Abstract
Using a high-resolution electron monochromator, we studied the formation of (H2O)n⩽19− cluster ions upon collisions of free low-energy electrons with water clusters embedded in helium droplets. The anion efficiency curves as a function of the initial electron energy were measured for the cluster sizes n = 2–8, 10, 13, 16, and 19. The present experimental results show that the shape of the resonance yields is dependent on the size of the water cluster anion. The results are discussed in terms of the different electron states available for the excess electron from a linear cluster structure to three-dimensional cluster structures as the number of water molecules within the cluster increases.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The water molecule is of fundamental interest in many fields of physics and chemistry and has been investigated for many years [1]. The studies of the presence of an excess electron in bulk water, commonly called hydrated electron, are important in understanding how an electron becomes solvated in such environment. The secondary electron seems to be responsible for a significant extent of radiation chemistry in living cells [2], as the ionizing radiation absorbed by the cells leads to formation of large amount of such secondary electrons in it. The hydrated electron has also been a central point of the studies in photochemistry, biochemistry [3–8] as well as nucleation and aerosol formation in upper atmosphere [9]. In this case, the hydrated electron state may be formed inside or the surface of a larger water cluster.
By investigating the structure of the water surrounding the excess electron, valuable insight has been added to the knowledge of the hydrated electron. It is well known that isolated water molecules do not bind an additional electron [10, 11]. Since bulk water readily solvates them, the process of electron attachment to water clusters may be understood as collective interaction of the electron with solvent molecules. Focusing on the spectral features of the excess electron in water clusters not only allows us to characterize the structural and dynamical aspects of the excess electron but also provides the observation of strong dependence of cluster size on the lifetimes of transitions [12–17].
Different experimental studies on the attachment of low-energy electrons to water clusters were performed by several groups. Early experiments by Haberland et al [18] showed that (H2O)n
− anions of  and
and  are formed by injection of low energy electrons into the condensation zone of a supersonic expansion of H2O vapor in argon. In contrast, Knapp et al [19] demonstrated that electron attachment to preformed water clusters, resulted in long lived water cluster anions only for sizes
are formed by injection of low energy electrons into the condensation zone of a supersonic expansion of H2O vapor in argon. In contrast, Knapp et al [19] demonstrated that electron attachment to preformed water clusters, resulted in long lived water cluster anions only for sizes  since in this crossed electron-cluster beams experiment no subsequent stabilization by further collisions was possible. Schermann et al [20–22] performed extended studies on Rydberg electron transfer to water clusters and mixed water clusters (see, e.g. [20]). Through experimental measurements combined with theoretical calculations, they suggested that the excess electron is dipole-bound to the cluster. Ayotte and Johnson [23] used electronic absorption spectroscopy and reported a strong size-dependence of absorption in water cluster anion spectra. Regarding size effects, the spectra were observed to be strongly redshifted, narrow and increased in peak intensity by going to small clusters. Through vibrational absorption spectroscopy, Johnson et al [24, 25] investigated how small and intermediate water cluster anions bind an excess electron. They found that for small water cluster anions, the water molecule hosting the excess electron is bound to the supporting water matrix through a double H-bond. By photo-electron spectroscopy, Coe et al [15] derived the binding energies of excess electrons to (H2O)n
− clusters. They identified three types of states due to their different vertical detachment energies (VDEs): Dipole-bound states (
since in this crossed electron-cluster beams experiment no subsequent stabilization by further collisions was possible. Schermann et al [20–22] performed extended studies on Rydberg electron transfer to water clusters and mixed water clusters (see, e.g. [20]). Through experimental measurements combined with theoretical calculations, they suggested that the excess electron is dipole-bound to the cluster. Ayotte and Johnson [23] used electronic absorption spectroscopy and reported a strong size-dependence of absorption in water cluster anion spectra. Regarding size effects, the spectra were observed to be strongly redshifted, narrow and increased in peak intensity by going to small clusters. Through vibrational absorption spectroscopy, Johnson et al [24, 25] investigated how small and intermediate water cluster anions bind an excess electron. They found that for small water cluster anions, the water molecule hosting the excess electron is bound to the supporting water matrix through a double H-bond. By photo-electron spectroscopy, Coe et al [15] derived the binding energies of excess electrons to (H2O)n
− clusters. They identified three types of states due to their different vertical detachment energies (VDEs): Dipole-bound states ( ) and two kinds of surface states, intermediate states and bulk embryont for
) and two kinds of surface states, intermediate states and bulk embryont for  and
and  , respectively. Verlet et al used time-resolved photoelectron imaging [17, 26, 27] to study the relaxation dynamics of excited hydrated electrons in size selected water cluster anions from clusters to bulk. They found that for
, respectively. Verlet et al used time-resolved photoelectron imaging [17, 26, 27] to study the relaxation dynamics of excited hydrated electrons in size selected water cluster anions from clusters to bulk. They found that for  (internally solvated electrons), the internal conversion is dominant and the life time scales as
(internally solvated electrons), the internal conversion is dominant and the life time scales as  . Over
. Over  (surface-bound electrons) the excited-state decays by auto-detachment of the electron with no obvious size dependence of the life time. They also found that for smaller anions (
(surface-bound electrons) the excited-state decays by auto-detachment of the electron with no obvious size dependence of the life time. They also found that for smaller anions ( ) and larger anions there is a competition between those two decay pathways.
) and larger anions there is a competition between those two decay pathways.
The studies mentioned above were carried out with bare water clusters in the gas phase. In our previous work, we also studied electron attachment to water clusters embedded in helium droplets [28, 29]. Using a high-resolution electron monochromator coupled to quadrupole mass spectrometry, we investigated the development of the electronic band structure for doped He droplets by means of measuring the (H2O)2
− ion yield at different initial He droplet sizes [28]. At the electron energy resolution of around 100 meV, we could determine  eV as the electronic surface barrier (V0), corresponding to the minimum droplet size of
eV as the electronic surface barrier (V0), corresponding to the minimum droplet size of  . In this work, we extend our previous experiments and report the anion efficiency curves of negatively charged water cluster (H2O)n
− for other cluster sizes. We detected anionic water clusters with sizes n = 2−8, 10, 13, 16, 19 by free electron attachment to preexisting water clusters formed in helium droplets. We show that for different anion cluster sizes, changes in the resonance shapes occur. As discussed below, we may tentatively attribute these changes to different excess electron states where the existence of these states depends on the structure of the water cluster.
. In this work, we extend our previous experiments and report the anion efficiency curves of negatively charged water cluster (H2O)n
− for other cluster sizes. We detected anionic water clusters with sizes n = 2−8, 10, 13, 16, 19 by free electron attachment to preexisting water clusters formed in helium droplets. We show that for different anion cluster sizes, changes in the resonance shapes occur. As discussed below, we may tentatively attribute these changes to different excess electron states where the existence of these states depends on the structure of the water cluster.
2. Experiment
For the present experiment, a helium droplet source based on supersonic expansion of precooled helium gas, a pick-up stage filled with water vapor, a hemispherical electron monochromator for generation of a well-defined low-energy electron beam and quadrupole mass spectrometry for detection of formed water cluster anions were used. This setup corresponded to the same apparatus previously used in [28]. Helium nanodroplets were produced by expanding helium (purity 99.99%) at a stagnation pressure of about 20 bar through a 5 μm pin hole nozzle, cooled by a closed-cycle cryostat (Sumitomo Heavy Industries LTD, model RDK-408D2) to about 9 K into a vacuum. The mean droplet size for these expansion conditions should be ∼105 helium atoms [30]. The resulting supersonic beam was skimmed by a 0.8 mm conical skimmer, located 10-mm downstream from the nozzle and passed through a pick-up cell into which H2O was introduced. Before measuring the ion yield as a function of the electron energy for a specific water cluster anion, the water pick-up pressure was set to a value where the ion yield for the cluster anion investigated was at the maximum. The most probable neutral water cluster size at each chosen condition corresponded to that of the anionic cluster size studied. The doped droplets passed another aperture of 2 mm diameter and entered the main vacuum chamber where they were crossed with an electron beam generated by a home-built hemispherical electron monochromator [31]. The energy resolution of electron beam was about 100 meV (full-width-half-maximum) at electron currents of about 30–50 nA. In the present study, the electron energy scale was calibrated via the well-known  eV resonance of SF6 leading to the formation of SF6
− [32]. This calibration procedure is equivalent to the one with CCl4 mentioned in [31]. Anions were extracted by a weak electric field into the entrance of a quadrupole mass filter. Mass analyzed anions were detected by a channel electron multiplier. The anion efficiency curves shown in the next section were obtained by selecting one specific water cluster anion with the mass filter and scanning the incident electron energy.
eV resonance of SF6 leading to the formation of SF6
− [32]. This calibration procedure is equivalent to the one with CCl4 mentioned in [31]. Anions were extracted by a weak electric field into the entrance of a quadrupole mass filter. Mass analyzed anions were detected by a channel electron multiplier. The anion efficiency curves shown in the next section were obtained by selecting one specific water cluster anion with the mass filter and scanning the incident electron energy.
3. Results and discussion
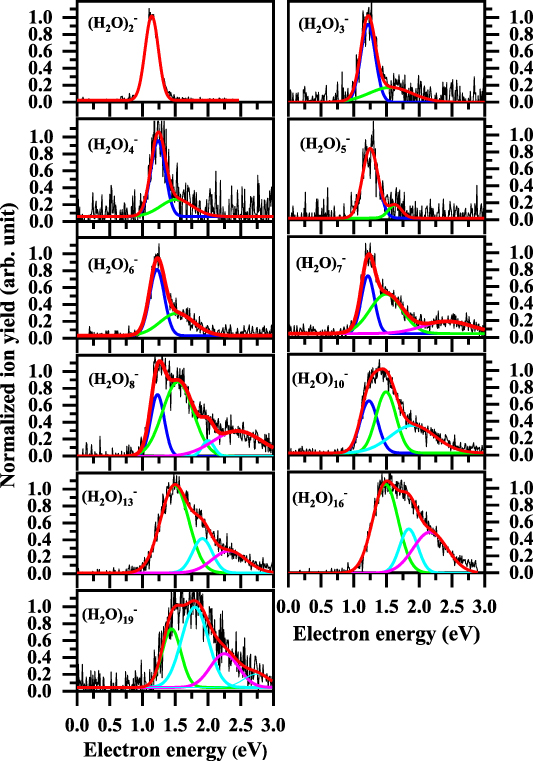
In figure 1, the anion efficiency curves of the studied water cluster anions (H2O)n − with n = 2–8, 10, 13, 16, and 19 formed upon low-energy electron attachment to water clusters embedded in helium droplets are shown in the electron energy range from about 0 to 3 eV. Table 1 summarizes the results from applying multiple Gaussian peak fits to the experimental data. Before the ion yields are discussed in detail, we briefly recall the underlying mechanism of electron attachment. If a low-energy electron interacts with a single neutral molecule, AB, in the gas phase, a temporary negative ion (TNI) may be formed by resonance electron capture,

Figure 1. Normalized anion efficiency curves of water cluster anions (H2O)n − with n = 2–8, 10, 13, 16 and 19 formed upon low-energy electron attachment to water clusters embedded in small helium droplets. The multi Gaussian fits shows the position of different peaks. Fit contributions at similar positions are indicated by the same color. The cumulative fit is displayed in red color. The non-normalized data is shown in supplementary material (figure S1).
Download figure:
Standard image High-resolution imageTable 1. Summary of the observed peak positions in the anion efficiency curves of the presently studied water cluster anions. The positions were obtained by applying multiple Gaussian fits on the ion yields.
| Peak position (in eV) | ||||
|---|---|---|---|---|
| Cluster | 1st | 2nd | 3rd | 4th |
| (H2O)2 – | 1.14 ± 0.05 | |||
| (H2O)3 – | 1.21 ± 0.05 | 1.55 ± 0.14 | ||
| (H2O)4 – | 1.23 ± 0.05 | 1.51 ± 0.14 | ||
| (H2O)5 – | 1.25 ± 0.05 | 1.63 ± 0.14 | ||
| (H2O)6 – | 1.22 ± 0.05 | 1.52 ± 0.10 | ||
| (H2O)7 – | 1.21 ± 0.05 | 1.49 ± 0.07 | 2.43 ± 0.40 | |
| (H2O)8 – | 1.23 ± 0.05 | 1.59 ± 0.07 | 2.00 ± 0.07 | 2.45 ± 0.43 |
| (H2O)10 – | 1.23 ± 0.05 | 1.49 ± 0.05 | 1.89 ± 0.80 | |
| (H2O)13 – | 1.49 ± 0.05 | 1.93 ± 0.07 | 2.32 ± 0.50 | |
| (H2O)16 – | 1.48 ± 0.05 | 1.83 ± 0.06 | 2.16 ± 0.50 | |
| (H2O)19 – | 1.44 ± 0.13 | 1.79 ± 0.24 | 2.25 ± 0.55 | |
It is important to note that the TNI formed in equation (1) is in an electronically or vibrationally excited state. The excess energy comprises the initial kinetic energy of the attached electron and the electron affinity of AB. The TNI can relax via autodetachment of the excess electron or via unimolecular decomposition into thermodynamically stable fragments  (so-called dissociative electron attachment). The electron attachment process as well as the relaxation processes may change significantly when going from isolated molecules to clusters. One main effect is that intramolecular fragmentation may be reduced due to fast distribution of the excess energy or caging effects, which prevents the emission of fragments [33]. These processes may affect the relative abundance of fragment anions but it may also modify the anion efficiency curve of a specific fragment anion [12, 14, 33]. Although the intramolecular dissociation may become reduced, the attachment process may be still a dissociative one since the excess energy distributed in the cluster may lead to evaporation of weakly bound cluster constituents. This evaporative cooling effect by the cluster environment may lead to formation of molecular anions, which are not observable by mass spectrometry for free electron attachment to the isolated molecules in the gas phase (like for example CO2) [34, 35].
(so-called dissociative electron attachment). The electron attachment process as well as the relaxation processes may change significantly when going from isolated molecules to clusters. One main effect is that intramolecular fragmentation may be reduced due to fast distribution of the excess energy or caging effects, which prevents the emission of fragments [33]. These processes may affect the relative abundance of fragment anions but it may also modify the anion efficiency curve of a specific fragment anion [12, 14, 33]. Although the intramolecular dissociation may become reduced, the attachment process may be still a dissociative one since the excess energy distributed in the cluster may lead to evaporation of weakly bound cluster constituents. This evaporative cooling effect by the cluster environment may lead to formation of molecular anions, which are not observable by mass spectrometry for free electron attachment to the isolated molecules in the gas phase (like for example CO2) [34, 35].
Regarding the initial electron attachment event, polarization effects within a cluster without significant dipole moment may lead to a red-shift of resonances [36]. However, water clusters possess such significant dipole moment, as discussed below, thus polarization effects will play a minor role. Instead, for the electrophobic helium droplets the opposite tendency leads to a pronounced blue-shift, since electrons require kinetic energy to enter the droplet [37, 38]. In our previous study on the formation of water dimer anion doped in He droplets, we observed a blue-shift of the onset of anion signal by at least 0.76 eV compared to the attachment reaction for bare water cluster in the gas phase [28]. This value is dependent on the size of the helium droplet and slightly increases for larger droplets. In addition to this expected shift, the ion yield of (H2O)2 − is further characterized by a single peak with symmetric shape located at the electron energy of 1.14 ± 0.05 eV. One important aspect in this context is the knowledge about the neutral precursor cluster. Since water clusters have a low electron affinity, which is lower than the binding energy of a water molecule to the cluster, the above mentioned scenario of cluster fragmentation after electron attachment can be excluded for small clusters n ⩽ 15 [39]. Thus, we propose that the (H2O)2 − signal results from electron attachment to the neutral water dimer in the following reaction (2)
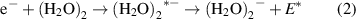
E* corresponds to the electron affinity released to bind the attached excess electron. This excess energy of the TNI is released to the surrounding He droplet. This additional energy dissipation corresponds to the second major modification of the electron attachment process compared to bare water clusters in the gas phase, in addition to the electronic surface barrier of the helium droplet, which causes a shift of the ion yield. The single peak observed here would correspond to the 0 eV resonance observed in [39] for electron attachment to the bare water dimer. For the used experimental condition, the onset of the dimer signal is found at about 0.9 eV. For the other cluster sizes studied, the onset is rather close, at about 0.98 eV, just for n = 19 the onset is found near 1.15 eV, see figure 1. These values indicate that a substantial amount of helium is surrounding the water clusters of these sizes at the moment of electron attachment (for the dimer about 6000 He atoms and for most other sizes about 14 000 He atoms; values derived as discussed in [28]). Therefore, the release of excess energy to the He droplet mentioned above will be complete for all cluster sizes studied and the formed water cluster anions will be long-lived (the detection time in the present experiment is ∼100–350 µs, depending of the cluster size). The observation of the bare water cluster anions further indicates that those are expelled out of the He droplet. We were not able to detect water cluster anions with a few helium atoms attached like in [29], which is a consequence of the lower detection sensitivity of the present apparatus.
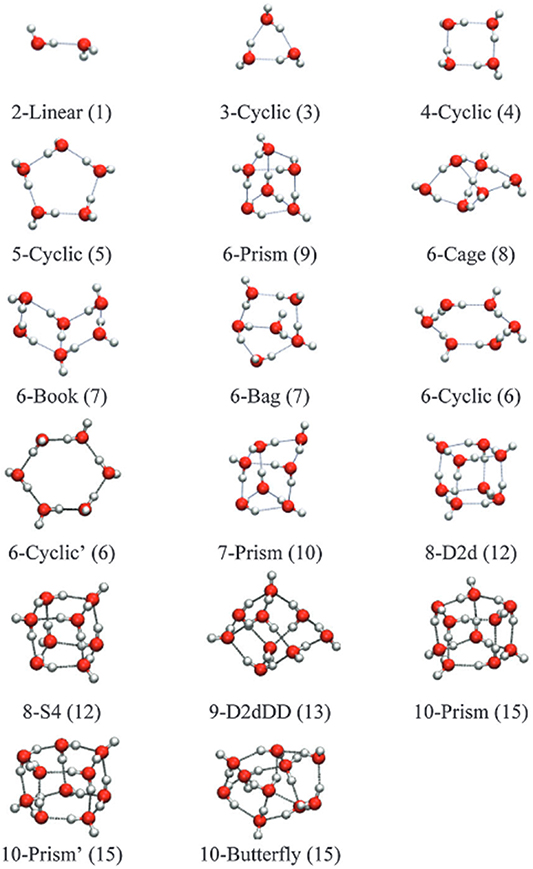
The structures of neutral water clusters with n = 2–10 were calculated by Miró and Cramer using density functional tight binding approach [40]. We replot these structures in figure 2. More recently, the water cluster structures for sizes n = 2–5 were also reported by Le Huyen et al using density functional theory ((U)B3LYP/6-311++G(3df,2p)) [41]. They provided the optimized structures for neutral as well as anionic water clusters. The water dimer has a dipole moment of 2.6 D [42] and the excess electron is weakly bound in a dipole state with electron affinity of 30 ± 4 meV [21]. Kim et al studied the dipole-bound water dimer anion in different configurations [43]. We just note that they suggested a different minimum structure of the anion compared to [41].
Figure 2. Structures for small water clusters (H2O)n − with n = 2–10 [40]. Color scheme: oxygen atoms are red and hydrogen atoms are white. Hydrogen bonds are shown in grey. Used with permission of Royal Society of Chemistry, from 'Water clusters to nanodrops: a tight-binding density functional study'. Reproduced from [40] with permission from the Royal Society of Chemistry.
Download figure:
Standard image High-resolution imageThe peak maximum of the water trimer anion (H2O)3
− is slightly shifted towards higher electron energies compared to (H2O)2
–. The maximum ion yield is found at  eV. Furthermore, the (H2O)3
− peak has a tail on the higher energy side with a contribution of a structure at
eV. Furthermore, the (H2O)3
− peak has a tail on the higher energy side with a contribution of a structure at  eV. It was proposed that the structure of the neutral water trimer is cyclic [40, 41], see also figure 2. However, Le Huyen et al suggested that upon the attachment of an electron, the nature of the intermolecular bonding changes from the classical O–H–O hydrogen bonding to H–H di–hydrogen bonding [41]. In addition, they pointed out that the (H2O)3
− cluster anion with such structure is almost isoenergetic to the one in a chain geometry (such structure was proposed by [44]). The anion efficiency curves of (H2O)4
− and (H2O)5
− reveal approximately the same shape as that for the (H2O)3
− cluster, see figure 1. The peaks for (H2O)4
− and (H2O)5
− appear at
eV. It was proposed that the structure of the neutral water trimer is cyclic [40, 41], see also figure 2. However, Le Huyen et al suggested that upon the attachment of an electron, the nature of the intermolecular bonding changes from the classical O–H–O hydrogen bonding to H–H di–hydrogen bonding [41]. In addition, they pointed out that the (H2O)3
− cluster anion with such structure is almost isoenergetic to the one in a chain geometry (such structure was proposed by [44]). The anion efficiency curves of (H2O)4
− and (H2O)5
− reveal approximately the same shape as that for the (H2O)3
− cluster, see figure 1. The peaks for (H2O)4
− and (H2O)5
− appear at  eV and
eV and  eV, respectively. Similarly, for both clusters we can see the contribution of a structure in the tail of the resonance visible at
eV, respectively. Similarly, for both clusters we can see the contribution of a structure in the tail of the resonance visible at  eV and
eV and  eV, respectively. The structure of neutral clusters with
eV, respectively. The structure of neutral clusters with  is limited to two-dimensional structures (linear for the dimer and cyclic for the rest [40]). For the anionic tetramers, it was proposed that the cyclic structure is retained [45, 46], while Le Huyen et al suggested that the cyclic shape becomes destroyed and molecules move out of the plane [41].
is limited to two-dimensional structures (linear for the dimer and cyclic for the rest [40]). For the anionic tetramers, it was proposed that the cyclic structure is retained [45, 46], while Le Huyen et al suggested that the cyclic shape becomes destroyed and molecules move out of the plane [41].
As figure 2 indicates, three dimensional structures of water clusters begin to form from the hexamer  . The (H2O)6 cluster may exist in a two-dimensional (cyclic) and three-dimensional (prism, cage, book, boat (bag)) form [40, 47]. The overall shape of the (H2O)6
− ion yield shown in figure 1 does not show a substantial difference in comparison to (H2O)n
− (
. The (H2O)6 cluster may exist in a two-dimensional (cyclic) and three-dimensional (prism, cage, book, boat (bag)) form [40, 47]. The overall shape of the (H2O)6
− ion yield shown in figure 1 does not show a substantial difference in comparison to (H2O)n
− ( ) though the intensity of the second peak at
) though the intensity of the second peak at  eV is slightly increased compared to the main feature at
eV is slightly increased compared to the main feature at  eV. The (H2O)6
− [48] and (H2O)7
− [49] have several isomer structures. These isomers were characterized according to their VDEs. The calculated VDE of (H2O)7
− isomers showed a nearly linear dependence on the dipole moments of the neutral scaffolds. The difference in the dipole moment may then also affect the electron attachment energy. Therefore, the structures contributing in the tail on the higher energy side may be resulting from the formation of different isomers of the (H2O)n
–. Such isomer effect was reported in a previous electron attachment study with isomers of aminobutanoic acid, which included long range interactions arising from the dipole moment in the theoretical calculations of ion yield features near threshold [50]. The recorded data for the formation of (H2O)7
− shows that the position of the maximum ion yield is almost at the same energy as for the formation (H2O)6
− with the value of
eV. The (H2O)6
− [48] and (H2O)7
− [49] have several isomer structures. These isomers were characterized according to their VDEs. The calculated VDE of (H2O)7
− isomers showed a nearly linear dependence on the dipole moments of the neutral scaffolds. The difference in the dipole moment may then also affect the electron attachment energy. Therefore, the structures contributing in the tail on the higher energy side may be resulting from the formation of different isomers of the (H2O)n
–. Such isomer effect was reported in a previous electron attachment study with isomers of aminobutanoic acid, which included long range interactions arising from the dipole moment in the theoretical calculations of ion yield features near threshold [50]. The recorded data for the formation of (H2O)7
− shows that the position of the maximum ion yield is almost at the same energy as for the formation (H2O)6
− with the value of  eV. The feature at the higher energy side of the main peak appears also for this cluster size but it has as higher relative intensity relative to the maximum. Additionally, a new peak is present at the electron energy of
eV. The feature at the higher energy side of the main peak appears also for this cluster size but it has as higher relative intensity relative to the maximum. Additionally, a new peak is present at the electron energy of  eV with about 20% intensity of the main peak.
eV with about 20% intensity of the main peak.
In 2014, Zakharov calculated the adiabatic bound state of an excess electron for the (H2O)8
− water cluster anion [51]. For the neutral cluster at the optimized structure of (H2O)8
− (coming close to a cube like structure, see also figure 2) he suggested a very high dipole moment of 10.95 D. Thus, the excess electron may bind via a strong electron-dipole interaction. In our results, the anion efficiency curve of (H2O)8
− has a different progression to the one seen from the formation of (H2O)3
− up to (H2O)7
–. As shown in figure 1, the single fits suggest that the first peak at  eV is not the dominant feature any more, whereas the peak at
eV is not the dominant feature any more, whereas the peak at  eV exceeds the other contributions. The fitting procedure indicates another peak at
eV exceeds the other contributions. The fitting procedure indicates another peak at  eV in addition to the peak at
eV in addition to the peak at  eV already showing up in the (H2O)7
− ion yield. Water clusters consisting of 7 water molecules may start to form a three-dimensional prism structure [49], see also figure 2. Thus, the enhancement of the higher energy resonance structure may be ascribed to such structures that become more stable with the increase of the number of water molecules within the water cluster. In the case of the (H2O)8
–, the cubic cage structure is maintained and the electron is bonded to four uncoordinated hydrogen atoms of 4 water molecules located on one face of the cube [51].
eV already showing up in the (H2O)7
− ion yield. Water clusters consisting of 7 water molecules may start to form a three-dimensional prism structure [49], see also figure 2. Thus, the enhancement of the higher energy resonance structure may be ascribed to such structures that become more stable with the increase of the number of water molecules within the water cluster. In the case of the (H2O)8
–, the cubic cage structure is maintained and the electron is bonded to four uncoordinated hydrogen atoms of 4 water molecules located on one face of the cube [51].
The experimental data discussed so far indicate that, till n = 7, the ion yields have their maximum near the electron energy of  eV. When the number of water molecules within the cluster reaches 8, the first peak is no longer dominant. This progression is also continued for (H2O)10
− as shown in figure 1. The structure also becomes broader and may have more features than the fitted three peaks. The recorded anion yield as a function of the electron energy for (H2O)13
− is presented in figure 1 as well. The resonance at ∼1.23 eV is no longer apparent for (H2O)13
–, since the features at higher electron energies prevail. This behavior also extends to the cluster anions (H2O)16
− and (H2O)19
− which is the largest cluster investigated in this study. As seen in figure 1, the ion yield structure continues to widen up for these cluster sizes and the maximum shifts towards higher electron energies. The ion yield maximum for (H2O)19
− can be found at
eV. When the number of water molecules within the cluster reaches 8, the first peak is no longer dominant. This progression is also continued for (H2O)10
− as shown in figure 1. The structure also becomes broader and may have more features than the fitted three peaks. The recorded anion yield as a function of the electron energy for (H2O)13
− is presented in figure 1 as well. The resonance at ∼1.23 eV is no longer apparent for (H2O)13
–, since the features at higher electron energies prevail. This behavior also extends to the cluster anions (H2O)16
− and (H2O)19
− which is the largest cluster investigated in this study. As seen in figure 1, the ion yield structure continues to widen up for these cluster sizes and the maximum shifts towards higher electron energies. The ion yield maximum for (H2O)19
− can be found at  eV. To briefly summarize, in the anion efficiency curves of (H2O)n
–, a succession in the evolution of the shapes is realized. Since the anion efficiency curves shown reflect the probability of electron attachment at different electron energies, we find that the attachment of the excess electron becomes more probable at higher electron energies with the increase of n (see figure 1).
eV. To briefly summarize, in the anion efficiency curves of (H2O)n
–, a succession in the evolution of the shapes is realized. Since the anion efficiency curves shown reflect the probability of electron attachment at different electron energies, we find that the attachment of the excess electron becomes more probable at higher electron energies with the increase of n (see figure 1).
4. Conclusion
Water cluster anions with sizes ranging from two water molecules up to 19 water molecules per cluster were formed in cold helium nanodroplets. The corresponding anion efficiency curves of water cluster anions show that the onset of resonances is shifted by the conduction band energy of helium droplets, which were the place of growth for these water clusters. Comparing the different cluster sizes , changes in the ion yield shapes can be derived. As discussed above, we may tentatively attribute these changes to different excess electron states where the existence of these states depends on the structure of the water cluster. They change from a linear structure to a planar structure ending up with a stable three-dimensional structure as the number of water molecules within the cluster increases. It can be expected that singly occupied molecular orbitals and their energies can be varying for each different structure as previously calculated for the tetramer anion [52]. However, we point out that this is just a first hypothesis and extensive further experimental and theoretical work is required in the future for confirmation. It also needs to be considered that the cold helium droplet environment (0.37 K [53]) would allow the formation of higher energy structures as demonstrated by Nauta and Miller [54, 55]. They found that the cyclic water hexamer cluster is formed by adding water molecules to smaller preformed cyclic complexes. This prevents formation of the more stable cage structure. Thus, the special properties of the helium droplets may complicate the situation further, e.g. when resonance calculations are aimed to reproduce the experimental anion efficiency curves. To provide better evidence about the cluster structure involved, experimental photoelectron spectroscopy at the specific peaks found presently at the different electron energies would reveal the VDE which could be compared with theoretical VDE´s for corresponding different isomeric structures.
Acknowledgments
This work was supported by the FWF, Vienna (P24443).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
Conflict of interest
The authors declare no conflict of interest.
Supplementary data (<0.1 MB DOCX)