well as the contamination of food processing equipment [41]. The transition of a planktonic bacterial cell to a surface adhered biofilm community develops in a highly conserved manner in a diverse range of microorganisms. Whilst there may be fundamental differences in the specific stages of the biofilm developmental life cycle between microorganisms, a generic developmental cycle involving several stages is still functional and used as a research platform and for basic understanding of a complex biological phenomenon as well as identification of opportunities for therapeutic intervention [42].
Beginning with the environment, biofilms may form on submerged surfaces, exposed to constant or intermittent fluid flow and perhaps, counter intuitively a major factor in stable biofilm formation can be high shear stress [43, 44]. A recent study demonstrated how high flow environments affect biofilm structure and what was once accepted as a sessile community, may be a dynamic community that is adapting to life in fluctuating flow environments [45]. As well as shear forces, other environmental cues for bacterial attachment include nutrient and oxygen availability and pH [46, 47]. The process of biofilm formation on a surface occurs in a sequential manner beginning with the formation of a conditioning film from passive settling of matter (organic and inorganic) within the bulk fluid. This results in modification of the surface properties making it more amenable to bacterial attachment. The characteristics of the surface and the cell are important, with hydrophobic-hydrophilic interactions key [48].
Adhesion occurs through the attachment of cellular appendages and adhesions such as pili, lectins and fimbriae in bacteria and fungi to a surface [49, 50]. Following attachment, adhesion is consolidated through cell division and the production of extracellular polymeric substances (EPS), such as polysaccharides, proteins and extracellular DNA (eDNA), creating the biofilm matrix. The EPS provides structural stability, viscoelasticity and contributes to the environmental persistence and tolerance of the biofilm in the environment. This stage is identified with micro-colony formation [51], cells enclosed in the matrix continue to grow and more complex 3D architecture develops. As the biofilm structure develops and extends; nutrients, pH and O2 availability within the biofilm varies resulting in metabolic heterogeneity within the biofilm consortia. This metabolic heterogeneity contributes to biofilms characteristic antimicrobial tolerance [52]. As the biofilm matures bacterial cell clumps, spores or fungal conidia disperse due to dynamic flow conditions colonizing new surfaces, environmental factors may also induce biofilm dispersal such as the important biological molecule nitric oxide, NO [53].
Antimicrobial tolerance and resistance of biofilms
The biofilm microenvironment is a privileged environment for bacterial cells providing protection and tolerance against antimicrobial agents, whereby tolerance is understood to describe a lack of susceptibility to antimicrobial agents due to an altered phenotypic state that is reversible and is related to the environment of exposure. However, resistance relates to the inherited ability of a microorganism to survive and grow in the presence of high concentrations of antimicrobial agents [54]. The characteristic biofilm tolerance is attributed to the biofilm matrix, the physical and genetic heterogeneity of the biofilm microenvironment and the presence of non-active persister cells within the biofilm community [12]. In addition, the increased density of multiple species within a biofilm creates an ideal environment for mutations and the transfer of resistance genes. Biofilms are thus described as reservoirs of infection and contamination, due to their tolerance and resistance to antimicrobials (reported to be 100–1000 times more tolerant than planktonic cultures [55]) and their growth/dispersal in dynamic conditions. This facilitate colonization and infection of new surfaces and tissues [56]. An in-depth discussion of the factors which contribute to bacterial biofilms resistance and tolerance is provided.
Heterogeneous microbial population within the biofilm
The spatial arrangement of cells within a biofilm results in a physical and chemical heterogeneity within the microbial population of a biofilm. Gradients of oxygen, nutrients and of waste metabolic products from actively respiring cells on the superficial layer of the biofilm produces a dynamic balance between consumption and diffusion [57]. A recent study by Stewart et al [58] provided insights into the chemical and physiological heterogeneity within the biofilm microenvironment in both in vitro and in vivo biofilms. The authors concluded that gradients of oxygen and free iron availability led to heterogeneous growth rates amongst cells in a biofilm with a proportion of cells within the stationary growth phase associated with phenotypic resistance/tolerance. The hypoxic environment in particular within biofilms has been shown to impact upon host healing [59, 60] with links to antimicrobial tolerance [52] and resistance [61] observed. There is a growing body of evidence indicating that other than actual drug interactions with biological targets, the induction of reactive oxygen species (ROS) contributes to the lethality of antibiotics [62–65]. This theory has evolved out of a proposed common mechanism of action [63, 66] proposing that the production of the hydroxyl radical was a factor in antibiotic induced cell death. The role of the hydroxyl radical was considered as bacterial cells have no detoxifying mechanism for the hydroxyl radical compared to superoxide or hydrogen peroxide. This proposal has generated much debate within the field of antimicrobial chemotherapy with studies providing contrary evidence; that ROS do not play a role in antibiotic's mechanism of action [67, 68]. Although, a study by Van Acker et al sought to investigate further, the role of ROS in antibiotic mediated cell death and the conflicting results within the literature [69]. Their study assessed several methods for measuring ROS including ROS specific stains, gene expression analyses of OxyR (a marker for oxidative stress), electron paramagnetic resonance, protein carbonylation and DNA oxidation. The authors attribute different experimental methods and conditions for the conflicting results. Further to these studies oxidative stress has been shown to increase persister cell formation in bacteria after exposure to antibiotics [70]. It has also been demonstrated in Burkholderia cepacia complex cells (BCC) that tobramycin treatment led to down regulation of the tricarboxylic acid (TCA)/citric acid pathway [71]. It was proposed that this metabolic pathway is hyper activated with antibiotic treatment of susceptible bacteria producing an increased concentration of superoxide that damages iron–sulphur clusters of proteins freeing Fe2+ and resulting in hydroxyl radical generation from a Fenton reaction. Generally, the field of microbiology and in particular the understanding of resistance development and antimicrobial mechanisms of action studies, require an appreciation of redox biology, an area not restricted to the biological application of non-thermal plasma.
Genetic diversity in biofilm communities
Within the biofilm community, growth of genetic variants can proceed in a stochastic manner without external stress or mutagens. These genetic variants have been observed in P. aeruginosa biofilms that produced genetically diverse subpopulations of cells that had specialized functions such as exopolysaccharide production and specific nutrient requirements. It was concluded these subpopulations of genetic variants would aid biofilm survival when encountering environmental stresses [72]. This process is viewed as an insurance policy for bacteria, providing a long-term survival option. Genetic variations may arise due to mutations (change in DNA sequence occurring randomly or through stress), recombination (process by which DNA is broken and repaired) or horizontal gene transfer between cells in close proximity. Horizontal gene transfer is particularly relevant within mixed-species biofilms and can be conducted via three mechanisms: direct cell–cell contact (conjugation), bacteriophage mediated DNA transfer (transduction) or the uptake of eDNA by competent cells (transformation) [73]. Biofilms provide an optimal spatial environment for horizontal gene transfer due to close proximity of cells and the presence of eDNA within the matrix. It has recently been demonstrated that conjugation of the blaNDM-1 gene (responsible for carbapenemase resistance) was transferred between Escherichia coli and both P. aeruginosa and Acinetobacter baumannii biofilms [74]. Although performed in vitro, it demonstrates the potential for antibiotic resistance to transfer between clinically relevant bacteria within biofilms. In addition to genetic variation stochastic gene expression increases the genetic diversity within a biofilm that is not influenced by environmental factors. An example of which is gene expression for matrix production in Bacillus subtilis. It was observed at an early stage of biofilm formation that genes encoding for matrix production were expressed in a small population of cells cited as a division of labour in a biofilm [72]. Another aspect of genetic heterogeneity within biofilms is related to quorum sensing. Quorum sensing is an evolutionarily conserved cell density-dependent signaling mechanism, controlling gene expression that results in phenotypic and group behaviours changes in microbial populations. There are several distinct quorum sensing pathways and signaling molecules in Gram-positive and Gram-negative bacteria [75], with the n-acylhomoserine lactone (AHL) system most intensively studied. P. aeruginosa is a well characterized model for bacterial biofilm formation and quorum sensing, with both virulence and biofilm maturation under quorum sensing control. Quorum sensing in P. aeruginosa is controlled by the production and sensing of AHL molecules produced by lasI and rhlI genes. It was observed that the genes controlling AHL molecule production were expressed at the substratum of biofilms with low expression on the periphery resulting in cells at the bottom of the biofilm with genes dependent on quorum sensing activated [76].
Extracellular matrix effects
One aspect of biofilm differentiation from planktonic cells is the existence of cells in a self-contained microenvironment typically referred to as the biofilm matrix. The biofilm matrix is typically conflated with EPS indicating that the matrix composition is a combination of biopolymer polysaccharides, proteins, extracellular (eDNA), lipids/glycolipids, humic substances and extracellular substances produced by bacterial cells [77]. The matrix has a diverse chemical composition, that creates a diffusion barrier contributing to biofilm tolerance to biocides and antimicrobial agents [78]. P. aeruginosa for example, produces at least three biopolymers: alginate (co-polymer of β-D-mannuronic acid and α-L-guluroninc acid), Psl and Pel. These carbohydrate based polymers interact with eDNA creating the biofilm matrix [79] and aid P. aeruginosa tolerance to antimicrobials [80]. Not limited to Gram-negative biofilms, eDNA has been demonstrated to impede the diffusion of vancomycin in Staphylococcus epidermidis biofilms [81]. This retardation of antimicrobials has been attributed to the binding of the matrix components to antimicrobials/antifungals [81, 82] with for example anionic alginate binding with cationic antimicrobials such as tobramycin and streptomycin [83–85]. Conversely, whilst evidence has demonstrated the matrix's ability to retard antimicrobial diffusion through the biofilm, cells within a biofilm are in various phases of bacterial growth from nutrient gradients creating a heterogeneous microenvironment (pH and oxygen limitation). This is another important aspect explaining biofilm resistance and tolerance to antimicrobials [52, 86]. As well as biofilms exhibiting greater tolerance to antibiotics, there is evidence that the biofilm phenotype protects bacterial cells from phagocytosis. Observations by Lam et al of P. aeruginosa cells surrounded by a 'slime' (mucoid extracellular matrix) within human lung tissue suggested a possible protection against an immune response [87]. Staphlyococcus aureus biofilms have been shown to reduce inflammatory markers (IL-1β, TNFα) in vivo, with repression of NO synthase in macrophages and restricted phagocytosis attributed to the biofilm matrix [88]. Gram-negative quorum sensing signaling molecules can also modulate host immune responses through induction of apoptosis in macrophages [89], the role of quorum-sensing in protecting biofilms from the immune response has been studied further with leukocytes penetrating a P. aeruginosa quorum sensing-deficient mutant more efficiently compared to the wild type [90].
Persister cell formation
Bacterial persistence/dormancy was first observed in the early 1940s by Hobby et al [91] and later by Bigger in 1944 [92]. Lewis and colleagues elaborated on these initial findings [93], developing the role and theory of persister cells in biofilms and infection [55]. Persister cells are dormant phenotypic variants of normal bacterial cells that are highly tolerant to antimicrobial agents and neither grow nor die in high concentrations of the antimicrobial which is sufficient to eradicate the majority of the bacterial population [92]. Resistance in microorganisms is understood as the ability of micro-organisms to grow in the presence of an elevated concentration of antimicrobials [94]. Unlike resistant cells, persister cells enter a dormant non-dividing state commonly associated with metabolic inactivity [95]. This state permits cell survival in elevated levels of antimicrobials, with bacteria exhibiting a characteristic tolerance to antibiotics. These subpopulation of altruistic cells play a role in the recalcitrant nature of biofilms [95], surviving antimicrobial chemotherapy through dormancy and reviving when the environmental conditions are more suitable. Formation of persister cells is influenced by toxin–antitoxin (TA) systems. TA systems are composed of a toxin protein responsible for targeting translation of mRNA and a complementary antitoxin that inhibits the toxin protein; increased production of the toxin protein results in persistence. Increased persister cell formation has been associated with pre-treatment of environmental stresses such as oxidative, osmotic and pH stresses. Following pre-exposure of E. coli to hydrogen peroxide as well incubation in acidic conditions, an increase in persister cell formation was observed following ampicillin treatment [96]. The authors demonstrated that a down regulation of stress resistance gene activation occurred from RpoS (a protein that controls transcription in E. coli leading to a general stress resistance) degradation via the toxin MqsR resulting in dormancy [96]. Persistance can be viewed as a hedge-betting strategy, whereby a proportion of bacteria invest in dormancy when stressed rather than activate genes to resist the stress. This has been demonstrated by Dörr et al and the identification of a dual role for the protein TisB in the development of antimicrobial resistance and persister cell formation [97]. Research into the mechanism(s) behind bacterial persistence is ongoing, as the mechanism(s) by which these toxins trigger dormancy is largely unknown. Consideration of persister formation when using non-thermal plasmas for biocide applications should be given, due to the production of oxidative species and pH changes induced by plasma exposure, leading to cellular stress and its implications for surviving cells.
Overview of plasma-biofilm control studies
Inactivation of microbial biofilms by non-thermal plasmas is an important application within the food industry [98], and the clinical setting including removal of biofilms in dentistry [99, 100], high level disinfection of surfaces [101–103] and eradication of biofilms from infected tissues [104–106]. Since the biofilm phenotype represents the predominant mode of growth of bacteria and given its role in pathogenicity; a more apt assessment of non-thermal plasma antimicrobial activity would with an appropriate biofilm model. In addition, the biofilm phenotype has exhibited greater tolerance to non-thermal plasma inactivation than planktonic cells [107–109]. Examples of biofilm models used to assess plasma's anti-biofilm efficacy include biofilms grown on glass surfaces [105, 108], 96-well microtitre plates [107, 109, 110], the Calgary biofilm (MBEC) device [111, 112] and flow devices such as the CDC biofilm reactor [113, 114] and continuous flow chambers [108, 111]. Each model has its own specific use and limitations with for example, the microtitre plate and CBD being suitable for testing anti-biofilm methods whereas the flow chamber facilitates biofilm visualization using microscopy and permits study of biofilm growth and dynamics. For a more technical discussion on biofilm models and their uses the interested reader is directed to Azeredo and colleagues who provide a critical review of biofilm methodologies [115].
Kill kinetics
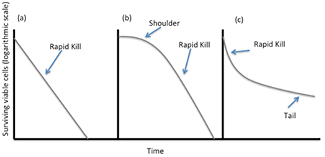
Microbial and biofilm inactivation kinetics are commonly depicted using semi-logarithmic exposure time versus survival curves (figure 2). Decimal reduction times (D values) are commonly reported in sterilization and disinfection studies as the time required to achieve a one-log (90%) reduction of viable cells under defined experimental conditions. Extrapolation of D values in heat sterilization is used to identify conditions necessary for sterilization. However, in plasma-microbe kill studies it is used as a comparison of the efficacy of plasma treatments and provides insight into various parameters (voltage, frequency, biofilm biomass, microbial species) that influence plasma's antimicrobial efficacy. With regards to disinfection and sterilization, survival-time curves are used to determine the inactivation kinetics of antimicrobials. Inactivation curves also provide information on the interaction of the biocide with the test organism. Figure 2 exhibits three types of inactivation kinetics commonly observed. Figure 2(b) has an initial slow phase followed by a rapid phase of kill typically referred to as a 'shoulder'. This shoulder may indicate an initial tolerant portion of cells that are less sensitive to the treatment, commonly attributed to cell aggregation or the time taken for accumulation of sufficient biocide concentrations within/surrounding the cell to result in cell death. This is particularly relevant for agents whose penetration is retarded by the outer membrane of Gram-negative bacteria, the cell envelope, or the biofilm matrix. With respect to plasma exposure of bacterial populations, an initial shoulder may be the result of the time-dependent production of plasma generated antimicrobial species, specifically within the liquid phase. Inactivation curves for plasma treatment biofilms typically produce bi-phasic kinetics with an initial rapid kill phase followed by a tail [111, 112, 116] as shown in figure 2(c). This type of kinetics is related to rapid killing of cells such as cells in the periphery of the biofilm followed by either the presence of a less susceptible population of cells (i.e. persister cells) or a decrease in the accessibility of the biocide to microorganisms due to the presence of aggregates from the biofilm matrix or cell debris presenting a physical barrier [116]. Such 'tailing' may also be associated with desiccation of the biofilm matrix, resulting in a reduced killing efficiency.
Figure 2. Inactivation kinetics (survival curves) commonly used to depict non-thermal plasma antimicrobial activity against microorganisms. (a) First order kinetics indicating exponential decline in viable cells. (b) Initial slow phase described as a shoulder, followed by rapid decline in cell viability (c) initial rapid kill followed by a reduction in the death rate (tail) indicating less susceptible or tolerant cells or a reduction in the accessibility of the biocide to the cells. [117] John Wiley & Sons. Copyright © 2013 Blackwell Publishing Ltd.
Download figure:
Standard image High-resolution imageMono-species biofilm studies
Examples of in vitro assessment studies of plasma's anti-biofilm activity have been performed across a wide variety of species such as inactivation of Chromobacterium violaceum [118]. Clinically relevant bacteria such as the ESKAPE pathogens (Enterococcus faecium, S. aureus, Klebsiella pneumonia, A. baumannii, P. aeruginosa, Enterobacter species) are susceptible to non-thermal plasma with complete eradication of planktonic and biofilm cells exhibited in under 6 min, except for A. baummannii biofilms whereby only a 4 log reduction was achieved with 6 min plasma treatment [107]. A. baummannii in particular has been identified as a 'priority pathogen' with critical status along with P. aeruginosa by the World Health Organization (WHO) as posing the greatest threat to human health. It is intrinsically resistant to antimicrobial therapy [119] and it is tolerant to desiccation, known to survive on inanimate surfaces for up to 33 days. A characteristic that facilitates its transmission within clinics [120]. A. baummannii tolerance to non-thermal plasma has been previously reported with a no log reduction noted following 40 seconds plasma exposure on inoculated agar compared to a 2 and 5.4 log reduction for P. aeruginosa and S. aureus. A. buamannii was reported to be the most susceptible to non-thermal plasma when inoculated on porcine skin [121]. Cahill et al also has observed a varied susceptibility of A. baumannii when inoculated on different surfaces and treated with a non-thermal plasma generated from air. A. baummannii was most tolerant to plasma treatment when inoculated on hard surfaces such as steel and polypropylene with a 2.97 and 2.73 log reduction compared to 3.18 and 3.12 on mattress and marmoleum [102]. Using these studies as examples this indicates the complexity in translating in vitro assessments into an applied setting with various surfaces. Potentially a solution for non-thermal plasma use for on-site decontamination would be identifying hotspots of surfaces and areas associated with microbial contamination such as hard/metal surfaces and skin with recommended treatment times/strategies for particular surfaces.
Multi-species biofilm studies
Multi-species biofilms consist of different bacterial and/or fungal species that reside amongst or in close proximity with each other [122]. Interactions through inter-species signaling between these species can result in emerging functions such as increased antimicrobial resistance [123, 124], increased virulence [125] and biofilm formation [126]. Studies utilizing single species biofilms provide a clear insight into the antimicrobial effectiveness within the laboratory setting, but do not necessarily replicate in vivo scenarios. For this reason, multi-species biofilm models are considered more appropriate models for in vivo testing, though the composition of the mixed-species model should be a key consideration when creating a biofilm model [122]. There are several investigations that have reported non-thermal plasma effect on multi-species biofilms. A clinically relevant study by Modic et al examined plasma's antimicrobial effect against multi-species biofilms grown using a CDC reactor. The effect of a surface barrier discharge was assessed against multi-species biofilms of P. aeruginosa, K. pneumonia, Enterococcus faecalis and S. aureus. Based upon comparisons to single species biofilms of P. aeruginosa and S. aureus tolerance to plasma treatment increased in the multi-species biofilm [113]. Koban et al compared the efficacy of various plasma configurations including the kINPen09 to chlorhexidine for treatment of Streptococcus mutans biofilms and biofilms derived from human saliva grown on titanium discs. Whilst the microbial composition of the saliva multi-species biofilm was not defined they reported that S. mutans single species biofilms were more susceptible to all the treatments than saliva derived biofilms, concluding that non-thermal plasma was more effective than chlorhexidine treatment [100]. Another study examining co-cultures of S. epidermidis and P. aeruginosa biofilms as examples of biofilm infections found in chronic wounds were treated with the kINPen Med. This investigation reported that P. aeruginosa was more susceptible to plasma when grown as a single species (3.6 log reduction), compared to a mixed-species biofilms with increased tolerance (2.6 log reduction) [127]. Regarding S. epidermidis a slightly higher 1.4 log reduction was noted as part of the multi-species biofilm compared to a 0.9 log reduction with the single species [127]. However, whether this variation of tolerance is due to physical shielding from an increased density of cells or by a beneficial symbiotic relationship between species is currently uninvestigated such as an increase in secretion of matrix components.
Tolerance to cold plasma treatment in mixed species biofilms has also been demonstrated with Salmonella typhimurium and cultivable indigenous microorganism grown on lettuce leafs [128]. Similar to Koban et al, the indigenous microbes were not characterized or defined, however it was reported that mixed species cultures led to greater internalization of cells within the lettuce than single species, accounting for tolerance to plasma treatment. Internalization of pathogens on foods such as lettuce appears to be a challenge for plasma decontamination of food products as previously reported [98], indicating the need for innovative strategies to overcome this.
The role of biomass
Biological factors that affect non-thermal plasmas antimicrobial efficacy include cell titer [129, 130] and the presence of biomass [131]. This latter study investigated the role of heat-treated S. typhimurium cells (as a model for bacterial biomass) on D values of plasma treated bacteria including the effect of various cell concentrations on the inactivation rate. It was demonstrated that heat-treated cells decreased the inactivation rate and played a role in shielding of cells, but that increasing the cell titer played a more significant role [131]. Within this study the model for biomass was not representative of biofilm biomass and matrix, thus the role of the biofilm matrix could not be inferred. However, the presence of matrix substituents may quench or dilute the reactive species responsible for plasma antimicrobial action. Alshraiedeh et al exposed eight different B. cepacia clinical isolates to a helium/oxygen 0.5% plasma jet. A correlation of increasing plasma tolerance relating to isolates with increasing biofilm biomass was identified [112]. Ermolaeva et al noted that susceptibility to argon plasma exposure was dependent on biofilm thickness, with cells readily inactivated on the upper surface of the biofilm and greater survival at deeper layers within the biofilm [105]. Implying a role for matrix components in biofilm tolerance and biofilm density.
It has been suggested that the reactive species produced by plasma can penetrate the layers of biofilms (based on the inactivation of biofilms of 40–80 µm thick after 240 seconds exposure) [111]. Also biofilm thickness of P. aeruginosa was reduced from 23 µm to 8 µm following 300 seconds of direct DBD treatment [132]. Evidence of a reduction in biofilm thickness would aid the penetration of plasma-derived reactive species over time. Using confocal microscopy and an indiscriminate fluorescent dye, a reduction of biomass of a Propionibacterium acnes was correlated with a reduction of viable cells [133]. These studies relied upon confocal laser scanning microscopy (CLSM) a technique that can be used to also assess the effects on the biofilm matrix using an appropriate fluorescent lectin probe [134].
Reduction of the biofilm matrix following plasma exposure has also been described using atomic force microscopy (AFM) observations. An insight into the adhesive properties of bacterial biofilms following plasma exposure was provided using force-displacement and adhesive step measurements (the force required to retract the cantilever was determined as the adhesive step). Non-thermal plasma treatment resulted in a reduction in the area of visible matrix and reduced adhesiveness of the bacterial cells concluding that plasma exposure was responsible for a loss of biofilm structural integrity leading to cell death [114].
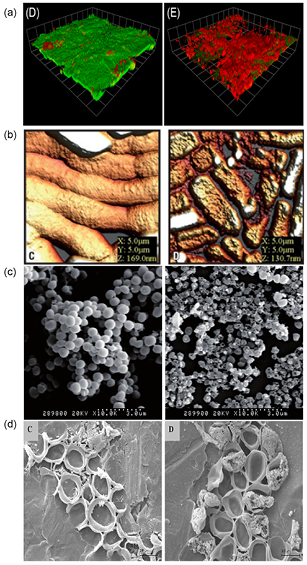
AFM has been used previously to assess damage induced by non-thermal plasma. A cohort of clinically relevant bacteria A. baumannii, E. coli, Methicillin resistant S. aureus (MRSA) and vancomycin resistant Enterococci were exposed to an air generated plasma with surface topography and damage assessed using AFM. Disruption of cell aggregates was observed with all treated bacteria and a general reduction of surface topography based upon the height distributions. However for MRSA an increase in the height distribution was noted potentially caused by the build-up of cell debris [102]. Other microscopic methods used to observe damage induced by plasma treatment include transmission electron microcopy (TEM) [108] and scanning electron microscopy (SEM) [110, 135]. Again these techniques allow visual appreciation of damaged cells with consistent observations of loss of cellular integrity. One aspect, which must be considered when using high-resolution vacuum microscopy for biofilm visualization, is sample preparation involving dehydration steps, which are destructive to the biofilm and can result in removal of the extracellular matrix. Figure 3 demonstrates examples of the various microscopic methods used to assess damage following non-thermal plasma treatment.
Figure 3. Various microscopy techniques used to visualize plasma-mediated kill and surface damage. (a) Confocal microscopy using LIVE/DEAD staining on 48 h P. aeruginosa biofilms unexposed (green viable cells) and exposed to the plume of kHz helium/oxygen plasma for 60 seconds (red fluorescence is the propidium iodide intercalating with DNA within the cell indicating the cell membrane is compromised). Reproduced from [111] © Alkwareek et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. (b) AFM of A. baumannii biofilms adhered to steel unexposed and exposed for 90 seconds. Plasma exposed cells show surface etching. Reproduced with permission from [102]. Copyright © 2014, American Society for Microbiology. All Rights Reserved. (c) SEM images of S. aureus biofilms unexposed and exposed (5 seconds) to a microwave argon plasma with plasma-surface mediated damage observed. Reproduced from [136]. © IOP Publishing Ltd. CC BY 3.0. (d) SEM images of C. albican biofilms exposed to a volume DBD and hollow DBD indicating gross damage to yeast cells induced by plasma exposure. Reproduced from [137]. © IOP Publishing Ltd. CC BY 3.0.
Download figure:
Standard image High-resolution imageFungal biofilm studies
Regarding fungal biofilms, Candida species (and in particular Candida albicans) is the most prevalent species found in the human microbiota and commonly associated with medical device and tissue infections, particularly in immunocompromised patients [138]. Similar to bacterial biofilms, the biofilm phenotype confers antifungal tolerance and resistance for Candida species [139]. Using titanium discs as biofilm substrates the antifungal efficacy of several configurations of non-thermal plasmas (kINPen 09, hollow electrode DBD and volume DBD) were assessed against C. albican biofilms. It was observed that device geometry impacted upon plasma's efficacy. The hollow and volume discharge configurations (whereby the samples were placed between the powered and grounded electrodes) provided the most effective inactivation of C. albicans biofilms (3.3 and 5.2 log reduction with 10 min treatment, respectively). An increased electric field was proposed to be responsible for this reduction compared to the precise nature of the kINPen plasma plume and large surface area of biofilm growth [137]. Maisch et al reported a greater than 5 log reduction for treatment of C. albican biofilms after 7 min using a surface microdischarge, the authors reported that electrical current measurements through the sample were negligible, inferring that antifungal activity was a result of the synergistic effects all plasma components including RONS and UV light [140]. These studies highlight how differences in plasma configuration can affect its efficacy and mechanisms of microbial inactivation which should be a consideration for applications for the various designs of plasma devices. A comparison of survival rates following argon plasma exposure of S. aureus, C. albicans and P. aeruginosa demonstrated that the fungal biofilm exhibited greater tolerance than bacterial biofilms [141].
Non-thermal plasma and adjunctive antimicrobials
Sun et al demonstrated the anti-fungal effect of a helium/oxygen (2%) microjet against various Candida strains (C. albicans, C. glabrata, C. krusei). Using colony enumeration and the XTT assay 60 s treatment was shown to result in rapid inactivation of Candida biofilms [142]. Interestingly, it was reported in this study that sub-lethal treatment (10–30 seconds) of Candida biofilms increased the fungistatic activity of amphotericin B, fluconazole and caspofungin with plasma pre-exposure. This activity was based on drug concentrations that resulted in a 50 or 80% reduction of formazon derivative turnover compared to untreated controls. The adjunctive use of conventional antifungal and emerging antimicrobial therapies is a new and exciting prospect, providing further scope for the use of non-thermal plasma against multi-drug resistant microbes. Bayliss et al examined non-thermal plasma pre-treatment of methicillin-resistant S. aureus (MRSA) and subsequent antibiotic treatment. They reported that treatments of up to 30 seconds exhibited faint increases in elliptical zones of antimicrobial activity around trimethoprim, kanamycin and oxacillin E strips compared to untreated bacteria [143]. These studies whilst promising, are in their infancy and require further investigations to discern whether these effects may be considered additive or synergistic with other antimicrobial agents and whether plasma treatment affects factors responsible for tolerance (i.e. metabolic rate changes, matrix composition and cell titer) or mechanisms relating to microbial resistance.
Mechanisms of antimicrobial activity of non thermal plasmas
As previously mentioned plasma has been utilized and developed for antimicrobial applications such as sterilization since the 1960s [5]. However, the antimicrobial mechanisms of action of atmospheric pressure non-thermal plasmas have only been given significant consideration in the last decade. It has been proposed that the mechanism of action is dependent on the plasma operating parameters dictated by the source device and with a multitude of non-thermal plasmas produced in various configurations and feed gases the result may be variations in its mechanism of cell destruction due to differences in the profile of plasma-derived active species produced [144]. Acknowledging these differences may be present, there is still a growing body of studies and experimental observations indicating common themes which are central to the elucidation of the plasma-derived species interaction with microorganisms. These commonalities of plasma mediated destruction of bacterial cells could allow engineers and physicists to tailor future parameters and designs of plasma devices for the most suitable applications and chemistry.
It is well known that RONS such as those generated by atmospheric plasmas occur naturally during respiration as part of the electron transport chain and in responses to infections by the innate immune system [145]. An early study by Laroussi et al provided early indication of the importance of plasma generated RONS for antimicrobial action [146]. The effect of RONS is implied from the use of antioxidants and radical scavengers. The addition of ascorbic acid as a RONS scavenger resulted in a reduction in the argon DBD antimicrobial effect, providing insight into the impact of plasma generated RONS. These findings confirmed earlier studies whereby another RONS scavenger; vitamin E decreased lipid peroxidation of E. coli and oxidative DNA damage during plasma exposure [147]. Unlike plasma treatment whilst in the presence of antioxidants such as vitamin E and C, pre-treatment of S. epidermidis, E. coli, and P. aeruginosa biofilms with vitamin C enhanced non-thermal plasma's anti-microbial activity [148]. The rationale for pre-treatment of biofilms with vitamin C is reliant on a previous study that exhibited vitamin C's antimicrobial activity [149] and is based upon its capacity to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) potentially driving Fenton-like reactions [150].
The role played by non-thermal plasma generated UV light was investigated by Taghizadeh et al [141], using quartz glass to block all species except UV light, they found that plasma exposure did not result in reduction of S. aureus biofilms. O'Connell et al used a MgFl2 filter allowing only UV light to penetrate, in order to assess how UV radiation produced by a RF plasma jet damaged DNA [151]. Their findings are consistent with Taghizadeh et al. Studies such as these provide evidence that UV light produced by plasma exposure is not wholly responsible for its bactericidal effect, but may play a role in generating reactive species [152] particularly the hydroxyl radical through plasma UV photolysis in aqueous solutions [153].
The presence of charged particles emitted from non-thermal plasmas has been attributed to membrane disruption of bacterial cells [154]. Morphological examination of bacterial cells and biofilms commonly exhibits cellular indentation and rupture [102, 108, 135]. Laroussi proposed that charge deposited from the plasma onto cell surfaces resulted in electrostatic disruption of bacterial cell structures [155]. Recent studies investigating this electrostatic disruption proposed plasma ion bombardment as the responsible species [135, 156]. Further to studies investigating the morphological changes through microscopy, experimental evidence was provided that physical disruption of bacterial cell surfaces was time and voltage dependent [156]. The authors provided evidence that bacterial inactivation may occur due to two mechanisms; physical destruction via plasma ions and programmable cell death due to ROS accumulation. That ROS induced programmable cell death was cited is in itself an interesting proposition, as programmable cell death in bacteria has been met with skepticism on whether it is a true manifestation [157]. These mechanisms were dependent on the voltage applied and the exposure time, short exposures led to programmable cell death with longer exposure times indicating a physical destruction mechanism [156]. As well as these new findings, their results also demonstrated greater planktonic Gram-positive tolerance compared to Gram-negative bacteria. These findings are in line with current findings regarding Gram-positive bacteria tolerance to plasma inactivation. Gram-positive tolerance has been attributed to the thicker peptidoglycan cell wall [154]. Gram-positive and Gram-negative related tolerance to plasma has been further supported by a study by Mai-Prochnow et al, correlating bacterial inactivation with cell wall thickness [127]. This trend however, may not necessarily be ubiquitous or relevant in the context of biofilms. Studies have reported greater tolerance to non-thermal plasma of Gram-negative bacteria in both planktonic and biofilms phenotypes of P. aeruginosa and A. baumannii than Bacillus cereus and E. faecium [107, 158, 159]. Suggesting that within a biofilm single cell factors have less of an influence compared to the biofilm composition and architecture.
The presence of charged particles is invariably linked to electric field production as well as the electric fields produced from the voltage pulses used to create plasmas. Electric fields have been shown to cause membrane permeability leading to cell death in bacteria [160]. The role played by electric fields would depend on the type of discharge and configuration of plasma treatment [137].
The bacterial surface is one obvious target affected by plasma-produced species. Several studies have demonstrated cell surface damage due to plasma exposure [147, 159, 161], although plasma inactivation of bacterial cells appears to involve multiple targets such as DNA and proteins. It has been suggested that proteins are less susceptible to plasma inactivation than plasmid DNA, likely due to the large structure of proteins requiring disruption of hydrogen bonds and protein carbonylation [159]. Plasma treatment, results in cell wall and membrane damage, proposed as one mechanism by which other cellular targets (DNA and proteins) can be oxidized from plasma exposure [159]. A study by Kvam et al found DNA damage or protein oxidation did not occur until bacterial membrane damage occurred [161]. The cell membrane is composed of polyunsaturated fatty acids, free radicals such as the hydroxyl radical can remove hydrogens from the unsaturated carbon chains by a process referred to as lipid peroxidation. This causes cell membranes perturbation and cellular damage. Increased levels of malondialdehyde (a final byproduct of lipid peroxidation and a marker of oxidative stress) have been observed following plasma treatment of bacteria [147, 159].
Resistance to non-thermal plasmas
It has been presumed that development of bacterial resistance to atmospheric pressure non-thermal plasma is unlikely, due to oxidation/physical destruction of multiple cellular targets and the multiple active species produced by plasma exposure that influence its antimicrobial activity (i.e. RONS, charged particles, electric field effects, UV). A study investigating development of resistance of bacteria to non-thermal plasma involved repeated sub-lethal exposures. This investigation concluded that repeated, short plasma exposures did not induce resistance [162]. This assumption has been challenged recently. Plasma treatment of P. aeruginosa inoculated agar plates identified resistant colonies within the zone of kill on the agar surface, these surviving colonies were isolated and re-inoculated onto plates with repeated treatments, resulting in reduced inhibition zones with repeated treatments [163]. The authors identified that these resistant colonies were able to grow in high concentrations of hydrogen peroxide and that phenazine production was correlated with increased plasma resistance [163]. Phenazines are heterocyclic redox active compounds, the quorum sensing controlled virulence factor pyocyanin is an example of a phenazine produced by P. aeruginosa [164]. This demonstration of resistance adaptation is an interesting and pertinent development challenging the current dogma that antimicrobial approaches which employ multiple active species, are unlikely to lead to the emergence of resistance. It is conceivable that adaptation to RONS over repeated exposures could lead to elevated tolerance and eventually genotypic resistance when considering the reliance of the production of RONS are for plasmas biological action. It may also provide further confirmation of the critical role played by plasma generated RONS in its antimicrobial activity, since phenazines such as pyocyanin provide protection from oxidative stress [165]. However, it may be possible, as in the case of disinfectant cycling protocols widely employed in healthcare and sterile manufacturing facilities that to avoid resistance development, alteration of the plasma operating parameters may avoid or delay resistance emergence.
Viable but non-culturable state (VBNC)
Bacteria are known to respond to a variety of environmental challenges by entering into a physiological state whereby cells are viable but are no longer culturable on standard laboratory media referred to as the viable but non-culturable state (VBNC) [166]. The capacity for bacterial cells to enter this VBNC state has important implications in assessing new antimicrobial strategies, contamination and infection persistence, environmental monitoring, food technology and medical microbiology with evidence suggesting their virulence is retained [167]. Conditions known to induce the VBNC state include antibiotic pressure, high/low temperatures, starvation, chlorination, change in pH and oxygen stress [167]. Non-thermal plasma exposure is a reactive cocktail of RONS, presenting a considerable environmental stress on microbes. Consideration of the VBNC state induced by cold plasma treatment has received some attention in the past decade [116, 168], and it is now accepted that the VBNC state occurs in bacteria following plasma exposure [169]. Commonly used assays for VBNC detection include metabolic assays such as XTT and resazurin, as well as the BacLight LIVE/DEAD fluorescence staining used alongside confocal laser microscopy. These assays are based on the premise VBNC cells are metabolically active with intact cell membranes. Molecular biology is increasingly being used to detect VBNC with reverse transcriptase (RT)-PCR used to detect RNA expression. RNA in comparison to DNA has a short half-life [170], with its presence due to the continual regeneration from active bacterial cells [166]. Whilst current understanding of the culturable to VBNC transition remain obscure, studies assessing the antimicrobial efficacy of atmospheric pressure non-thermal plasma should include appropriate methods for measuring viability of organisms other than colony enumeration.
Control of bacterial cell–cell signaling and virulence
Whilst the antimicrobial activity of plasma is well established, the consequence of non-thermal plasma on bacterial signaling and the consequence for surviving cells virulence has received less attention. Indeed, how bacteria and their signaling respond to non-thermal plasma treatment was highlighted in the 2012 plasma roadmap [171]. The presence of auto-inducer molecules and bacterial virulence factors within the biological milieu such as the biofilm matrix, would likely encounter reactive species and charged particles produced by non-thermal plasma exposure, with the reactive nature of plasma produced species interacting with these components as depicted in figure 1.
Indirect and direct DBD treatment of P. aeruginosa supernatants by Ziuzana et al was the first demonstration of non-thermal plasma to attenuate bacterial virulence factors pyocyanin and elastase. An almost complete inactivation of pyocyanin was achieved after 300 seconds treatment with a 70% and 88% reduction in pyocyanin levels after 60 seconds treatment for both direct and indirect treatments. Slower reductions were observed for elastase activity with under 50% and approximately 60% activity following 300 seconds direct and indirect treatments respectively [172]. In relation to respective susceptibilities of pyocyanin and elastase, elastase is a large zinc metalloproteinase with 301 amino acids, compared to the relatively small, non-proteinaceous phenazine pyocyanin. Minor modifications of the pyocyanin structure may have a greater impact whilst the activity of elastase is dependent on the protein's tertiary structure, which imparts unique active site geometry and arrangement of the catalytic amino acid residues. The active site residues could be shielded from plasma reactive species by the tertiary structure of the protease, which may require several chemical modifications before the protein loses structural integrity and activity.
Regarding plasma attenuation of bacterial virulence, Vandervoort and Brelles–Marino measured bacterial virulence in vivo using a lettuce leaf virulence assay. They reported that P. aeruginosa biofilms exposed for 1 min resulting in approximately a 4 log reduction of cells, retained the virulent capacity causing brown rot on lettuce leaf stems compared to 30 min treatment whereby complete eradication was achieved and no observed brown rot occurred [114].
The relationship between quorum sensing and virulence of in particular P. aeruginosa is well characterized and thus is used extensively as a model for quorum sensing dependent virulence. Following on from these investigations, it was demonstrated that non-thermal plasma attenuated virulence factor production through plasma-mediated modifications of AHL molecules. Using a P. aeruginosa double lasI/rhlI knock out, plasma exposure of AHL molecules 3-oxo-dodecanoyl homoserine lactone and butyrl homoserine lactone were unable to induce production of virulence factors pyoverdin (las controlled) and pyocyanin (rhl controlled). Attenuation of bacterial virulence was also exhibited in an in vivo virulence lettuce leaf model at exposure times relevant to in vitro reduction of virulence factor reduction. This study demonstrated that susceptibility of AHL molecules to plasma exposure was carbon chain length dependent, with greater susceptibility observed with longer carbon chains [173]. It had previously been identified that AHL molecules were susceptible to hydroxyl radical attack in a similar carbon chain length dependent manner [174], demonstrating the potential for plasma produced chemistry to modify bacterially-derived molecules.
These initial studies have demonstrated the capabilities of non-thermal plasma to not only inactivate bacteria cells, but also to modify and attenuate extracellular products secreted by the bacteria themselves. The consequence of which has yet to be realized in a clinical scenario or within the food industry where non-thermal plasma disinfection may be readily utilized. It is necessary to consider these effects as plasma exposure times required for complete eradication may be impractical and short exposure times appearing to induce the viable and non-culturable state. Thus further investigations are necessary to ascertain the cytotoxicity of plasma modified extracellular products and how the immune system may respond to these modifications.
Biological interactions of plasma—the immune system
Stimulation of macrophage activity following plasma treatment has recently been demonstrated [175, 176]. Macrophages are released into the blood stream from the bone marrow as monocytes differentiating into macrophages (Mϕ) at inflamed tissue sites [177]. Further polarization dependent on microenvironment signals occurs into M1 and M2 types. M1 types have powerful cytotoxic activity involved in the elimination of viruses, bacteria and cancer cells. Whereas, M2 types are involved in pro-angiogenesis, wound healing, allergic inflammation and parasitic helminthes. Lin et al demonstrated that plasma treatment of Mϕ macrophages and separate treatment of the tumor cells enhanced the anti-tumor effect of macrophages. In addition stress signaling from plasma treated tumor cells stimulated macrophage activity [176]. Inferring that plasma treatment may induce macrophage anti-tumor activity via two processes: direct macrophage stimulation and plasma treated tumor mediated stimulation. These results would indicate the opportunity for non-thermal plasma to not only modulate macrophage activation for tumor cells, but also for bacterial infections typically caused by microbial biofilms. S. aureus biofilms have demonstrated the capacity to interfere with macrophage phagocytosis through the production of proteinaceous toxins [178] as well as skewing M1 activation to M2 polarization [179]. With the potential for plasma exposure to modulate macrophage activation and its known effects on components such as the biofilm matrix and virulence factors necessary for evading the immune system, it would be of interest to investigate how non-thermal plasma may aid the body's fight against biofilm-related infections.
Future perspectives
Undoubtedly non-thermal plasma has demonstrated potential for the control of biofilm related infections and virulence. With the rise in antimicrobial resistance and the recalcitrance of biofilm infections to conventional antibiotics and biocides, innovative strategies and technologies, such as non-thermal plasma, are urgently required. The potential of such technology however is tempered somewhat by engineering challenges and translation of in vitro treatment times to real clinical scenarios. It could not be expected that one single plasma device/configuration is a panacea for the many potential applications of cold plasma systems; from decontamination of food products to applications in dentistry and decontamination of medical devices and intricate anatomical areas. Thus a plasma toolkit, containing various configurations of plasma devices designed for specific applications may be a solution. Already there is evidence of the development of such plasma toolkits [33]. The translation of treatment times for effective microbial decontamination will require clarification. The feasibility of plasma as an on-site biofilm sterilization tool is a step too far perhaps when accounting for exposure times required for complete eradication, however a defined log reduction of microorganisms in a defined period as a disinfectant is more realistic. This could be used to inform users on treatment times for surfaces with work already being implemented on such, with standards for assessment of medical plasma sources [180]. Although, these studies were implemented on planktonic species, biofilms exhibit greater tolerance to antimicrobial challenge compared to planktonic cells. Further understanding of how this tolerance is induced and maintained is necessary. In the biofilm, antibiotic tolerance and resistance is linked to the extracellular matrix and its influence in creating physical and genetic heterogeneity in the microbial population. What role the matrix plays in tolerance to plasma requires further research. This would inform therapeutic strategies such as the use of adjunctive treatments for the removal of matrix components. Indeed, a combination approach with physical or chemical disinfectants/conventional antibiotics/antiseptics and plasma exposure may be a suitable way forward for this technology to reach the clinic and could provide initial findings on its effectiveness within the clinic. The prospect of the emergence of antimicrobial resistance to non-thermal plasma warrants further consideration, and research efforts to elucidate the mechanisms of resistance development, if actual, are urgently required.
Conclusion
This review has introduced to the reader basic concepts in microbiology with particular focus on biofilms and their well characterized antimicrobial tolerance and resistance. With regard to the plasma-biofilm interaction, common themes emerge including the influence of the type of surface, the nature of the biofilm matrix in sequestration of reactive species and subsequent tolerance, and the differences in tolerance between mono-species and multispecies biofilms. The putative mechanistic overlap between traditional antimicrobial chemotherapies and the antimicrobial activity of plasma, highlighting the role of RONS, is also noteworthy and may provide future therapeutic opportunities. Basic antimicrobial characterization of non-thermal plasma sources has been a mainstay of plasma source characterization, however biofilm eradication represents a more realistic assessment, albeit a more difficult challenge. As well as extensive characterization of its antimicrobial effects, studies are beginning to investigate how plasma species may interact with extracellular products of bacteria responsible for the pathogenicity of bacteria and the consequence of this interaction. This is key to the understanding of the plasma-biofilm interaction required for further advances of non-thermal plasma as an antimicrobial application. Whilst there are several challenges to the translation of findings into a clinical application, these will no doubt be addressed by asking the correct questions of non-thermal plasma systems as an antimicrobial approach.
Acknowledgments
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) grant BB/P008496/1 "EnvironSafe: Cold Plasma Innovations for Food Safety and Sustainability" and Engineering and Physical Sciences Research Council (EPSRC) grant EP/M027473/1 "Building the Queen's University Belfast AMR Network".